Deck 40: One-Dimensional Quantum Mechanics
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/32
Play
Full screen (f)
Deck 40: One-Dimensional Quantum Mechanics
1
An electron is in an infinite square well that is 2.6 nm wide.What is the smallest value of the state quantum number n for which the energy level exceeds 100 eV? (h = 6.626 × 10-34 J ∙ s, mel = 9.11 × 10-31 kg,1 eV = 1.60 × 10-19)
A)43
B)44
C)45
D)42
E)41
A)43
B)44
C)45
D)42
E)41
43
2
The wave function of an electron in a rigid box (infinite well)is shown in the figure.If the electron energy 98.0 eV,what is the energy of the electron's ground state? (mel = 9.11 × 10-31 kg) 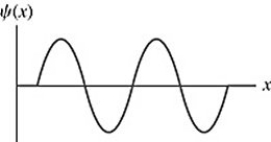
A)6.13 eV
B)3.92 eV
C)10.9 eV
D)24.5 eV

A)6.13 eV
B)3.92 eV
C)10.9 eV
D)24.5 eV
6.13 eV
3
A 10.0-g bouncy ball is confined in a 8.3-cm-long box (an infinite well).If we model the ball as a point particle,what is the minimum kinetic energy of the ball? (h = 6.626 × 10-34 J ∙ s)
A)8.0 × 10-64 J
B)3.2 × 10-46 J
C)1.3 × 10-20 J
D)zero
A)8.0 × 10-64 J
B)3.2 × 10-46 J
C)1.3 × 10-20 J
D)zero
8.0 × 10-64 J
4
You want to confine an electron in a box (an infinite well)so that its ground state energy is 5.0 × 10-18 J.What should be the length of the box? (h = 6.626 × 10-34 J ∙ s, mel = 9.11 × 10-31 kg)
A)0.11 nm
B)0.22 nm
C)0.15 nm
D)0.18 nm
A)0.11 nm
B)0.22 nm
C)0.15 nm
D)0.18 nm

Unlock Deck
Unlock for access to all 32 flashcards in this deck.
Unlock Deck
k this deck
5
An electron is in an infinite square well (a box)that is 2.0 nm wide.The electron makes a transition from the 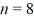 to the
to the 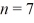 state,what is the wavelength of the emitted photon? (h = 6.626 × 10-34 J ∙ s,mel = 9.11 × 10-31 kg,1 eV = 1.60 × 10-19)
state,what is the wavelength of the emitted photon? (h = 6.626 × 10-34 J ∙ s,mel = 9.11 × 10-31 kg,1 eV = 1.60 × 10-19)
A)880 nm
B)750 nm
C)610 nm
D)1000 nm
E)1100 nm
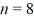 to the
to the 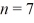 state,what is the wavelength of the emitted photon? (h = 6.626 × 10-34 J ∙ s,mel = 9.11 × 10-31 kg,1 eV = 1.60 × 10-19)
state,what is the wavelength of the emitted photon? (h = 6.626 × 10-34 J ∙ s,mel = 9.11 × 10-31 kg,1 eV = 1.60 × 10-19)A)880 nm
B)750 nm
C)610 nm
D)1000 nm
E)1100 nm

Unlock Deck
Unlock for access to all 32 flashcards in this deck.
Unlock Deck
k this deck
6
An electron is bound in an infinite well (a box)of width 0.10 nm.If the electron is initially in the n = 8 state and falls to the n = 7 state,find the wavelength of the emitted photon.
(c = 3.00 × 108 m/s,h = 6.626 × 10-34 J ∙ s,mel = 9.11 × 10-31 kg)
(c = 3.00 × 108 m/s,h = 6.626 × 10-34 J ∙ s,mel = 9.11 × 10-31 kg)

Unlock Deck
Unlock for access to all 32 flashcards in this deck.
Unlock Deck
k this deck
7
An electron is in the ground state of an infinite well (a box)where its energy is 5.00 eV.In the next higher level,what would its energy be? (1 eV = 1.60 × 10-19 J)
A)1.25 eV
B)2.50 eV
C)10.0 eV
D)15.0 eV
E)20.0 eV
A)1.25 eV
B)2.50 eV
C)10.0 eV
D)15.0 eV
E)20.0 eV

Unlock Deck
Unlock for access to all 32 flashcards in this deck.
Unlock Deck
k this deck
8
An electron is confined in a one-dimensional box (an infinite well).Two adjacent allowed energies of the electron are 1.068 × 10-18 J and 1.352 × 10-18 J.What is the length of the box? (h = 6.626 × 10-34 J ∙ s,mel = 9.11 × 10-31 kg)
A)1.9 nm
B)0.93 nm
C)1.1 nm
D)2.3 nm
A)1.9 nm
B)0.93 nm
C)1.1 nm
D)2.3 nm

Unlock Deck
Unlock for access to all 32 flashcards in this deck.
Unlock Deck
k this deck
9
The lowest energy level of a particle confined to a one-dimensional region of space (a box,or infinite well)with fixed length L is E0.If an identical particle is confined to a similar region with fixed length L/6,what is the energy of the lowest energy level that the particles have in common? Express your answer in terms of E0.

Unlock Deck
Unlock for access to all 32 flashcards in this deck.
Unlock Deck
k this deck
10
A particle is confined to a one-dimensional box (an infinite well)on the x-axis between x = 0 and x = L.The potential height of the walls of the box is infinite.The normalized wave function of the particle,which is in the ground state,is given by ψ(x)= 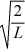 sin
sin 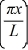 ,with 0 ≤ x ≤ L.What is the maximum probability per unit length of finding the particle?
,with 0 ≤ x ≤ L.What is the maximum probability per unit length of finding the particle?
A)1/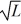
B)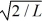
C)2/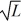
D)1/L
E)2/L
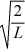 sin
sin 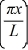 ,with 0 ≤ x ≤ L.What is the maximum probability per unit length of finding the particle?
,with 0 ≤ x ≤ L.What is the maximum probability per unit length of finding the particle?A)1/
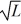
B)
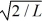
C)2/
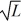
D)1/L
E)2/L

Unlock Deck
Unlock for access to all 32 flashcards in this deck.
Unlock Deck
k this deck
11
If an atom in a crystal is acted upon by a restoring force that is directly proportional to the distance of the atom from its equilibrium position in the crystal,then it is impossible for the atom to have zero kinetic energy.

Unlock Deck
Unlock for access to all 32 flashcards in this deck.
Unlock Deck
k this deck
12
A particle is confined to a one-dimensional box (an infinite well)on the x-axis between x = 0 and x = L.The potential height of the walls of the box is infinite.The normalized wave function of the particle,which is in the ground state,is given by ψ(x)= 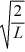 sin
sin 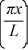 ,with 0 ≤ x ≤ L.What is the probability of finding the particle between x = 0 and x = L/3?
,with 0 ≤ x ≤ L.What is the probability of finding the particle between x = 0 and x = L/3?
A)0.20
B)0.22
C)0.24
D)0.26
E)0.28
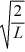 sin
sin 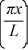 ,with 0 ≤ x ≤ L.What is the probability of finding the particle between x = 0 and x = L/3?
,with 0 ≤ x ≤ L.What is the probability of finding the particle between x = 0 and x = L/3?A)0.20
B)0.22
C)0.24
D)0.26
E)0.28

Unlock Deck
Unlock for access to all 32 flashcards in this deck.
Unlock Deck
k this deck
13
You want to have an electron in an energy level where its speed is no more than 66 m/s.What is the length of the smallest box (an infinite well)in which you can do this? (h = 6.626 × 10-34 J ∙ s,mel = 9.11 × 10-31 kg)
A)5.5 µm
B)11 µm
C)2.8 µm
D)1.4 µm
A)5.5 µm
B)11 µm
C)2.8 µm
D)1.4 µm

Unlock Deck
Unlock for access to all 32 flashcards in this deck.
Unlock Deck
k this deck
14
An electron is in an infinite square well (a box)that is 8.9 nm wide.What is the ground state energy of the electron? (h = 6.626 × 10-34 J ∙ s,mel = 9.11 × 10-31 kg,1 eV = 1.60 × 10-19)
A)0.0048 eV
B)0.0057 eV
C)0.0066 eV
D)0.0076 eV
E)0.0085 eV
A)0.0048 eV
B)0.0057 eV
C)0.0066 eV
D)0.0076 eV
E)0.0085 eV

Unlock Deck
Unlock for access to all 32 flashcards in this deck.
Unlock Deck
k this deck
15
An electron is bound in an infinite square-well potential (a box)on the x-axis.The width of the well is L and the well extends from x = 0.00 nm to 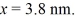 In its present state,the normalized wave function of the electron is given by: ψ(x)=
In its present state,the normalized wave function of the electron is given by: ψ(x)= 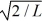 sin (2πx/L).What is the energy of the electron in this state? (h = 6.626 × 10-34 J ∙ s,mel = 9.11 × 10-31 kg,
sin (2πx/L).What is the energy of the electron in this state? (h = 6.626 × 10-34 J ∙ s,mel = 9.11 × 10-31 kg,
1 eV = 1.60 × 10-19)
A)0.10 eV
B)0.052 eV
C)0.13 eV
D)0.078 eV
E)0.026 eV
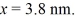 In its present state,the normalized wave function of the electron is given by: ψ(x)=
In its present state,the normalized wave function of the electron is given by: ψ(x)= 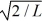 sin (2πx/L).What is the energy of the electron in this state? (h = 6.626 × 10-34 J ∙ s,mel = 9.11 × 10-31 kg,
sin (2πx/L).What is the energy of the electron in this state? (h = 6.626 × 10-34 J ∙ s,mel = 9.11 × 10-31 kg,1 eV = 1.60 × 10-19)
A)0.10 eV
B)0.052 eV
C)0.13 eV
D)0.078 eV
E)0.026 eV

Unlock Deck
Unlock for access to all 32 flashcards in this deck.
Unlock Deck
k this deck
16
An electron in an infinite potential well (a box)makes a transition from the n = 3 level to the ground state and in so doing emits a photon of wavelength 20.9 nm.(c = 3.00 × 108 m/s,
h = 6.626 × 10-34J ∙ s,mel = 9.11 × 10-31 kg)
(a)What is the width of this well?
(b)What wavelength photon would be required to excite the electron from its original level to the next higher one?
h = 6.626 × 10-34J ∙ s,mel = 9.11 × 10-31 kg)
(a)What is the width of this well?
(b)What wavelength photon would be required to excite the electron from its original level to the next higher one?

Unlock Deck
Unlock for access to all 32 flashcards in this deck.
Unlock Deck
k this deck
17
The smallest kinetic energy that an electron in a box (an infinite well)can have is zero.

Unlock Deck
Unlock for access to all 32 flashcards in this deck.
Unlock Deck
k this deck
18
A particle confined in a rigid one-dimensional box (an infinite well)of length 17.0 fm has an energy level 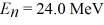 and an adjacent energy level En+1 = 37.5 MeV.What is the value of the ground state energy? (1 eV = 1.60 × 10-19 J)
and an adjacent energy level En+1 = 37.5 MeV.What is the value of the ground state energy? (1 eV = 1.60 × 10-19 J)
A)1.50 MeV
B)13.5 MeV
C)0.500 MeV
D)4.50 MeV
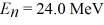 and an adjacent energy level En+1 = 37.5 MeV.What is the value of the ground state energy? (1 eV = 1.60 × 10-19 J)
and an adjacent energy level En+1 = 37.5 MeV.What is the value of the ground state energy? (1 eV = 1.60 × 10-19 J)A)1.50 MeV
B)13.5 MeV
C)0.500 MeV
D)4.50 MeV

Unlock Deck
Unlock for access to all 32 flashcards in this deck.
Unlock Deck
k this deck
19
An electron is trapped in an infinite square well (a box)of width 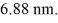 Find the wavelength of photons emitted when the electron drops from the n = 5 state to the n = 1 state in this system.(c = 3.00 × 108 m/s,h = 6.626 × 10-34 J ∙ s,mel = 9.11 × 10-31 kg)
Find the wavelength of photons emitted when the electron drops from the n = 5 state to the n = 1 state in this system.(c = 3.00 × 108 m/s,h = 6.626 × 10-34 J ∙ s,mel = 9.11 × 10-31 kg)
A)6.49 μm
B)5.45 μm
C)5.91 μm
D)7.07 μm
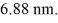 Find the wavelength of photons emitted when the electron drops from the n = 5 state to the n = 1 state in this system.(c = 3.00 × 108 m/s,h = 6.626 × 10-34 J ∙ s,mel = 9.11 × 10-31 kg)
Find the wavelength of photons emitted when the electron drops from the n = 5 state to the n = 1 state in this system.(c = 3.00 × 108 m/s,h = 6.626 × 10-34 J ∙ s,mel = 9.11 × 10-31 kg)A)6.49 μm
B)5.45 μm
C)5.91 μm
D)7.07 μm

Unlock Deck
Unlock for access to all 32 flashcards in this deck.
Unlock Deck
k this deck
20
One fairly crude method of determining the size of a molecule is to treat the molecule as an infinite square well (a box)with an electron trapped inside,and to measure the wavelengths of emitted photons.If the photon emitted during the n = 2 to n = 1 transition has wavelength 1940 nm,what is the width of the molecule? (c = 3.00 × 108 m/s,h = 6.626 × 10-34 J ∙ s,
Mel = 9.11 × 10-31 kg)
A)1.33 nm
B)1.12 nm
C)1.21 nm
D)1.45 nm
Mel = 9.11 × 10-31 kg)
A)1.33 nm
B)1.12 nm
C)1.21 nm
D)1.45 nm

Unlock Deck
Unlock for access to all 32 flashcards in this deck.
Unlock Deck
k this deck
21
Find the wavelength of the photon emitted during the transition from the second EXCITED state to the ground state in a harmonic oscillator with a classical frequency of 3.72 × 1013 Hz. (c = 3.00 × 108 m/s,1 eV = 1.60 × 10-19 J,h = 1.055 × 10-34 J ∙ s,h = 6.626 × 10-34 J ∙ s)
A)4.03 μm
B)2.26 μm
C)2.98 μm
D)5.24 μm
A)4.03 μm
B)2.26 μm
C)2.98 μm
D)5.24 μm

Unlock Deck
Unlock for access to all 32 flashcards in this deck.
Unlock Deck
k this deck
22
The atoms in a nickel crystal vibrate as harmonic oscillators with an angular frequency of 2.3 × 1013 rad/s.The mass of a nickel atom is 9.75 × 10-26 kg.What is the difference in energy between adjacent vibrational energy levels of nickel? (h = 6.626 × 10-34 J ∙ s,
H = 1.055 × 10-34 J ∙ s,1 eV = 1.60 × 10-19 J)
A)0.015 eV
B)0.019 eV
C)0.023 eV
D)0.027 eV
E)0.031 eV
H = 1.055 × 10-34 J ∙ s,1 eV = 1.60 × 10-19 J)
A)0.015 eV
B)0.019 eV
C)0.023 eV
D)0.027 eV
E)0.031 eV

Unlock Deck
Unlock for access to all 32 flashcards in this deck.
Unlock Deck
k this deck
23
An 80-eV electron impinges upon a potential barrier 100 eV high and 0.20 nm thick.What is the probability the electron will tunnel through the barrier? (1 eV = 1.60 × 10-19 J, mproton = 1.67 × 10-27 kg,h = 1.055 × 10-34 J ∙ s,h = 6.626 × 10-34 J ∙ s)
A)0.011%
B)1.1%
C)0.11%
D)1.1 × 10-4 %
E)7.7 × 10-10 %
A)0.011%
B)1.1%
C)0.11%
D)1.1 × 10-4 %
E)7.7 × 10-10 %

Unlock Deck
Unlock for access to all 32 flashcards in this deck.
Unlock Deck
k this deck
24
Calculate the ground state energy of a harmonic oscillator with a classical frequency of 3.68 × 1015 Hz.(1 eV = 1.60 × 10-19 J,h = 1.055 × 10-34 J ∙ s,h = 6.626 × 10-34 J ∙ s)
A)7.62 eV
B)15.2 eV
C)11.4 eV
D)5.71 eV
A)7.62 eV
B)15.2 eV
C)11.4 eV
D)5.71 eV

Unlock Deck
Unlock for access to all 32 flashcards in this deck.
Unlock Deck
k this deck
25
The energy of a particle in the second EXCITED state of a harmonic oscillator potential is 5.45 eV.What is the classical angular frequency of oscillation of this particle? (1 eV = 1.60 × 10-19 J,h = 1.055 × 10-34 J ∙ s,h = 6.626 × 10-34 J ∙ s)
A)3.31 × 1015 rad/s
B)2.08 × 1016 rad/s
C)4.97 × 1015 rad/s
D)6.95 × 1015 rad/s
A)3.31 × 1015 rad/s
B)2.08 × 1016 rad/s
C)4.97 × 1015 rad/s
D)6.95 × 1015 rad/s

Unlock Deck
Unlock for access to all 32 flashcards in this deck.
Unlock Deck
k this deck
26
The energy of a proton is 1.0 MeV below the top of a 6.8-fm-wide energy barrier.What is the probability that the proton will tunnel through the barrier? (1 eV = 1.60 × 10-19 J, mproton = 1.67 × 10-27 kg,h = 1.055 × 10-34 J ∙ s,h = 6.626 × 10-34 J ∙ s)
A)0.051
B)0.048
C)0.056
D)0.053
A)0.051
B)0.048
C)0.056
D)0.053

Unlock Deck
Unlock for access to all 32 flashcards in this deck.
Unlock Deck
k this deck
27
A 3.10-eV electron is incident on a 0.40-nm barrier that is 5.67 eV high.What is the probability that this electron will tunnel through the barrier? (1 eV = 1.60 × 10-19 J, mel = 9.11 × 10-31 kg,h = 1.055 × 10-34 J ∙ s,h = 6.626 × 10-34 J ∙ s)
A)1.4 × 10-3
B)9.0 × 10-4
C)1.0 × 10-3
D)1.5 × 10-3
E)1.2 × 10-3
A)1.4 × 10-3
B)9.0 × 10-4
C)1.0 × 10-3
D)1.5 × 10-3
E)1.2 × 10-3

Unlock Deck
Unlock for access to all 32 flashcards in this deck.
Unlock Deck
k this deck
28
An electron is confined in a harmonic oscillator potential well.A photon is emitted when the electron undergoes a 3→1 quantum jump.What is the wavelength of the emission if the net force on the electron behaves as though it has a spring constant of 9.6 N/m? (mel = 9.11 × 10-31 kg, c = 3.00 × 108 m/s,1 eV = 1.60 × 10-19 J,h = 1.055 × 10-34 J ∙ s,h = 6.626 × 10-34 J ∙ s)
A)290 nm
B)150 nm
C)190 nm
D)580 nm
A)290 nm
B)150 nm
C)190 nm
D)580 nm

Unlock Deck
Unlock for access to all 32 flashcards in this deck.
Unlock Deck
k this deck
29
An electron with kinetic energy 2.80 eV encounters a potential barrier of height 4.70 eV.If the barrier width is 0.40 nm,what is the probability that the electron will tunnel through the barrier? (1 eV = 1.60 × 10-19 J,mel = 9.11 × 10-31 kg,h = 1.055 × 10-34 J ∙ s, h = 6.626 × 10-34 J ∙ s)
A)3.5 × 10-3
B)7.3 × 10-3
C)1.4 × 10-2
D)2.9 × 10-3
E)3.5 × 10-2
A)3.5 × 10-3
B)7.3 × 10-3
C)1.4 × 10-2
D)2.9 × 10-3
E)3.5 × 10-2

Unlock Deck
Unlock for access to all 32 flashcards in this deck.
Unlock Deck
k this deck
30
An electron is confined in a harmonic oscillator potential well.What is the longest wavelength of light that the electron can absorb if the net force on the electron behaves as though it has a spring constant of 74 N/m? (mel = 9.11 × 10-31 kg,c = 3.00 × 108 m/s, 1 eV = 1.60 × 10-19 J,h = 1.055 × 10-34 J ∙ s,h = 6.626 × 10-34 J ∙ s)
A)210 nm
B)200 nm
C)220 nm
D)230 nm
A)210 nm
B)200 nm
C)220 nm
D)230 nm

Unlock Deck
Unlock for access to all 32 flashcards in this deck.
Unlock Deck
k this deck
31
The lowest energy level of a certain quantum harmonic oscillator is 5.00 eV.What is the energy of the next higher level?
A)7.50 eV
B)10.0 eV
C)15.0 eV
D)20.0 eV
E)50.0 eV
A)7.50 eV
B)10.0 eV
C)15.0 eV
D)20.0 eV
E)50.0 eV

Unlock Deck
Unlock for access to all 32 flashcards in this deck.
Unlock Deck
k this deck
32
A lithium atom,mass 1.17 × 10-26 kg,vibrates with simple harmonic motion in a crystal lattice,where the effective force constant of the forces on the atom is k = 49.0 N/m.
(c = 3.00 × 108 m/s,h = 6.626 × 10-34 J ∙ s,h = 1.055 × 10-34 J ∙ s,1 eV = 1.60 × 10-19 J)
(a)What is the ground state energy of this system,in eV?
(b)What is the wavelength of the photon that could excite this system from the ground state to the first excited state?
(c = 3.00 × 108 m/s,h = 6.626 × 10-34 J ∙ s,h = 1.055 × 10-34 J ∙ s,1 eV = 1.60 × 10-19 J)
(a)What is the ground state energy of this system,in eV?
(b)What is the wavelength of the photon that could excite this system from the ground state to the first excited state?

Unlock Deck
Unlock for access to all 32 flashcards in this deck.
Unlock Deck
k this deck


