Deck 5: Matter and Heat
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/65
Play
Full screen (f)
Deck 5: Matter and Heat
1
A Celsius thermometer and an absolute thermometer are used to measure the temperature of the same gas sample. The readings on the thermometers are respectively TC and TK. Which of these statements is correct?
A) TC is smaller than TK.
B) TC is larger than TK.
C) TC is equal to TK.
D) Any of these choices could be correct, depending on the gas temperature.
A) TC is smaller than TK.
B) TC is larger than TK.
C) TC is equal to TK.
D) Any of these choices could be correct, depending on the gas temperature.
TC is smaller than TK.
2
Of these substances, the one with the lowest specific heat is
A) water.
B) ice.
C) concrete.
D) gold.
A) water.
B) ice.
C) concrete.
D) gold.
gold.
3
The pressure at the bottom of a barrel filled with liquid does not depend on the
A) acceleration of gravity.
B) liquid density.
C) height of the liquid.
D) area of the liquid surface.
A) acceleration of gravity.
B) liquid density.
C) height of the liquid.
D) area of the liquid surface.
area of the liquid surface.
4
Which of the following quantities is independent of the size and shape of an object composed of a given material?
A) volume
B) mass
C) weight
D) density
A) volume
B) mass
C) weight
D) density

Unlock Deck
Unlock for access to all 65 flashcards in this deck.
Unlock Deck
k this deck
5
Of the following, a molecule is best described as
A) any very tiny particle.
B) the smallest particle found in nature.
C) the smallest particle of a substance that is representative of the substance.
D) the ultimate particle of which all matter is composed.
A) any very tiny particle.
B) the smallest particle found in nature.
C) the smallest particle of a substance that is representative of the substance.
D) the ultimate particle of which all matter is composed.

Unlock Deck
Unlock for access to all 65 flashcards in this deck.
Unlock Deck
k this deck
6
The average pressure of the atmosphere at sea level corresponds to which one or more of the following?
A) 101 Pa
B) 101 kPa
C) 98 kPa
D) 15 lb/in2
E) Both B and D
A) 101 Pa
B) 101 kPa
C) 98 kPa
D) 15 lb/in2
E) Both B and D

Unlock Deck
Unlock for access to all 65 flashcards in this deck.
Unlock Deck
k this deck
7
A barometer measures
A) atmospheric density.
B) atmospheric pressure.
C) water density.
D) water pressure.
A) atmospheric density.
B) atmospheric pressure.
C) water density.
D) water pressure.

Unlock Deck
Unlock for access to all 65 flashcards in this deck.
Unlock Deck
k this deck
8
The pressure on the bottom of an object inside a liquid is
A) less than the pressure on its top.
B) equal to the pressure on its top.
C) more than the pressure on its top.
D) Any of these choices, depending on the nature of the liquid.
A) less than the pressure on its top.
B) equal to the pressure on its top.
C) more than the pressure on its top.
D) Any of these choices, depending on the nature of the liquid.

Unlock Deck
Unlock for access to all 65 flashcards in this deck.
Unlock Deck
k this deck
9
Which of the following formulas expresses the relationship between the pressure and absolute temperature of a gas sample whose volume is fixed?
A)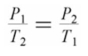
B)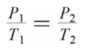
C)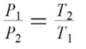
D)
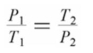
A)
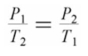
B)
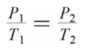
C)
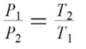
D)
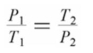

Unlock Deck
Unlock for access to all 65 flashcards in this deck.
Unlock Deck
k this deck
10
Ice floats in water because
A) its density is less than that of water.
B) its specific heat is less than that of water.
C) it is a solid whereas water is a liquid.
D) it is colder than water.
A) its density is less than that of water.
B) its specific heat is less than that of water.
C) it is a solid whereas water is a liquid.
D) it is colder than water.

Unlock Deck
Unlock for access to all 65 flashcards in this deck.
Unlock Deck
k this deck
11
A typical metabolic rate while walking is
A) 10 W.
B) 50 W.
C) 300 W.
D) 1.2 kW.
A) 10 W.
B) 50 W.
C) 300 W.
D) 1.2 kW.

Unlock Deck
Unlock for access to all 65 flashcards in this deck.
Unlock Deck
k this deck
12
A thermometer calibrated in the Celsius scale and a thermometer calibrated in the Fahrenheit scale are used to measure the same temperature. The numerical reading on the Fahrenheit thermometer
A) is proportional to that on the Celsius thermometer.
B) is smaller than that on the Celsius thermometer.
C) is larger than that on the Celsius thermometer.
D) may be smaller or larger than that on the Celsius thermometer.
A) is proportional to that on the Celsius thermometer.
B) is smaller than that on the Celsius thermometer.
C) is larger than that on the Celsius thermometer.
D) may be smaller or larger than that on the Celsius thermometer.

Unlock Deck
Unlock for access to all 65 flashcards in this deck.
Unlock Deck
k this deck
13
Of the substances below, the one with the highest specific heat is
A) water.
B) ice.
C) concrete.
D) gold.
A) water.
B) ice.
C) concrete.
D) gold.

Unlock Deck
Unlock for access to all 65 flashcards in this deck.
Unlock Deck
k this deck
14
The properties of several different materials are being compared. If the samples all have the same volume, the one with the greatest mass also has the greatest
A) density.
B) buoyancy.
C) pressure.
D) temperature.
A) density.
B) buoyancy.
C) pressure.
D) temperature.

Unlock Deck
Unlock for access to all 65 flashcards in this deck.
Unlock Deck
k this deck
15
When heat is added to a body of matter, the resulting temperature increase does not depend upon
A) its mass.
B) its shape.
C) what kind of material it consists of.
D) whether it is in the solid, liquid, or gaseous state.
A) its mass.
B) its shape.
C) what kind of material it consists of.
D) whether it is in the solid, liquid, or gaseous state.

Unlock Deck
Unlock for access to all 65 flashcards in this deck.
Unlock Deck
k this deck
16
A cake of soap is placed in a bathtub sink. The buoyant force on the soap is
A) 0.
B) less than its weight.
C) equal to its weight.
D) more than its weight.
A) 0.
B) less than its weight.
C) equal to its weight.
D) more than its weight.

Unlock Deck
Unlock for access to all 65 flashcards in this deck.
Unlock Deck
k this deck
17
In order for an object to sink when placed in water, the object's average density must be
A) less than that of water.
B) equal to that of water.
C) more than that of water.
D) Any of these choices are correct, depending on the object's shape.
A) less than that of water.
B) equal to that of water.
C) more than that of water.
D) Any of these choices are correct, depending on the object's shape.

Unlock Deck
Unlock for access to all 65 flashcards in this deck.
Unlock Deck
k this deck
18
Which of the following is not true of molecular motion in a gas?
A) There is no order in the motion.
B) There is no uniformity of speed or direction.
C) There is a definite average speed at a given temperature.
D) There is a definite average direction of motion at a given temperature.
A) There is no order in the motion.
B) There is no uniformity of speed or direction.
C) There is a definite average speed at a given temperature.
D) There is a definite average direction of motion at a given temperature.

Unlock Deck
Unlock for access to all 65 flashcards in this deck.
Unlock Deck
k this deck
19
Which of the following formulas relating temperatures on the Celsius and Fahrenheit scales is correct?
A) Tc = (5/9)(TF - 32 )
B) Tc = (5/9)(TF + 32 )
C) Tc = (9/5)(TF - 32 )
D) Tc = (9/5)(TF + 32 )
A) Tc = (5/9)(TF - 32 )
B) Tc = (5/9)(TF + 32 )
C) Tc = (9/5)(TF - 32 )
D) Tc = (9/5)(TF + 32 )

Unlock Deck
Unlock for access to all 65 flashcards in this deck.
Unlock Deck
k this deck
20
Molecules are, in general, farthest apart from one another in
A) gases.
B) liquids.
C) crystalline solids.
D) amorphous solids.
A) gases.
B) liquids.
C) crystalline solids.
D) amorphous solids.

Unlock Deck
Unlock for access to all 65 flashcards in this deck.
Unlock Deck
k this deck
21
The temperature of a gas sample in a rigid container is reduced. The pressure the gas exerts on the container walls decreases because
A) its molecules are in contact with the walls for briefer intervals.
B) its molecular masses decrease.
C) its molecules have lower average speeds and so strike the walls less often with less momentum.
D) its molecules lose less energy each time they strike the walls.
A) its molecules are in contact with the walls for briefer intervals.
B) its molecular masses decrease.
C) its molecules have lower average speeds and so strike the walls less often with less momentum.
D) its molecules lose less energy each time they strike the walls.

Unlock Deck
Unlock for access to all 65 flashcards in this deck.
Unlock Deck
k this deck
22
Molecular motion in a gas is the minimum possible at
A) 0 F.
B) 0 C.
C) 0 K.
D) -273 K.
A) 0 F.
B) 0 C.
C) 0 K.
D) -273 K.

Unlock Deck
Unlock for access to all 65 flashcards in this deck.
Unlock Deck
k this deck
23
Steam at 100 C is more dangerous than the same mass of water at 100 C because the steam
A) is less dense.
B) moves faster.
C) has a higher specific heat.
D) releases a great deal of heat when it condenses.
A) is less dense.
B) moves faster.
C) has a higher specific heat.
D) releases a great deal of heat when it condenses.

Unlock Deck
Unlock for access to all 65 flashcards in this deck.
Unlock Deck
k this deck
24
When a gas is forced into a smaller volume without a change in temperature, its pressure increases because its molecules
A) strike the container walls more often.
B) strike the container walls at higher speeds.
C) strike the container walls with greater force.
D) have more energy.
A) strike the container walls more often.
B) strike the container walls at higher speeds.
C) strike the container walls with greater force.
D) have more energy.

Unlock Deck
Unlock for access to all 65 flashcards in this deck.
Unlock Deck
k this deck
25
Heat transfer by conduction occurs
A) only in liquids.
B) only in solids.
C) only in liquids and solids.
D) in liquids, solids, and gases.
A) only in liquids.
B) only in solids.
C) only in liquids and solids.
D) in liquids, solids, and gases.

Unlock Deck
Unlock for access to all 65 flashcards in this deck.
Unlock Deck
k this deck
26
The maximum possible efficiency of a heat engine
A) is 100%.
B) depends on the intake temperature.
C) depends on the difference between the exhaust and intake temperatures.
D) depends on the ratio between the exhaust and intake temperatures.
A) is 100%.
B) depends on the intake temperature.
C) depends on the difference between the exhaust and intake temperatures.
D) depends on the ratio between the exhaust and intake temperatures.

Unlock Deck
Unlock for access to all 65 flashcards in this deck.
Unlock Deck
k this deck
27
The physics of a heat engine cannot be used to understand the operation of a
A) nuclear reactor.
B) heat pump.
C) refrigerator.
D) diesel engine.
A) nuclear reactor.
B) heat pump.
C) refrigerator.
D) diesel engine.

Unlock Deck
Unlock for access to all 65 flashcards in this deck.
Unlock Deck
k this deck
28
Heat transfer in a vacuum
A) can take place only by radiation.
B) can take place only by radiation and convection.
C) can take place by radiation, convection, and conduction.
D) cannot take place.
A) can take place only by radiation.
B) can take place only by radiation and convection.
C) can take place by radiation, convection, and conduction.
D) cannot take place.

Unlock Deck
Unlock for access to all 65 flashcards in this deck.
Unlock Deck
k this deck
29
The melting point of water is 0 C. Its freezing point is
A) slightly less than 0 C.
B) 0 C.
C) slightly more than 0 C.
D) 32 C.
A) slightly less than 0 C.
B) 0 C.
C) slightly more than 0 C.
D) 32 C.

Unlock Deck
Unlock for access to all 65 flashcards in this deck.
Unlock Deck
k this deck
30
The first law of thermodynamics is the same as the
A) second law of thermodynamics.
B) law of conservation of energy.
C) law of conservation of momentum.
D) first law of motion.
A) second law of thermodynamics.
B) law of conservation of energy.
C) law of conservation of momentum.
D) first law of motion.

Unlock Deck
Unlock for access to all 65 flashcards in this deck.
Unlock Deck
k this deck
31
Radiation is emitted
A) only by liquids.
B) only by solids.
C) only by liquids and solids.
D) by liquids, solids, and gases.
A) only by liquids.
B) only by solids.
C) only by liquids and solids.
D) by liquids, solids, and gases.

Unlock Deck
Unlock for access to all 65 flashcards in this deck.
Unlock Deck
k this deck
32
In order to operate, a heat engine must have
A) either a hot or a cold reservoir.
B) both a hot and a cold reservoir.
C) a boiler.
D) a supply of fuel that can be burned, such as coal or oil.
A) either a hot or a cold reservoir.
B) both a hot and a cold reservoir.
C) a boiler.
D) a supply of fuel that can be burned, such as coal or oil.

Unlock Deck
Unlock for access to all 65 flashcards in this deck.
Unlock Deck
k this deck
33
The work output of every heat engine
A) equals its heat intake.
B) equals the difference between its heat intake and heat exhaust.
C) depends only on its intake temperature.
D) depends only on its exhaust temperature.
A) equals its heat intake.
B) equals the difference between its heat intake and heat exhaust.
C) depends only on its intake temperature.
D) depends only on its exhaust temperature.

Unlock Deck
Unlock for access to all 65 flashcards in this deck.
Unlock Deck
k this deck
34
When a liquid freezes to become a solid,
A) its temperature rises.
B) its temperature falls.
C) it absorbs heat.
D) it gives off heat.
A) its temperature rises.
B) its temperature falls.
C) it absorbs heat.
D) it gives off heat.

Unlock Deck
Unlock for access to all 65 flashcards in this deck.
Unlock Deck
k this deck
35
When a liquid becomes a vapor,
A) its temperature must decrease.
B) its temperature must increase.
C) it must absorb heat.
D) it must give off heat.
A) its temperature must decrease.
B) its temperature must increase.
C) it must absorb heat.
D) it must give off heat.

Unlock Deck
Unlock for access to all 65 flashcards in this deck.
Unlock Deck
k this deck
36
A sample of a gas is expanded to twice its original volume while its temperature is held constant. Relative to their original average energy, the new average energy of the molecules is
A) half as great.
B) the same.
C) twice as great.
D) four times as great.
A) half as great.
B) the same.
C) twice as great.
D) four times as great.

Unlock Deck
Unlock for access to all 65 flashcards in this deck.
Unlock Deck
k this deck
37
Suppose there were molecules that had no attraction whatsoever for one another. A collection of such molecules would form a
A) gas.
B) liquid.
C) amorphous solid.
D) crystalline solid.
A) gas.
B) liquid.
C) amorphous solid.
D) crystalline solid.

Unlock Deck
Unlock for access to all 65 flashcards in this deck.
Unlock Deck
k this deck
38
Sublimation refers to
A) the vaporization of a solid without first becoming a liquid.
B) the melting of a solid.
C) the vaporization of a liquid.
D) the condensation of a gas into a liquid.
A) the vaporization of a solid without first becoming a liquid.
B) the melting of a solid.
C) the vaporization of a liquid.
D) the condensation of a gas into a liquid.

Unlock Deck
Unlock for access to all 65 flashcards in this deck.
Unlock Deck
k this deck
39
Heat transfer by convection occurs
A) only in liquids.
B) only in gases.
C) only in liquids and gases.
D) in liquids, gases, and solids.
A) only in liquids.
B) only in gases.
C) only in liquids and gases.
D) in liquids, gases, and solids.

Unlock Deck
Unlock for access to all 65 flashcards in this deck.
Unlock Deck
k this deck
40
The freezing point of a substance is always lower than its
A) melting point.
B) boiling point.
C) heat of fusion.
D) heat of vaporization.
A) melting point.
B) boiling point.
C) heat of fusion.
D) heat of vaporization.

Unlock Deck
Unlock for access to all 65 flashcards in this deck.
Unlock Deck
k this deck
41
The "heat death" of the universe refers to a possible (although improbable) future in which all of its molecules are
A) at rest.
B) as close together as possible
C) moving in the same direction
D) moving with the same average speed
A) at rest.
B) as close together as possible
C) moving in the same direction
D) moving with the same average speed

Unlock Deck
Unlock for access to all 65 flashcards in this deck.
Unlock Deck
k this deck
42
The heat a refrigerator absorbs from its contents is
A) less than it gives off.
B) the same amount it gives off.
C) more than it gives off.
D) Any of these choices, depending on its design.
A) less than it gives off.
B) the same amount it gives off.
C) more than it gives off.
D) Any of these choices, depending on its design.

Unlock Deck
Unlock for access to all 65 flashcards in this deck.
Unlock Deck
k this deck
43
The pressure on 100 liters of helium is increased from 100 kPa to 400 kPa. The new volume of the helium is
A) 25 liters.
B) 50 liters.
C) 400 liters.
D) 1600 liters.
A) 25 liters.
B) 50 liters.
C) 400 liters.
D) 1600 liters.

Unlock Deck
Unlock for access to all 65 flashcards in this deck.
Unlock Deck
k this deck
44
A 400-kg concrete block is 1 m long, 0.6 m wide, and 0.3 m high. Its density is
A) 72 kg/m.
B) 222 kg/m.
C) 667 kg/m.
D) 2222 kg/m.
A) 72 kg/m.
B) 222 kg/m.
C) 667 kg/m.
D) 2222 kg/m.

Unlock Deck
Unlock for access to all 65 flashcards in this deck.
Unlock Deck
k this deck
45
In an isolated system, entropy
A) cannot decrease.
B) must remain constant.
C) cannot increase.
D) may increase, decrease, or remain constant.
A) cannot decrease.
B) must remain constant.
C) cannot increase.
D) may increase, decrease, or remain constant.

Unlock Deck
Unlock for access to all 65 flashcards in this deck.
Unlock Deck
k this deck
46
The specific heat of water is 4.2 kJ/kg C. The heat needed to warm 8 kg of water from 20 C to 70 C is
A) 400 kJ.
B) 420 kJ.
C) 1680 kJ.
D) 2016 kJ.
A) 400 kJ.
B) 420 kJ.
C) 1680 kJ.
D) 2016 kJ.

Unlock Deck
Unlock for access to all 65 flashcards in this deck.
Unlock Deck
k this deck
47
The specific heat of water is 4.2 kJ/kg C. How long will it take for a 2-kW heating element to raise the temperature of 30 kg of water from 20 C to 80 C?
A) 6.3 min
B) 15 min
C) 63 min
D) 84 min
A) 6.3 min
B) 15 min
C) 63 min
D) 84 min

Unlock Deck
Unlock for access to all 65 flashcards in this deck.
Unlock Deck
k this deck
48
A 400-kg concrete block is 1 m long, 0.6 m wide, and 0.3 m high. It can exert three different pressures on a horizontal surface, depending on which face it rests on. The highest pressure is
A) 0.7 kPa.
B) 2.2 kPa.
C) 13.1 kPa.
D) 21.8 kPa.
A) 0.7 kPa.
B) 2.2 kPa.
C) 13.1 kPa.
D) 21.8 kPa.

Unlock Deck
Unlock for access to all 65 flashcards in this deck.
Unlock Deck
k this deck
49
The density of brass is 8 103 kg/m3. The volume occupied by 320 g of brass is
A) 0.038 cm3.
B) 3.2 cm3.
C) 38 cm3.
D) 380 cm3.
A) 0.038 cm3.
B) 3.2 cm3.
C) 38 cm3.
D) 380 cm3.

Unlock Deck
Unlock for access to all 65 flashcards in this deck.
Unlock Deck
k this deck
50
When 90 kJ is removed from a 2-kg copper bar, its temperature drops from 200 C to 85 C. The specific heat of copper is
A) 0.16 kJ/kg C.
B) 0.19 kJ/kg C.
C) 0.23 kJ/kg C.
D) 0.39 kJ/kg C.
A) 0.16 kJ/kg C.
B) 0.19 kJ/kg C.
C) 0.23 kJ/kg C.
D) 0.39 kJ/kg C.

Unlock Deck
Unlock for access to all 65 flashcards in this deck.
Unlock Deck
k this deck
51
The specific heat of ice is 2.1 kJ/kg C. When 50 kJ of heat is removed from 2 kg of ice initially at -5 C, the final temperature of the ice is
A) -6 C.
B) -11 C.
C) -12 C.
D) -17 C.
A) -6 C.
B) -11 C.
C) -12 C.
D) -17 C.

Unlock Deck
Unlock for access to all 65 flashcards in this deck.
Unlock Deck
k this deck
52
An absolute temperature of 100 K is the same as a Celsius temperature of
A) -173 C.
B) 32 C.
C) 212 C.
D) 373 C.
A) -173 C.
B) 32 C.
C) 212 C.
D) 373 C.

Unlock Deck
Unlock for access to all 65 flashcards in this deck.
Unlock Deck
k this deck
53
The entropy of a system is a measure of its
A) temperature.
B) heat content.
C) density.
D) disorder.
A) temperature.
B) heat content.
C) density.
D) disorder.

Unlock Deck
Unlock for access to all 65 flashcards in this deck.
Unlock Deck
k this deck
54
At which of the following Celsius temperatures will a Fahrenheit thermometer show the same reading in degrees?
A) -40 C
B) 0 C
C) 32 C
D) 40 C
A) -40 C
B) 0 C
C) 32 C
D) 40 C

Unlock Deck
Unlock for access to all 65 flashcards in this deck.
Unlock Deck
k this deck
55
A temperature of 20 C is the same as
A) -20.9 F.
B) -6.4 F.
C) 68 F.
D) 94 F.
A) -20.9 F.
B) -6.4 F.
C) 68 F.
D) 94 F.

Unlock Deck
Unlock for access to all 65 flashcards in this deck.
Unlock Deck
k this deck
56
A refrigerator
A) produces cold.
B) changes heat to cold.
C) causes heat to disappear.
D) removes heat from a region and carries it elsewhere.
A) produces cold.
B) changes heat to cold.
C) causes heat to disappear.
D) removes heat from a region and carries it elsewhere.

Unlock Deck
Unlock for access to all 65 flashcards in this deck.
Unlock Deck
k this deck
57
One liter of hydrogen gas at atmospheric pressure is allowed to expand to a volume of 3 liters with the temperature held constant. If the average speed of the hydrogen molecules was originally v, their new average speed is
A) v/9.
B) v/3.
C) v.
D) 3 v.
A) v/9.
B) v/3.
C) v.
D) 3 v.

Unlock Deck
Unlock for access to all 65 flashcards in this deck.
Unlock Deck
k this deck
58
A container holds 1 g of air at atmospheric pressure. When an additional gram of air is pumped into the container and there is no change in temperature, the new pressure is
A) 0.5 atm.
B) 1 atm.
C) 2 atm.
D) 4 atm.
A) 0.5 atm.
B) 1 atm.
C) 2 atm.
D) 4 atm.

Unlock Deck
Unlock for access to all 65 flashcards in this deck.
Unlock Deck
k this deck
59
The density of air is 1.3 kg/m3. The air in a room 5 m long, 4 m wide, and 2.5 m high has a mass of
A) 0.26 kg.
B) 6.5 kg.
C) 38 kg.
D) 65 kg.
A) 0.26 kg.
B) 6.5 kg.
C) 38 kg.
D) 65 kg.

Unlock Deck
Unlock for access to all 65 flashcards in this deck.
Unlock Deck
k this deck
60
Ethanol boils at 172 F. The Celsius equivalent of this temperature is
A) 64 C.
B) 78 C.
C) 140 C.
D) 278 C.
A) 64 C.
B) 78 C.
C) 140 C.
D) 278 C.

Unlock Deck
Unlock for access to all 65 flashcards in this deck.
Unlock Deck
k this deck
61
In order to double the average energy of the molecules in a gas at 200 K, its temperature must be changed to
A) 100 K.
B) 400 K.
C) 800 K.
D) 1600 K.
A) 100 K.
B) 400 K.
C) 800 K.
D) 1600 K.

Unlock Deck
Unlock for access to all 65 flashcards in this deck.
Unlock Deck
k this deck
62
A heat engine absorbs heat at 127 C and exhausts heat at 77 C. Its maximum efficiency is
A) 13 percent.
B) 39 percent.
C) 61 percent.
D) 88 percent.
A) 13 percent.
B) 39 percent.
C) 61 percent.
D) 88 percent.

Unlock Deck
Unlock for access to all 65 flashcards in this deck.
Unlock Deck
k this deck
63
Fifty kJ of heat is added to a 10-kg piece of lead at its melting point of 330 C and 2 kg of lead melts. The heat of fusion of lead is
A) 2.5 kJ/kg.
B) 3.3 kJ/kg.
C) 5 kJ/kg.
D) 25 kJ/kg.
A) 2.5 kJ/kg.
B) 3.3 kJ/kg.
C) 5 kJ/kg.
D) 25 kJ/kg.

Unlock Deck
Unlock for access to all 65 flashcards in this deck.
Unlock Deck
k this deck
64
An ideal frictionless engine takes in 10 kJ of heat per second when it operates between 500 K and 400 K. The work that the engine does per second is
A) 2 kJ.
B) 2.5 kJ.
C) 8 kJ.
D) 10 kJ.
A) 2 kJ.
B) 2.5 kJ.
C) 8 kJ.
D) 10 kJ.

Unlock Deck
Unlock for access to all 65 flashcards in this deck.
Unlock Deck
k this deck
65
If it is to be 40 percent efficient, a heat engine that exhausts heat at 350 K must absorb heat at a minimum temperature of
A) 210 K.
B) 583 K.
C) 875 K.
D) 1038 K.
A) 210 K.
B) 583 K.
C) 875 K.
D) 1038 K.

Unlock Deck
Unlock for access to all 65 flashcards in this deck.
Unlock Deck
k this deck


