Deck 2: Matter and Minerals
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/115
Play
Full screen (f)
Deck 2: Matter and Minerals
1
Which electrons are responsible for most chemical bonding?
A) innermost electron shell because the electrons can be transferred to the nucleus
B) middle electron shell because they are intermediate in distance between the nucleus and the adjacent atom that bonds with the atom
C) outer electron shell because these electrons can be readily exchanged with adjacent atoms
D) Any electron can exchange with adjacent atoms to form a bond; there is no preference.
A) innermost electron shell because the electrons can be transferred to the nucleus
B) middle electron shell because they are intermediate in distance between the nucleus and the adjacent atom that bonds with the atom
C) outer electron shell because these electrons can be readily exchanged with adjacent atoms
D) Any electron can exchange with adjacent atoms to form a bond; there is no preference.
outer electron shell because these electrons can be readily exchanged with adjacent atoms
2
The first minerals to be mined over 6000 years ago. were _______ and_______ .
A) lead; quartz
B) gold; silver
C) copper; bronze
D) flint; chert
A) lead; quartz
B) gold; silver
C) copper; bronze
D) flint; chert
flint; chert
3
Which of the following is not a fundamental particle found in atoms?
A) electron
B) neutron
C) protons
D) selectron
A) electron
B) neutron
C) protons
D) selectron
selectron
4
Which of the following best defines a mineral and a rock?
A) A rock consists of atoms bonded in a regular, geometrically predictable arrangement; a mineral is a consolidated aggregate of different rock particles.
B) A rock has an orderly, repetitive, geometrical, internal arrangement of minerals; a mineral is a lithified or consolidated aggregate of rocks.
C) A mineral consists of its constituent atoms arranged in a geometrically repetitive structure; in a rock, the atoms are randomly bonded without any geometric pattern.
D) In a mineral the constituent atoms are bonded in a regular, repetitive, internal structure; a rock is a lithified or consolidated aggregate of different mineral grains.
A) A rock consists of atoms bonded in a regular, geometrically predictable arrangement; a mineral is a consolidated aggregate of different rock particles.
B) A rock has an orderly, repetitive, geometrical, internal arrangement of minerals; a mineral is a lithified or consolidated aggregate of rocks.
C) A mineral consists of its constituent atoms arranged in a geometrically repetitive structure; in a rock, the atoms are randomly bonded without any geometric pattern.
D) In a mineral the constituent atoms are bonded in a regular, repetitive, internal structure; a rock is a lithified or consolidated aggregate of different mineral grains.

Unlock Deck
Unlock for access to all 115 flashcards in this deck.
Unlock Deck
k this deck
5
Atoms of the same element, zinc for example, have the same number of ____.
A) protons in the nucleus
B) neutrons in the outer nuclear shell
C) electrons in the valence bond level
D) electrons in the nucleus
A) protons in the nucleus
B) neutrons in the outer nuclear shell
C) electrons in the valence bond level
D) electrons in the nucleus

Unlock Deck
Unlock for access to all 115 flashcards in this deck.
Unlock Deck
k this deck
6
Use the Periodic table below to answer the following questions:
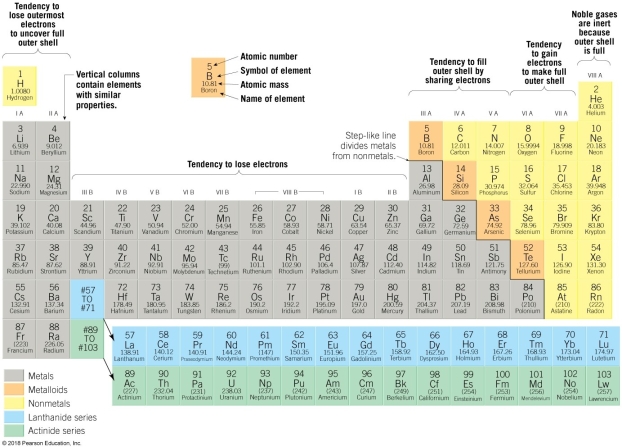
-Element 20, Ca, has what chemical property?
A) It tends to form covalent bonds and an ion with a charge of +1.
B) It behaves as a nonmetal, accepting electrons to form an ion with charge - 2.
C) It tends to be inert, and thus is dispersed throughout the crust.
D) It behaves as a metal ion, giving up two electrons to form a +2 ion.

-Element 20, Ca, has what chemical property?
A) It tends to form covalent bonds and an ion with a charge of +1.
B) It behaves as a nonmetal, accepting electrons to form an ion with charge - 2.
C) It tends to be inert, and thus is dispersed throughout the crust.
D) It behaves as a metal ion, giving up two electrons to form a +2 ion.

Unlock Deck
Unlock for access to all 115 flashcards in this deck.
Unlock Deck
k this deck
7
When Calcium Ca. bonds with oxygen, it gives up two electrons. What is the charge of the Ca ion in this compound?
A) - 2
B) - 1
C) +2
D) +1
A) - 2
B) - 1
C) +2
D) +1

Unlock Deck
Unlock for access to all 115 flashcards in this deck.
Unlock Deck
k this deck
8
Heavy elements like Pb lead. and U Uranium. were generated_______ .
A) by the Sun and expelled to the solar system via the solar wind
B) during collapse of a star and subsequent nuclear synthesis in a supernova
C) by humans in nuclear reactors
D) during the big band when the universe was formed
A) by the Sun and expelled to the solar system via the solar wind
B) during collapse of a star and subsequent nuclear synthesis in a supernova
C) by humans in nuclear reactors
D) during the big band when the universe was formed

Unlock Deck
Unlock for access to all 115 flashcards in this deck.
Unlock Deck
k this deck
9
The basic building blocks for halite________ are and______ .
A) Na; Cl
B) Ca; K
C) Al; O
D) C; Si
A) Na; Cl
B) Ca; K
C) Al; O
D) C; Si

Unlock Deck
Unlock for access to all 115 flashcards in this deck.
Unlock Deck
k this deck
10
Why are boulders not a mineral?
A) They are organic.
B) They are not naturally occurring.
C) They are not solid.
D) They do not have an orderly crystalline structure.
E) They do not have a well- defined chemical composition.
A) They are organic.
B) They are not naturally occurring.
C) They are not solid.
D) They do not have an orderly crystalline structure.
E) They do not have a well- defined chemical composition.

Unlock Deck
Unlock for access to all 115 flashcards in this deck.
Unlock Deck
k this deck
11
An atom's mass number is 13 and its atomic number is 6. How many neutrons are in its nucleus?
A) 13
B) 6
C) 7
D) 19
A) 13
B) 6
C) 7
D) 19

Unlock Deck
Unlock for access to all 115 flashcards in this deck.
Unlock Deck
k this deck
12
Be is to Mg as_________ .
A) K is to Mg
B) K is to Rb
C) Ti is to F
D) Ti is to V
A) K is to Mg
B) K is to Rb
C) Ti is to F
D) Ti is to V

Unlock Deck
Unlock for access to all 115 flashcards in this deck.
Unlock Deck
k this deck
13
Which the following denotes the positively charged particles in an atom's nucleus?
A) protons
B) electrons
C) neutrons
D) isotrons
A) protons
B) electrons
C) neutrons
D) isotrons

Unlock Deck
Unlock for access to all 115 flashcards in this deck.
Unlock Deck
k this deck
14
Limestone is composed almost entirely of calcite, which has the chemical formula CaCO3. As a result, limestone is classified as____________ .
A) a rock
B) a mineral
C) both a mineral and a rock
D) neither a mineral nor a rock because it is organic
A) a rock
B) a mineral
C) both a mineral and a rock
D) neither a mineral nor a rock because it is organic

Unlock Deck
Unlock for access to all 115 flashcards in this deck.
Unlock Deck
k this deck
15
Which one of the following is not true for minerals?
A) They can be identified by characteristic physical properties.
B) They can be a liquid, solid, or gas.
C) They have a specific, internal, crystalline structure.
D) Many have a specific, predictable chemical composition.
A) They can be identified by characteristic physical properties.
B) They can be a liquid, solid, or gas.
C) They have a specific, internal, crystalline structure.
D) Many have a specific, predictable chemical composition.

Unlock Deck
Unlock for access to all 115 flashcards in this deck.
Unlock Deck
k this deck
16
Which of the following minerals is not a chemical compound?
A) quartz SiO2.
B) graphite C.
C) pyrite FeS.
D) halite NaCl.
A) quartz SiO2.
B) graphite C.
C) pyrite FeS.
D) halite NaCl.

Unlock Deck
Unlock for access to all 115 flashcards in this deck.
Unlock Deck
k this deck
17
Use the Periodic table below to answer the following questions:

-Element 17 Cl. and 9
A) behave as metals because they lie on the right side of the periodic table
B) are chemically similar because they lie directly below each other on the periodic table
C) are chemically relatively inert because they adjacent to the inert gases on the periodic table
D) are chemically very different because they lie directly below each other on the periodic table

-Element 17 Cl. and 9
A) behave as metals because they lie on the right side of the periodic table
B) are chemically similar because they lie directly below each other on the periodic table
C) are chemically relatively inert because they adjacent to the inert gases on the periodic table
D) are chemically very different because they lie directly below each other on the periodic table

Unlock Deck
Unlock for access to all 115 flashcards in this deck.
Unlock Deck
k this deck
18
Which of the following is a mineral as defined by a geologist?
A) concrete
B) salt
C) sugar
D) boulder
E) water
A) concrete
B) salt
C) sugar
D) boulder
E) water

Unlock Deck
Unlock for access to all 115 flashcards in this deck.
Unlock Deck
k this deck
19
Which of the following is not a criterion for defining something as a mineral?
A) naturally occurring
B) generally inorganic
C) orderly crystalline structure
D) hard
A) naturally occurring
B) generally inorganic
C) orderly crystalline structure
D) hard

Unlock Deck
Unlock for access to all 115 flashcards in this deck.
Unlock Deck
k this deck
20
In 1960 the largest gold producer was South Africa. Now it is____________ .
A) Russia
B) China
C) India
D) Brazil
A) Russia
B) China
C) India
D) Brazil

Unlock Deck
Unlock for access to all 115 flashcards in this deck.
Unlock Deck
k this deck
21
What mineral is the hardest known substance in nature?
A) muscovite
B) silicate
C) native gold
D) diamond
A) muscovite
B) silicate
C) native gold
D) diamond

Unlock Deck
Unlock for access to all 115 flashcards in this deck.
Unlock Deck
k this deck
22
Geologists may choose to lick a mineral to identify it. What mineral is the geologist expecting with this test?
A) NaCl halite. or KCl sylvite.
B) a sulfide bearing rock which will taste like rotten eggs
C) None, it clears the dust off the sample so he/she can see if more clearly.
D) None, they are clearing the hydrochloric acid from the sample to rerun a test for calcite.
A) NaCl halite. or KCl sylvite.
B) a sulfide bearing rock which will taste like rotten eggs
C) None, it clears the dust off the sample so he/she can see if more clearly.
D) None, they are clearing the hydrochloric acid from the sample to rerun a test for calcite.

Unlock Deck
Unlock for access to all 115 flashcards in this deck.
Unlock Deck
k this deck
23
Which mineral reacts readily with cool, dilute hydrochloric acid to produce visible bubbles of carbon dioxide gas?
A) calcite
B) gypsum
C) quartz
D) plagioclase
A) calcite
B) gypsum
C) quartz
D) plagioclase

Unlock Deck
Unlock for access to all 115 flashcards in this deck.
Unlock Deck
k this deck
24
The bond between two hydrogen atoms a covalent bond. is based on the force of attraction between____________ .
A) two nuclei
B) two ions
C) two atoms
D) protons and electrons in the same atom
A) two nuclei
B) two ions
C) two atoms
D) protons and electrons in the same atom

Unlock Deck
Unlock for access to all 115 flashcards in this deck.
Unlock Deck
k this deck
25
The strong tendency of certain minerals to break along smooth, parallel planes is known as_________ .
A) cleavage
B) habit
C) cracking luster
D) streak
A) cleavage
B) habit
C) cracking luster
D) streak

Unlock Deck
Unlock for access to all 115 flashcards in this deck.
Unlock Deck
k this deck
26
Atoms that have an electrical charge due to a gain or loss of electrons are called _____
A) isotopes
B) ions
C) periodic elements
D) isochrons
A) isotopes
B) ions
C) periodic elements
D) isochrons

Unlock Deck
Unlock for access to all 115 flashcards in this deck.
Unlock Deck
k this deck
27
When electrons are shared amongst all atoms, the resulting bond is an__________ bond.
A) covalent
B) ionic
C) metallic
D) partial
A) covalent
B) ionic
C) metallic
D) partial

Unlock Deck
Unlock for access to all 115 flashcards in this deck.
Unlock Deck
k this deck
28
When a mineral fractures along a cleavage plane, what does this suggest about the crystal structure of the mineral?
A) The crystal grows only planar faces that become weak zones that form cleavage.
B) The crystal contains warped planes called twin planes that weaken the crystal structure and allow it fracture along a planar surface, causing cleavage.
C) The atoms are arranged in a simple orderly arrangement with uniform bonding.
D) The crystal structure contains planes along which chemical bonding is much weaker than other directions.
A) The crystal grows only planar faces that become weak zones that form cleavage.
B) The crystal contains warped planes called twin planes that weaken the crystal structure and allow it fracture along a planar surface, causing cleavage.
C) The atoms are arranged in a simple orderly arrangement with uniform bonding.
D) The crystal structure contains planes along which chemical bonding is much weaker than other directions.

Unlock Deck
Unlock for access to all 115 flashcards in this deck.
Unlock Deck
k this deck
29
The bond between sodium Na. and Chlorine Cl. to form halite salt. is an___________ bond.
A) covalent
B) valent
C) ionic
D) metallic
A) covalent
B) valent
C) ionic
D) metallic

Unlock Deck
Unlock for access to all 115 flashcards in this deck.
Unlock Deck
k this deck
30
Angles are important when looking at which physical properties of minerals?
A) cleavage
B) fracture
C) luster
D) streak
A) cleavage
B) fracture
C) luster
D) streak

Unlock Deck
Unlock for access to all 115 flashcards in this deck.
Unlock Deck
k this deck
31
Which of the following physical properties is not generally used to identify most minerals?
A) cleavage
B) smell
C) hardness
D) luster
A) cleavage
B) smell
C) hardness
D) luster

Unlock Deck
Unlock for access to all 115 flashcards in this deck.
Unlock Deck
k this deck
32
The resistance of a mineral to abrasion is known as ____________.
A) luster
B) hardness
C) streak
D) cleavage
A) luster
B) hardness
C) streak
D) cleavage

Unlock Deck
Unlock for access to all 115 flashcards in this deck.
Unlock Deck
k this deck
33
The most unreliable variable. diagnostic property of minerals such as quartz is__________ .
A) habit
B) color
C) specific gravity
D) hardness
A) habit
B) color
C) specific gravity
D) hardness

Unlock Deck
Unlock for access to all 115 flashcards in this deck.
Unlock Deck
k this deck
34
The columns of the periodic table divide atoms by their_____________ .
A) number of valence electrons
B) number of neutrons
C) atomic mass
D) number of protons
A) number of valence electrons
B) number of neutrons
C) atomic mass
D) number of protons

Unlock Deck
Unlock for access to all 115 flashcards in this deck.
Unlock Deck
k this deck
35
1 gram is defined as the mass of 1 cubic centimeter of water. A cubic centimeter of quartz weighs 2.65 g and a cubic centimeter of galena weighs 7.5 g. The density of these materials from highest to lowest is__________ .
A) galena, quartz, water
B) water, quartz, galena
C) galena, water, quartz
D) quartz, galena, water
A) galena, quartz, water
B) water, quartz, galena
C) galena, water, quartz
D) quartz, galena, water

Unlock Deck
Unlock for access to all 115 flashcards in this deck.
Unlock Deck
k this deck
36
Which of the following describes the light reflecting and transmission characteristics of a mineral?
A) luster
B) fluorescence
C) color streak
D) virtual absorption
A) luster
B) fluorescence
C) color streak
D) virtual absorption

Unlock Deck
Unlock for access to all 115 flashcards in this deck.
Unlock Deck
k this deck
37
Atoms that share electrons have an___________ bond.
A) metallic
B) covalent
C) ionic
D) partial
A) metallic
B) covalent
C) ionic
D) partial

Unlock Deck
Unlock for access to all 115 flashcards in this deck.
Unlock Deck
k this deck
38
Changing the number of neutrons in an atom will affect its_______________ .
A) charge
B) atomic number
C) atomic weight
D) All of the above
A) charge
B) atomic number
C) atomic weight
D) All of the above

Unlock Deck
Unlock for access to all 115 flashcards in this deck.
Unlock Deck
k this deck
39
What does the tendency of micas to produce thin cleavage flakes suggest about its crystal structure?
A) The structure is characterized by rings that form an interlocking network, forming planar sheets.
B) The crystal structure is characterized by complex polymerized mats that form a sheetlike structure.
C) The structure is produced by flow in the igneous rock, aligning glass layers within the crystal structure.
D) The atoms are arranged in orderly arrangements that form strongly bonded sheets separated by weak bonds between the sheets.
A) The structure is characterized by rings that form an interlocking network, forming planar sheets.
B) The crystal structure is characterized by complex polymerized mats that form a sheetlike structure.
C) The structure is produced by flow in the igneous rock, aligning glass layers within the crystal structure.
D) The atoms are arranged in orderly arrangements that form strongly bonded sheets separated by weak bonds between the sheets.

Unlock Deck
Unlock for access to all 115 flashcards in this deck.
Unlock Deck
k this deck
40
Atoms tend to gain, lose, or share electrons until they are surrounded by ____________valence electrons.
A) 8
B) 2
C) 5
D) 4
A) 8
B) 2
C) 5
D) 4

Unlock Deck
Unlock for access to all 115 flashcards in this deck.
Unlock Deck
k this deck
41
Sulfates always include__________ .
A) Cl- 1, F- 1, or Br- 1
B) SO4- 2
C) CO3- 2
D) SiO4- 4
A) Cl- 1, F- 1, or Br- 1
B) SO4- 2
C) CO3- 2
D) SiO4- 4

Unlock Deck
Unlock for access to all 115 flashcards in this deck.
Unlock Deck
k this deck
42
Why do the minerals calcite and dolomite bubble with the mineral or its powder are placed in hydrochloric acid?
A) Both minerals are metal hydrides, and when placed in hydrochloric acid they give off hydrogen gas.
B) Both minerals are sulfides, and the acid reacts to release sulfur dioxide gas.
C) The acid reacts with the mineral to release CO2 gas that is bound into the crystal as carbonate ion.
D) The acid and the mineral together react with oxygen in the air, releasing CO2 gas.
A) Both minerals are metal hydrides, and when placed in hydrochloric acid they give off hydrogen gas.
B) Both minerals are sulfides, and the acid reacts to release sulfur dioxide gas.
C) The acid reacts with the mineral to release CO2 gas that is bound into the crystal as carbonate ion.
D) The acid and the mineral together react with oxygen in the air, releasing CO2 gas.

Unlock Deck
Unlock for access to all 115 flashcards in this deck.
Unlock Deck
k this deck
43
Clay is an example of_____________ .
A) a sulfate that forms from weathering of other sulfates
B) a carbonate that forms from weathering of other carbonates
C) a silicate that forms from weathering of other silicates
D) a halide that forms from weathering of other halides
A) a sulfate that forms from weathering of other sulfates
B) a carbonate that forms from weathering of other carbonates
C) a silicate that forms from weathering of other silicates
D) a halide that forms from weathering of other halides

Unlock Deck
Unlock for access to all 115 flashcards in this deck.
Unlock Deck
k this deck
44
Your fingernail will scratch
A) talc
B) orthoclase
C) calcite
D) corundum
A) talc
B) orthoclase
C) calcite
D) corundum

Unlock Deck
Unlock for access to all 115 flashcards in this deck.
Unlock Deck
k this deck
45
Which mineral will "double refract" written text?
A) flourite
B) apatite
C) quartz
D) calcite
A) flourite
B) apatite
C) quartz
D) calcite

Unlock Deck
Unlock for access to all 115 flashcards in this deck.
Unlock Deck
k this deck
46
Which of the following is not a common rock forming mineral but by contrast is always found in living things.
A) iron
B) potassium
C) magnesium
D) carbon
A) iron
B) potassium
C) magnesium
D) carbon

Unlock Deck
Unlock for access to all 115 flashcards in this deck.
Unlock Deck
k this deck
47
The most common group of silicates is___________ .
A) granite
B) mica
C) quartz
D) feldspar
A) granite
B) mica
C) quartz
D) feldspar

Unlock Deck
Unlock for access to all 115 flashcards in this deck.
Unlock Deck
k this deck
48
Clay minerals are light silicates that form __________.
A) from chemical weathering of igneous rocks
B) from mechanical weathering of any rock
C) from pressure and heat
D) from molten rock
A) from chemical weathering of igneous rocks
B) from mechanical weathering of any rock
C) from pressure and heat
D) from molten rock

Unlock Deck
Unlock for access to all 115 flashcards in this deck.
Unlock Deck
k this deck
49
Light colored silicates have a specific gravity of about 2.7 grams/cm3 are composed primarily of the silica tetrahedra and __________.
A) iron, magnesium, calcium, and sodium
B) potassium, calcium, sodium, and aluminum
C) aluminum, magnesium, calcium, and iron
D) potassium, aluminum, magnesium, and sodium
E) magnesium, aluminum, sodium, and calcium
A) iron, magnesium, calcium, and sodium
B) potassium, calcium, sodium, and aluminum
C) aluminum, magnesium, calcium, and iron
D) potassium, aluminum, magnesium, and sodium
E) magnesium, aluminum, sodium, and calcium

Unlock Deck
Unlock for access to all 115 flashcards in this deck.
Unlock Deck
k this deck
50
The most common group of rock forming minerals is_________ .
A) the halides
B) the silicates
C) the sulfates
D) carbonate
A) the halides
B) the silicates
C) the sulfates
D) carbonate

Unlock Deck
Unlock for access to all 115 flashcards in this deck.
Unlock Deck
k this deck
51
Dark silicates have a specific gravity of 3.2 to 3.6 and are composed primarily of silica tetrahedral and____________ .
A) aluminum and sodium
B) iron and magnesium
C) aluminum and magnesium
D) potassium and iron
E) potassium and calcium
A) aluminum and sodium
B) iron and magnesium
C) aluminum and magnesium
D) potassium and iron
E) potassium and calcium

Unlock Deck
Unlock for access to all 115 flashcards in this deck.
Unlock Deck
k this deck
52
Halides always include_____ .
A) Cl- 1, F- 1, or Br- 1
B) SO4- 2
C) CO3- 2
D) SiO4- 4
A) Cl- 1, F- 1, or Br- 1
B) SO4- 2
C) CO3- 2
D) SiO4- 4

Unlock Deck
Unlock for access to all 115 flashcards in this deck.
Unlock Deck
k this deck
53
The basic building block of a silicate is composed of___________ .
A) 1 oxygen and 1 silicon
B) 3 oxygens and 1 silicon
C) 2 oxygens and 1 silicon
D) 4 oxygens and 1 silicon
A) 1 oxygen and 1 silicon
B) 3 oxygens and 1 silicon
C) 2 oxygens and 1 silicon
D) 4 oxygens and 1 silicon

Unlock Deck
Unlock for access to all 115 flashcards in this deck.
Unlock Deck
k this deck
54
The mineral fluorite is commonly _______ .
A) conchoidal
B) octohedral
C) sheetlike
D) cubical
A) conchoidal
B) octohedral
C) sheetlike
D) cubical

Unlock Deck
Unlock for access to all 115 flashcards in this deck.
Unlock Deck
k this deck
55
Which two elements combine to make most of the common rock forming minerals in the crust?
A) silicon and oxygen
B) silicon and nitrogen
C) carbon and nitrogen
D) nitrogen and oxygen
E) carbon and oxygen
A) silicon and oxygen
B) silicon and nitrogen
C) carbon and nitrogen
D) nitrogen and oxygen
E) carbon and oxygen

Unlock Deck
Unlock for access to all 115 flashcards in this deck.
Unlock Deck
k this deck
56
Carbonates always include_________ .
A) SiO4- 4
B) SO4- 2
C) Cl- 1, F- 1, or Br- 1
D) CO3- 2
A) SiO4- 4
B) SO4- 2
C) Cl- 1, F- 1, or Br- 1
D) CO3- 2

Unlock Deck
Unlock for access to all 115 flashcards in this deck.
Unlock Deck
k this deck
57
A cubic centimeter of quartz, olivine, and gold weighs 2.5, 3.0, and 19.8 grams, respectively. This indicates that______ .
A) gold has a higher specific gravity than quartz and olivine
B) olivine and quartz powders are harder than metallic gold
C) gold and olivine are silicates, whereas quartz is elemental silicon
D) gold is 6 to 7 times harder than olivine and quartz
A) gold has a higher specific gravity than quartz and olivine
B) olivine and quartz powders are harder than metallic gold
C) gold and olivine are silicates, whereas quartz is elemental silicon
D) gold is 6 to 7 times harder than olivine and quartz

Unlock Deck
Unlock for access to all 115 flashcards in this deck.
Unlock Deck
k this deck
58
Silicates most commonly form ________.
A) at the surface of Earth
B) under extreme pressure
C) from other silicates
D) from cooling molten rock
A) at the surface of Earth
B) under extreme pressure
C) from other silicates
D) from cooling molten rock

Unlock Deck
Unlock for access to all 115 flashcards in this deck.
Unlock Deck
k this deck
59
Although it is relatively common, limestone is an economically important rock type because its major constituent mineral,__________ , is used in the production of ________.
A) calcite; cement
B) halite; halogen
C) halite; salt
D) calcite; calcium
A) calcite; cement
B) halite; halogen
C) halite; salt
D) calcite; calcium

Unlock Deck
Unlock for access to all 115 flashcards in this deck.
Unlock Deck
k this deck
60
Quartz has a characteristic conchoidal fracture, yet rock shops often sell quartz as elongate six sided objects with a pointed termination. What causes this shape?
A) You should never buy a crystal like this because it is clearly fake, only artificial crystals grow this way.
B) Quartz usually is amorphous, consistent with its conchoidal fracture, but when it grows it grows against minerals with planar faces, causing this shape.
C) The rock shop cuts them that way with abrasives. The facets are cut to give the crystals more "power" for the crystal people.
D) The planar faces that form the object are crystal faces that grow when the crystals grew into a void.
A) You should never buy a crystal like this because it is clearly fake, only artificial crystals grow this way.
B) Quartz usually is amorphous, consistent with its conchoidal fracture, but when it grows it grows against minerals with planar faces, causing this shape.
C) The rock shop cuts them that way with abrasives. The facets are cut to give the crystals more "power" for the crystal people.
D) The planar faces that form the object are crystal faces that grow when the crystals grew into a void.

Unlock Deck
Unlock for access to all 115 flashcards in this deck.
Unlock Deck
k this deck
61
Examine the words and/or phrases below and determine the relationship among the majority of words/phrases. Choose the option that does not fit the pattern.
A) cubic
B) amorphous
C) bladed
D) tabular
A) cubic
B) amorphous
C) bladed
D) tabular

Unlock Deck
Unlock for access to all 115 flashcards in this deck.
Unlock Deck
k this deck
62
Dolomite is a magnesium- rich member of the______ group.
A) halide
B) carbonate
C) sulfate
D) silicate
A) halide
B) carbonate
C) sulfate
D) silicate

Unlock Deck
Unlock for access to all 115 flashcards in this deck.
Unlock Deck
k this deck
63
Gypsum, which is widely used in plaster and wallboard, is a member of the __________group.
A) silicate
B) halide
C) carbonate
D) sulfate
A) silicate
B) halide
C) carbonate
D) sulfate

Unlock Deck
Unlock for access to all 115 flashcards in this deck.
Unlock Deck
k this deck
64
This element is classified as an ore even in average concentrations because it is so abundant.
A) uranium
B) carbon
C) aluminum
D) boron
A) uranium
B) carbon
C) aluminum
D) boron

Unlock Deck
Unlock for access to all 115 flashcards in this deck.
Unlock Deck
k this deck
65
In the late 20th century most metal prices were very low but metal prices increased dramatically in the early 21st century. Simultaneously, the early 21st century saw extensive "brown fields exploration" in which companies went to old mining areas and extracted old mine wastes or reopened old mine workings. What is the primary explanation for this activity?
A) The companies were only interested acquiring properties through a sleazy land grab, and had no intention of doing anything with the deposits assuming no one cared about brown fields.
B) Environmental regulations make it impossible to explore anywhere but old mining areas, so this was the only place the companies could look for deposits.
C) The increase of metal prices made mineral resources that were previously uneconomic into ores that could potentially be extracted profitably.
D) The old miners were wasteful and left large amounts of ore in the ground.
A) The companies were only interested acquiring properties through a sleazy land grab, and had no intention of doing anything with the deposits assuming no one cared about brown fields.
B) Environmental regulations make it impossible to explore anywhere but old mining areas, so this was the only place the companies could look for deposits.
C) The increase of metal prices made mineral resources that were previously uneconomic into ores that could potentially be extracted profitably.
D) The old miners were wasteful and left large amounts of ore in the ground.

Unlock Deck
Unlock for access to all 115 flashcards in this deck.
Unlock Deck
k this deck
66
Below is a picture of the enormous Bingham Canyon copper mine in Utah. What is the reason that the mine is so large? 
A) It is unknown if any copper underlies this deposit, so miners must continue to dig to find it.
B) Copper is economically valuable even in small quantities, so it is considered to be worth creating a large hole to extract as much as possible.
C) Copper exists in abundant quantities, so miners are trying to extract as much as possible.
D) The mine is the only location on Earth where copper is found, so the hole needs to be large.

A) It is unknown if any copper underlies this deposit, so miners must continue to dig to find it.
B) Copper is economically valuable even in small quantities, so it is considered to be worth creating a large hole to extract as much as possible.
C) Copper exists in abundant quantities, so miners are trying to extract as much as possible.
D) The mine is the only location on Earth where copper is found, so the hole needs to be large.

Unlock Deck
Unlock for access to all 115 flashcards in this deck.
Unlock Deck
k this deck
67
Which of the following is a renewable resource?
A) water
B) helium gas
C) coal
D) rock salt
A) water
B) helium gas
C) coal
D) rock salt

Unlock Deck
Unlock for access to all 115 flashcards in this deck.
Unlock Deck
k this deck
68
Examine the words and/or phrases below and determine the relationship among the majority of words/phrases. Choose the option that does not fit the pattern.
A) feldspar
B) quartz
C) olivine
D) calcite
A) feldspar
B) quartz
C) olivine
D) calcite

Unlock Deck
Unlock for access to all 115 flashcards in this deck.
Unlock Deck
k this deck
69
Which of the following minerals is a silicate?
A) halite
B) feldspar
C) hematite
D) calcite
A) halite
B) feldspar
C) hematite
D) calcite

Unlock Deck
Unlock for access to all 115 flashcards in this deck.
Unlock Deck
k this deck
70
Which common mineral is composed entirely of silicon and oxygen?
A) quartz
B) calcite
C) diamond
D) olivine
A) quartz
B) calcite
C) diamond
D) olivine

Unlock Deck
Unlock for access to all 115 flashcards in this deck.
Unlock Deck
k this deck
71
Examine the words and/or phrases below and determine the relationship among the majority of words/phrases. Choose the option that does not fit the pattern.
A) feldspar
B) quartz
C) olivine
D) calcite
A) feldspar
B) quartz
C) olivine
D) calcite

Unlock Deck
Unlock for access to all 115 flashcards in this deck.
Unlock Deck
k this deck
72
What is true of three- dimensional networks of silicates?
A) they form sheets
B) they form complex masses
C) they are bonded equally strong in all directions
D) they tend to be separate tetrahedral units
A) they form sheets
B) they form complex masses
C) they are bonded equally strong in all directions
D) they tend to be separate tetrahedral units

Unlock Deck
Unlock for access to all 115 flashcards in this deck.
Unlock Deck
k this deck
73
Which one of the following mineral groups exhibits a sheet- like silicate structure?
A) carbonates
B) micas
C) pyroxenes
D) feldspars
A) carbonates
B) micas
C) pyroxenes
D) feldspars

Unlock Deck
Unlock for access to all 115 flashcards in this deck.
Unlock Deck
k this deck
74
Examine the words and/or phrases below and determine the relationship among the majority of words/phrases. Choose the option that does not fit the pattern.
A) valence
B) hydrogen
C) covalent
D) ionic
A) valence
B) hydrogen
C) covalent
D) ionic

Unlock Deck
Unlock for access to all 115 flashcards in this deck.
Unlock Deck
k this deck
75
Examine the words and/or phrases below and determine the relationship among the majority of words/phrases. Choose the option that does not fit the pattern.
A) carbon
B) aluminum
C) oxygen
D) iron
A) carbon
B) aluminum
C) oxygen
D) iron

Unlock Deck
Unlock for access to all 115 flashcards in this deck.
Unlock Deck
k this deck
76
A naturally occurring concentration of one or more metallic minerals that can be extracted economically is an__________ .
A) ore
B) reserve
C) tailing
D) resource
A) ore
B) reserve
C) tailing
D) resource

Unlock Deck
Unlock for access to all 115 flashcards in this deck.
Unlock Deck
k this deck
77
What time span is required to produce most mineral deposits?
A) billions of years
B) tens of thousands to millions of years
C) 1- 100 years, or about a human life span
D) We have no way of knowing this, but most were formed at the same time as Earth.
A) billions of years
B) tens of thousands to millions of years
C) 1- 100 years, or about a human life span
D) We have no way of knowing this, but most were formed at the same time as Earth.

Unlock Deck
Unlock for access to all 115 flashcards in this deck.
Unlock Deck
k this deck
78
Examine the words and/or phrases below and determine the relationship among the majority of words/phrases. Choose the option that does not fit the pattern.
A) neutron
B) atom
C) proton
D) electron
A) neutron
B) atom
C) proton
D) electron

Unlock Deck
Unlock for access to all 115 flashcards in this deck.
Unlock Deck
k this deck
79
What theory dramatically improved geologist's ability to predict where certain ore deposits were formed?
A) quantum mechanics
B) plate tectonics
C) faulting theory
D) geosynclines
A) quantum mechanics
B) plate tectonics
C) faulting theory
D) geosynclines

Unlock Deck
Unlock for access to all 115 flashcards in this deck.
Unlock Deck
k this deck
80
Deposits of which of the following minerals would never be considered an ore due to their relatively low market value?
A) chalcopyrite
B) galena
C) hematite
D) quartz
A) chalcopyrite
B) galena
C) hematite
D) quartz

Unlock Deck
Unlock for access to all 115 flashcards in this deck.
Unlock Deck
k this deck


