Deck 10: Solutions
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/52
Play
Full screen (f)
Deck 10: Solutions
1
Silver chloride is a relatively insoluble salt.Only 1.92 mg of AgCl will dissolve per liter of water at 25°C.What concentration of Ag+,in molarity units,can be present in water at 25°C?
A) 3.35 × 10−6 M
B) 6.70 × 10−6 M
C) 1.34 × 10−5 M
D) 9.60 × 10−4 M
E) 1.92 × 10−3 M
A) 3.35 × 10−6 M
B) 6.70 × 10−6 M
C) 1.34 × 10−5 M
D) 9.60 × 10−4 M
E) 1.92 × 10−3 M
1.34 × 10−5 M
2
What is the definition of molarity?
A) Mass of solute per liter of solvent
B) Mass of solute per kg of solvent
C) Moles of solute per kg of solvent
D) Moles of solute in one liter of solvent
E) Moles of solute per liter of solution
A) Mass of solute per liter of solvent
B) Mass of solute per kg of solvent
C) Moles of solute per kg of solvent
D) Moles of solute in one liter of solvent
E) Moles of solute per liter of solution
Moles of solute per liter of solution
3
A 15 meter by 12 meter pool of water has a depth of 2.2 meters.What mass of silver ion is present in the reservoir if the concentration of silver ion is 0.14 ppm? (1 m3 = 1000 L;assume the density of the solution is 1.00 g/mL)
A) 5.5 × 10-4 g
B) 5.5 × 10-2 g
C) 0.55 g
D) 5.5 g
E) 55 g
A) 5.5 × 10-4 g
B) 5.5 × 10-2 g
C) 0.55 g
D) 5.5 g
E) 55 g
55 g
4
Concentrated nitric acid is 70.4% HNO3 by mass.What is the mole fraction of nitric acid?
A) 0.0112
B) 0.0620
C) 0.171
D) 0.377
E) 0.405
A) 0.0112
B) 0.0620
C) 0.171
D) 0.377
E) 0.405

Unlock Deck
Unlock for access to all 52 flashcards in this deck.
Unlock Deck
k this deck
5
Concentrated sodium hydroxide is 19.4 M and has a density of 1.54 g/mL.What is the molality of concentrated NaOH?
A) 12.6 m
B) 19.8 m
C) 25.4 m
D) 29.9 m
E) 50.4 m
A) 12.6 m
B) 19.8 m
C) 25.4 m
D) 29.9 m
E) 50.4 m

Unlock Deck
Unlock for access to all 52 flashcards in this deck.
Unlock Deck
k this deck
6
What concentration of silver nitrate (in ppm)is present in 7.1 × 10-7 M AgNO3(aq)? For very dilute aqueous solutions,you can assume the solution's density is 1.0 g/mL.The molar mass of AgNO3 is 169.9 g/mol.
A) 0.0071 ppm
B) 0.12 ppm
C) 0.71 ppm
D) 1.7 ppm
E) 8.3 ppm
A) 0.0071 ppm
B) 0.12 ppm
C) 0.71 ppm
D) 1.7 ppm
E) 8.3 ppm

Unlock Deck
Unlock for access to all 52 flashcards in this deck.
Unlock Deck
k this deck
7
To prepare a solution that is 15.0% aqueous KCl by mass,one should
A) dissolve 15.0 g KCl in 85.0 g H2O.
B) dissolve 15.0 g KCl in 1.00 × 102 g H2O.
C) dissolve 15.0 g KCl in 0.850 mol H2O.
D) dissolve 0.150 mol KCl in 0.850 mol H2O.
E) dissolve 0.150 mol KCl in 1.00 mol H2O.
A) dissolve 15.0 g KCl in 85.0 g H2O.
B) dissolve 15.0 g KCl in 1.00 × 102 g H2O.
C) dissolve 15.0 g KCl in 0.850 mol H2O.
D) dissolve 0.150 mol KCl in 0.850 mol H2O.
E) dissolve 0.150 mol KCl in 1.00 mol H2O.

Unlock Deck
Unlock for access to all 52 flashcards in this deck.
Unlock Deck
k this deck
8
What volume of 6.0 M HNO3 is required to prepare 250 mL of 0.40 M HNO3?
A) 9.7 mL
B) 17 mL
C) 27 mL
D) 38 mL
E) 270 L
A) 9.7 mL
B) 17 mL
C) 27 mL
D) 38 mL
E) 270 L

Unlock Deck
Unlock for access to all 52 flashcards in this deck.
Unlock Deck
k this deck
9
If sea water contains 15 ppm gold,how many kilograms of sea water must be processed to remove 1.00 g of gold?
A) 67 kg
B) 97 kg
C) 150 kg
D) 6.7 × 103 kg
E) 15 × 104 kg
A) 67 kg
B) 97 kg
C) 150 kg
D) 6.7 × 103 kg
E) 15 × 104 kg

Unlock Deck
Unlock for access to all 52 flashcards in this deck.
Unlock Deck
k this deck
10
Concentrated phosphoric acid is 85.0% by mass H3PO4.If the molarity of concentrated H3PO4 is 14.5 M,what is the density?
A) 0.60 g/mL
B) 1.67 g/mL
C) 1.87 g/mL
D) 1.95 g/mL
E) 2.07 g/mL
A) 0.60 g/mL
B) 1.67 g/mL
C) 1.87 g/mL
D) 1.95 g/mL
E) 2.07 g/mL

Unlock Deck
Unlock for access to all 52 flashcards in this deck.
Unlock Deck
k this deck
11
What mass of HCl is required to prepare 1.00 kg of 5.5% by mass aqueous HCl?
A) 0.018 g
B) 5.5 g
C) 18 g
D) 55 g
E) 550 g
A) 0.018 g
B) 5.5 g
C) 18 g
D) 55 g
E) 550 g

Unlock Deck
Unlock for access to all 52 flashcards in this deck.
Unlock Deck
k this deck
12
What is the mole fraction of water in a solution that is 33.3% by mass ethylene glycol? The molar mass of ethylene glycol,HOCH2CH2OH,is 62.07 g/mol.
A) 0.127
B) 0.290
C) 0.368
D) 0.667
E) 0.873
A) 0.127
B) 0.290
C) 0.368
D) 0.667
E) 0.873

Unlock Deck
Unlock for access to all 52 flashcards in this deck.
Unlock Deck
k this deck
13
Concentrated sulfuric acid is 18.0 M and has a density of 1.84 g/mL.Calculate the percent mass of sulfuric acid in concentrated H2SO4.
A) 17.7%
B) 32.5%
C) 78.2%
D) 96.0%
E) 99.4%
A) 17.7%
B) 32.5%
C) 78.2%
D) 96.0%
E) 99.4%

Unlock Deck
Unlock for access to all 52 flashcards in this deck.
Unlock Deck
k this deck
14
Pure acetic acid,CH3CO2H(l),has a density of 1.049 g/mL.To prepare 1.00 L of 6.00 M CH3CO2H(aq),one may dilute:
A) 175 g of acetic acid to a volume of 1.00 L.
B) 343 mL of acetic acid to a volume of 1.00 L.
C) 360 mL of acetic acid to a volume of 1.00 L.
D) 382 mL of acetic acid to a volume of 1.00 L.
E) 1049 g of acetic acid to a volume of 1.00 L.
A) 175 g of acetic acid to a volume of 1.00 L.
B) 343 mL of acetic acid to a volume of 1.00 L.
C) 360 mL of acetic acid to a volume of 1.00 L.
D) 382 mL of acetic acid to a volume of 1.00 L.
E) 1049 g of acetic acid to a volume of 1.00 L.

Unlock Deck
Unlock for access to all 52 flashcards in this deck.
Unlock Deck
k this deck
15
Silver chloride is a relatively insoluble salt.Only 1.92 mg of AgCl will dissolve per liter of water at 25°C.How many parts per million of Ag+ can be present in water at 25°C? Assume the density of the solution equals the density of water,1.00 g/mL.
A) 0.52 ppm
B) 0.93 ppm
C) 1.45 ppm
D) 9.30 ppm
E) 52.0 ppm
A) 0.52 ppm
B) 0.93 ppm
C) 1.45 ppm
D) 9.30 ppm
E) 52.0 ppm

Unlock Deck
Unlock for access to all 52 flashcards in this deck.
Unlock Deck
k this deck
16
What mass of Cu(NO3)2 (187.6 g/mol)is present in 25.0 g of 1.00 m Cu(NO3)2(aq)?
A) 3.95 g
B) 4.69 g
C) 13.8 g
D) 25.0 g
E) 63.5 g
A) 3.95 g
B) 4.69 g
C) 13.8 g
D) 25.0 g
E) 63.5 g

Unlock Deck
Unlock for access to all 52 flashcards in this deck.
Unlock Deck
k this deck
17
The mole fraction of calcium chloride in an aqueous solution is 0.0724.What is the percent mass of CaCl2 in the solution?
A) 4.46%
B) 7.24%
C) 32.5%
D) 36.2%
E) 50.0%
A) 4.46%
B) 7.24%
C) 32.5%
D) 36.2%
E) 50.0%

Unlock Deck
Unlock for access to all 52 flashcards in this deck.
Unlock Deck
k this deck
18
A bottle of phosphoric acid is labeled "85.0% H3PO4 by mass;density = 1.689 g/cm3." Calculate the molarity of phosphoric acid in the solution.
A) 16.7 M
B) 14.6 M
C) 12.2 M
D) 8.67 M
E) 0.146 M
A) 16.7 M
B) 14.6 M
C) 12.2 M
D) 8.67 M
E) 0.146 M

Unlock Deck
Unlock for access to all 52 flashcards in this deck.
Unlock Deck
k this deck
19
If 26.5 g of methanol (CH3OH)is added to 735 g of water,what is the molality of the methanol?
A) 0.0348 m
B) 2.03 m
C) 1.13 m
D) 3.61 m
E) 36.1 m
A) 0.0348 m
B) 2.03 m
C) 1.13 m
D) 3.61 m
E) 36.1 m

Unlock Deck
Unlock for access to all 52 flashcards in this deck.
Unlock Deck
k this deck
20
Pure acetic acid,often called glacial acetic acid,is a liquid with a density of 1.049 g/mL.Which calculation correctly shows how to determine the volume of glacial acetic acid necessary to prepare 250 mL of 0.400 M CH3CO2H(aq)?
A)
B)
C)
D)
E) .
A)
B)
C)
D)
E) .

Unlock Deck
Unlock for access to all 52 flashcards in this deck.
Unlock Deck
k this deck
21
The Henry's law constant for O2 in water at 25 °C is 1.26 × 10-3 M/atm.What partial pressure of O2 is necessary to achieve an equilibrium concentration of 1.5 × 10-3 M O2?
A) 1.9 × 10-6 atm
B) 0.24 atm
C) 0.84 atm
D) 1.2 atm
E) 5.3 × 105 atm
A) 1.9 × 10-6 atm
B) 0.24 atm
C) 0.84 atm
D) 1.2 atm
E) 5.3 × 105 atm

Unlock Deck
Unlock for access to all 52 flashcards in this deck.
Unlock Deck
k this deck
22
In 1.00 atm of pure oxygen,the solubility of O2(g)in water is 1.26 × 10−3 M at 25.0°C.The mole fraction of oxygen in air is 0.210.If the atmospheric pressure is 0.979 atm,what is the solubility of oxygen in air at 25.0°C?
A) 2.59 × 10−4 M
B) 2.65 × 10−3 M
C) 5.87 × 10−3 M
D) 6.00 × 10−3 M
E) 3.86 × 103 M
A) 2.59 × 10−4 M
B) 2.65 × 10−3 M
C) 5.87 × 10−3 M
D) 6.00 × 10−3 M
E) 3.86 × 103 M

Unlock Deck
Unlock for access to all 52 flashcards in this deck.
Unlock Deck
k this deck
23
Which of the following aqueous solutions will freeze at the lowest temperature?
A) 0.10 m KCl
B) 0.20 m C6H12O6 (glucose)
C) 0.050 m AlCl3
D) 0.15 m SrBr2
E) All of the above freeze at the same temperature.
A) 0.10 m KCl
B) 0.20 m C6H12O6 (glucose)
C) 0.050 m AlCl3
D) 0.15 m SrBr2
E) All of the above freeze at the same temperature.

Unlock Deck
Unlock for access to all 52 flashcards in this deck.
Unlock Deck
k this deck
24
Henry's law states that gas solubility is
A) directly proportional to temperature of the solution.
B) directly proportional to the molar mass of the gas.
C) inversely proportional to the combined pressure of all gases over the solution.
D) inversely proportional to the pressure of the gas over the solution.
E) directly proportional to the pressure of the gas over the solution.
A) directly proportional to temperature of the solution.
B) directly proportional to the molar mass of the gas.
C) inversely proportional to the combined pressure of all gases over the solution.
D) inversely proportional to the pressure of the gas over the solution.
E) directly proportional to the pressure of the gas over the solution.

Unlock Deck
Unlock for access to all 52 flashcards in this deck.
Unlock Deck
k this deck
25
All of the following statements are correct EXCEPT
A) the solubility of a gas in water decreases as the water temperature increases.
B) dissolving a solid in water is usually an exothermic process.
C) when an equilibrium is established between molecules in a solid and a solution,the solution is said to be saturated.
D) if a precipitate forms when a solution is cooled,the solution is supersaturated.
E) solubility decreases with increasing molar mass.
A) the solubility of a gas in water decreases as the water temperature increases.
B) dissolving a solid in water is usually an exothermic process.
C) when an equilibrium is established between molecules in a solid and a solution,the solution is said to be saturated.
D) if a precipitate forms when a solution is cooled,the solution is supersaturated.
E) solubility decreases with increasing molar mass.

Unlock Deck
Unlock for access to all 52 flashcards in this deck.
Unlock Deck
k this deck
26
A gas mixture has mole fractions of 0.24 oxygen and 0.76 nitrogen.If the total pressure of the gases is 1.44 atm at 325 K,what is the concentration,in molarity,of oxygen? (R = 0.0821 L⋅atm/mol⋅K)
A) 0.013 M
B) 0.017M
C) 0.041 M
D) 0.54 M
E) 0.069 M
A) 0.013 M
B) 0.017M
C) 0.041 M
D) 0.54 M
E) 0.069 M

Unlock Deck
Unlock for access to all 52 flashcards in this deck.
Unlock Deck
k this deck
27
An aqueous solution contains 11.5 g of NaCl in 250.2 g of water.Calculate the vapor pressure of this solution at 25.0°C.The vapor pressure of pure water is 23.8 mm Hg at 25.0°C.
A) 3.38 mm Hg
B) 21.8 mm Hg
C) 23.5 mm Hg
D) 24.1 mm Hg
E) 31.3 mm Hg
A) 3.38 mm Hg
B) 21.8 mm Hg
C) 23.5 mm Hg
D) 24.1 mm Hg
E) 31.3 mm Hg

Unlock Deck
Unlock for access to all 52 flashcards in this deck.
Unlock Deck
k this deck
28
Which of the following liquids will be miscible with water in any proportions: ethanol (CH3CH2OH),carbon tetrachloride (CCl4),hexane (C6H14),and/or formic acid (HCO2H)?
A) ethanol and carbon tetrachloride
B) carbon tetrachloride and hexane
C) ethanol and formic acid
D) ethanol,carbon tetrachloride,and benzene
E) carbon tetrachloride,and formic acid
A) ethanol and carbon tetrachloride
B) carbon tetrachloride and hexane
C) ethanol and formic acid
D) ethanol,carbon tetrachloride,and benzene
E) carbon tetrachloride,and formic acid

Unlock Deck
Unlock for access to all 52 flashcards in this deck.
Unlock Deck
k this deck
29
The molal boiling point constant for water is 0.52°C/m.At what temperature will a mixture of 45.0 g of NaCl and 0.500 kg of water boil?
A) 98.4°C
B) 99.2°C
C) 100.0°C
D) 100.8°C
E) 101.6°C
A) 98.4°C
B) 99.2°C
C) 100.0°C
D) 100.8°C
E) 101.6°C

Unlock Deck
Unlock for access to all 52 flashcards in this deck.
Unlock Deck
k this deck
30
At 1.00 atm,1.64 × 10−3 g H2(g)will dissolve in 1.0 L of water.What pressure of gas is necessary to obtain a concentration of 1.0 × 10−3 M H2(g)?
A) 6.1 × 10−4 atm
B) 4.4 × 10−2 atm
C) 1.2 atm
D) 2.2 × 103 atm
E) 1.6 × 103 atm
A) 6.1 × 10−4 atm
B) 4.4 × 10−2 atm
C) 1.2 atm
D) 2.2 × 103 atm
E) 1.6 × 103 atm

Unlock Deck
Unlock for access to all 52 flashcards in this deck.
Unlock Deck
k this deck
31
All of the following are colligative properties EXCEPT
A) gas solubility as a function of partial pressure above a solution (Henry's law).
B) osmotic pressure.
C) vapor pressure lowering.
D) boiling point elevation.
E) freezing point depression.
A) gas solubility as a function of partial pressure above a solution (Henry's law).
B) osmotic pressure.
C) vapor pressure lowering.
D) boiling point elevation.
E) freezing point depression.

Unlock Deck
Unlock for access to all 52 flashcards in this deck.
Unlock Deck
k this deck
32
Arrange the molecules below in order of increasing solubility in water. 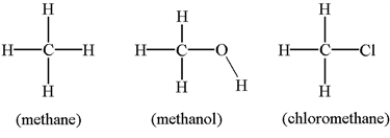
A) methane < methanol < chloromethane
B) methane < chloromethane < methanol
C) chloromethane < methanol < methane
D) chloromethane < methane < methanol
E) methanol < methane < chloromethane

A) methane < methanol < chloromethane
B) methane < chloromethane < methanol
C) chloromethane < methanol < methane
D) chloromethane < methane < methanol
E) methanol < methane < chloromethane

Unlock Deck
Unlock for access to all 52 flashcards in this deck.
Unlock Deck
k this deck
33
What is the equilibrium partial pressure of water vapor above a mixture of 44.0 g H2O and 56.0 g HOCH2CH2OH at 35°C.The partial pressure of pure water at 35.0°C is 42.2 mm Hg.Assume ideal behavior for the solution.
A) 0.730 Hg
B) 18.7 mm Hg
C) 23.6 mm Hg
D) 30.8 mm Hg
E) 58.8 mm Hg
A) 0.730 Hg
B) 18.7 mm Hg
C) 23.6 mm Hg
D) 30.8 mm Hg
E) 58.8 mm Hg

Unlock Deck
Unlock for access to all 52 flashcards in this deck.
Unlock Deck
k this deck
34
The Henry's law constant for the solubility of nitrogen in water is 6.4 × 10−4 M/atm at 25°C.At 0.75 atm of N2,what mass of N2(g)dissolves in 1.0 L of water at 25°C?
A) 4.8 × 10−4 g
B) 8.5 × 10−4 g
C) 4.5 × 10−3 g
D) 1.3 × 10−2 g
E) 2.4 × 10−2 g
A) 4.8 × 10−4 g
B) 8.5 × 10−4 g
C) 4.5 × 10−3 g
D) 1.3 × 10−2 g
E) 2.4 × 10−2 g

Unlock Deck
Unlock for access to all 52 flashcards in this deck.
Unlock Deck
k this deck
35
What concentration unit is necessary for the calculation of boiling point elevation?
A) Molarity
B) Molality
C) Mass fraction
D) Mole fraction
E) Parts per million
A) Molarity
B) Molality
C) Mass fraction
D) Mole fraction
E) Parts per million

Unlock Deck
Unlock for access to all 52 flashcards in this deck.
Unlock Deck
k this deck
36
Arrange the molecules below in order of increasing solubility in water. 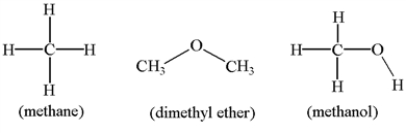
A) dimethyl ether < methane < methanol
B) dimethyl ether < methanol < methane
C) methane < methanol< dimethyl ether
D) methanol < dimethyl ether < methane
E) methane < dimethyl ether < methanol

A) dimethyl ether < methane < methanol
B) dimethyl ether < methanol < methane
C) methane < methanol< dimethyl ether
D) methanol < dimethyl ether < methane
E) methane < dimethyl ether < methanol

Unlock Deck
Unlock for access to all 52 flashcards in this deck.
Unlock Deck
k this deck
37
What mass of ethylene glycol,when mixed with 225 g H2O,will reduce the equilibrium vapor pressure of H2O from 1.00 atm to 0.800 atm at 100°C? The molar masses of water and ethylene glycol are 18.02 g/mol and 62.07 g/mol,respectively.Assume ideal behavior for the solution.
A) 15.6 g
B) 49.9 g
C) 194 g
D) 969 g
E) 3.10 × 103 g
A) 15.6 g
B) 49.9 g
C) 194 g
D) 969 g
E) 3.10 × 103 g

Unlock Deck
Unlock for access to all 52 flashcards in this deck.
Unlock Deck
k this deck
38
If 77.5 g of ethylene glycol (HOCH2CH2OH)is added to 422.5 g of water,what is the mole fraction of ethylene glycol?
A) 0.00296
B) 0.0506
C) 0.183
D) 0.949
E) 2.96
A) 0.00296
B) 0.0506
C) 0.183
D) 0.949
E) 2.96

Unlock Deck
Unlock for access to all 52 flashcards in this deck.
Unlock Deck
k this deck
39
What concentration unit is necessary for the calculation of vapor pressure lowering?
A) Molarity
B) Molality
C) Mass fraction
D) Mole fraction
E) Density
A) Molarity
B) Molality
C) Mass fraction
D) Mole fraction
E) Density

Unlock Deck
Unlock for access to all 52 flashcards in this deck.
Unlock Deck
k this deck
40
A substance that dissolves in water and conducts electricity when present in an aqueous solution is
A) metallic.
B) ionic.
C) molecular.
D) network covalent.
E) none of the above.
A) metallic.
B) ionic.
C) molecular.
D) network covalent.
E) none of the above.

Unlock Deck
Unlock for access to all 52 flashcards in this deck.
Unlock Deck
k this deck
41
At 25°C,what is the osmotic pressure of a homogeneous solution consisting of 18.0 g urea (CON2H4)diluted with water to 3.00 L? (R = 0.0821 L⋅atm/mol⋅K)
A) 0.205 atm
B) 2.44 atm
C) 7.33 atm
D) 12.3 atm
E) 14.7 atm
A) 0.205 atm
B) 2.44 atm
C) 7.33 atm
D) 12.3 atm
E) 14.7 atm

Unlock Deck
Unlock for access to all 52 flashcards in this deck.
Unlock Deck
k this deck
42
An aqueous solution is composed of 7.50 g NaCl (MM = 58.44 g/mol)diluted to 0.100 L.Calculate the osmotic pressure of the solution at 298 K.(R = 0.0821 L⋅atm/mol⋅K)
A) 5.83 atm
B) 9.22 atm
C) 18.3 atm
D) 31.4 atm
E) 62.8 atm
A) 5.83 atm
B) 9.22 atm
C) 18.3 atm
D) 31.4 atm
E) 62.8 atm

Unlock Deck
Unlock for access to all 52 flashcards in this deck.
Unlock Deck
k this deck
43
Equal masses of water and ethylene glycol (C2H6O2)are mixed.At what temperature will the mixture freeze? The molal freezing point constant for water is −1.86°C/m.
A) −115°C
B) −93.0°C
C) −42.0°C
D) −30.0°C
E) −0.93°C
A) −115°C
B) −93.0°C
C) −42.0°C
D) −30.0°C
E) −0.93°C

Unlock Deck
Unlock for access to all 52 flashcards in this deck.
Unlock Deck
k this deck
44
The molal freezing point constant for water is -1.86°C/m.At what temperature will a solution containing 8.27 g CaCl2 and 45.0 g H2O begin to freeze? Assume that no ion-pairing occurs between Ca2+ and Cl-.
A) -9.24°C
B) -4.62°C
C) -0.804°C
D) -0.749°C
E) +4.62°C
A) -9.24°C
B) -4.62°C
C) -0.804°C
D) -0.749°C
E) +4.62°C

Unlock Deck
Unlock for access to all 52 flashcards in this deck.
Unlock Deck
k this deck
45
Which of the following electrolytes is likely to have a van't Hoff factor equal to 3?
A) CaI2
B) Na3PO4
C) KCl
D) answers a and b
E) answers a,b,and c
A) CaI2
B) Na3PO4
C) KCl
D) answers a and b
E) answers a,b,and c

Unlock Deck
Unlock for access to all 52 flashcards in this deck.
Unlock Deck
k this deck
46
A 0.230 m solution of an unknown electrolyte depresses the freezing point of water by 0.821°C.What is the Van't Hoff factor for this electrolyte? The freezing point depression constant for water is 1.86°C/m.
A) 0.521
B) 1.92
C) 2.00
D) 2.30
E) 4.41
A) 0.521
B) 1.92
C) 2.00
D) 2.30
E) 4.41

Unlock Deck
Unlock for access to all 52 flashcards in this deck.
Unlock Deck
k this deck
47
A solution is prepared by dissolving 4.21 g of a nonelectrolyte in 50.0 g of water.If the boiling point increases by 0.203°C,what is the molar mass of the solute? The boiling point elevation constant for water is 0.512°C/m.
A) 33.4 g/mol
B) 111 g/mol
C) 172 g/mol
D) 212 g/mol
E) 810 g/mol
A) 33.4 g/mol
B) 111 g/mol
C) 172 g/mol
D) 212 g/mol
E) 810 g/mol

Unlock Deck
Unlock for access to all 52 flashcards in this deck.
Unlock Deck
k this deck
48
What is the boiling point of a solution containing 0.80 g caffeine,C8H10N4O2,dissolved in 13.20 g benzene? The boiling point of pure benzene is 80.1°C and the molal boiling point constant,Kb,is 2.53°C/m.
A) 79.8°C
B) 80.4°C
C) 80.9°C
D) 85.2°C
E) 88.2°C
A) 79.8°C
B) 80.4°C
C) 80.9°C
D) 85.2°C
E) 88.2°C

Unlock Deck
Unlock for access to all 52 flashcards in this deck.
Unlock Deck
k this deck
49
What is the molar mass of a nonpolar molecular compound if 3.42 grams dissolved in 41.8 grams benzene begins to freeze at 1.17°C? The freezing point of pure benzene is 5.50°C and the molal freezing point constant,Kf,is -5.12°C/m.
A) 2.89 g/mol
B) 69.2 g/mol
C) 96.7 g/mol
D) 126 g/mol
E) 358 g/mol
A) 2.89 g/mol
B) 69.2 g/mol
C) 96.7 g/mol
D) 126 g/mol
E) 358 g/mol

Unlock Deck
Unlock for access to all 52 flashcards in this deck.
Unlock Deck
k this deck
50
Maple syrup is made from the sap of the maple tree.When the sap is tapped from the maple tree it is 2.0% by mass sucrose.Maple syrup is 66% by mass sucrose.What mass of sap would be required to make 0.5 kg of maple syrup?
A) 0.50 kg
B) 2.0 kg
C) 66 kg
D) 15 kg
E) 17 kg
A) 0.50 kg
B) 2.0 kg
C) 66 kg
D) 15 kg
E) 17 kg

Unlock Deck
Unlock for access to all 52 flashcards in this deck.
Unlock Deck
k this deck
51
A solution is prepared by dissolving 5.00 g of an unknown molecular solid in water to make 1.00 L of solution.The osmotic pressure of the solution is 1.61 atm at 25°C.What is the molar mass of the solute? (R = 0.0821 L⋅atm/mol⋅K)
A) 6.37 g/mol
B) 58.44 g/mol
C) 76.0 g/mol
D) 102 g/mol
E) 180.2 g/mol
A) 6.37 g/mol
B) 58.44 g/mol
C) 76.0 g/mol
D) 102 g/mol
E) 180.2 g/mol

Unlock Deck
Unlock for access to all 52 flashcards in this deck.
Unlock Deck
k this deck
52
A solution is prepared by dissolving 4.78 g of an unknown nonelectrolyte in enough water to make 375 mL of solution.The osmotic pressure of the solution is 1.33 atm at 27°C.What is the molar mass of the solute? (R = 0.0821 L⋅atm/mol⋅K)
A) 0.0203 g/mol
B) 21.2 g/mol
C) 49.4 g/mol
D) 96.8 g/mol
E) 236 g/mol
A) 0.0203 g/mol
B) 21.2 g/mol
C) 49.4 g/mol
D) 96.8 g/mol
E) 236 g/mol

Unlock Deck
Unlock for access to all 52 flashcards in this deck.
Unlock Deck
k this deck


