Deck 7: Covalent Bonding
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/59
Play
Full screen (f)
Deck 7: Covalent Bonding
1
How many possible resonance structures exist for carbonate ion,CO32-?
A) 0
B) 1
C) 3
D) 6
E) 8
A) 0
B) 1
C) 3
D) 6
E) 8
3
2
How many possible resonance structures exist for the formate ion,HCO2−?
A) 0
B) 2
C) 3
D) 4
E) 8
A) 0
B) 2
C) 3
D) 4
E) 8
2
3
Which of the following elements is able to form a molecular structure that exceeds the octet rule?
A) C
B) B
C) N
D) F
E) S
A) C
B) B
C) N
D) F
E) S
S
4
Which of the following is a correct Lewis structure for oxygen?
A)
B)
C)
D)
E)
A)

B)

C)

D)

E)


Unlock Deck
Unlock for access to all 59 flashcards in this deck.
Unlock Deck
k this deck
5
Which of the following is a correct Lewis structure for sulfur dioxide,SO2?
A)
B)
C)
D)
E)
A)

B)

C)

D)

E)


Unlock Deck
Unlock for access to all 59 flashcards in this deck.
Unlock Deck
k this deck
6
How many different molecules have the formula C5H12?
A) 1
B) 2
C) 3
D) 4
E) 6
A) 1
B) 2
C) 3
D) 4
E) 6

Unlock Deck
Unlock for access to all 59 flashcards in this deck.
Unlock Deck
k this deck
7
Which of the following species has a Lewis structure with a molecular geometry similar to SO3?
A) NH3
B) ICl3
C) CO32−
D) SO32−
E) PCl3
A) NH3
B) ICl3
C) CO32−
D) SO32−
E) PCl3

Unlock Deck
Unlock for access to all 59 flashcards in this deck.
Unlock Deck
k this deck
8
Which of the following molecules or ions will have a Lewis structure most like that of phosphorus trichloride,PCl3?
A) ClO3-
B) SO3
C) CO32-
D) BF3
E) Cl2CO
A) ClO3-
B) SO3
C) CO32-
D) BF3
E) Cl2CO

Unlock Deck
Unlock for access to all 59 flashcards in this deck.
Unlock Deck
k this deck
9
A fluoride ion,F-,has _____ valence electrons.
A) 6
B) 7
C) 8
D) 9
E) 10
A) 6
B) 7
C) 8
D) 9
E) 10

Unlock Deck
Unlock for access to all 59 flashcards in this deck.
Unlock Deck
k this deck
10
Which of the following species will have a Lewis structure with a molecular geometry similar to IF4-?
A) XeF4
B) SO42-
C) PF4+
D) SF4
E) IO4-
A) XeF4
B) SO42-
C) PF4+
D) SF4
E) IO4-

Unlock Deck
Unlock for access to all 59 flashcards in this deck.
Unlock Deck
k this deck
11
All of the following Lewis structures of nitrogen oxides are possible EXCEPT: 
A) N2O.
B) N2O4.
C) N2O3.
D) N2O5.
E) All of the above are correct structures.

A) N2O.
B) N2O4.
C) N2O3.
D) N2O5.
E) All of the above are correct structures.

Unlock Deck
Unlock for access to all 59 flashcards in this deck.
Unlock Deck
k this deck
12
Which of the following is a correct Lewis structure for PH3?
A)
B)
C)
D)
E)
A)

B)

C)

D)

E)


Unlock Deck
Unlock for access to all 59 flashcards in this deck.
Unlock Deck
k this deck
13
Which of the following is/are possible Lewis structures for C2H6O? 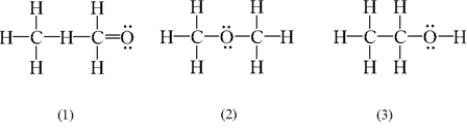
A) 1
B) 2
C) 3
D) 2 and 3
E) 1,2,and 3

A) 1
B) 2
C) 3
D) 2 and 3
E) 1,2,and 3

Unlock Deck
Unlock for access to all 59 flashcards in this deck.
Unlock Deck
k this deck
14
What is the correct Lewis structure of SF4?
A)
B)
C)
D)
E)
A)

B)

C)

D)

E)


Unlock Deck
Unlock for access to all 59 flashcards in this deck.
Unlock Deck
k this deck
15
What is the correct Lewis structure for IF3?
A)
B)
C)
D)
E)
A)

B)

C)

D)

E)


Unlock Deck
Unlock for access to all 59 flashcards in this deck.
Unlock Deck
k this deck
16
A nitrogen atom has _____ valence electrons.
A) 0
B) 3
C) 5
D) 7
E) 8
A) 0
B) 3
C) 5
D) 7
E) 8

Unlock Deck
Unlock for access to all 59 flashcards in this deck.
Unlock Deck
k this deck
17
Which of the following are correct resonance structures of N2O4? 
A) (1)and (2)
B) (2)and (3)
C) (1), (2),and (3)
D) (2), (3),and (4)
E) (1), (2), (3),and (4)

A) (1)and (2)
B) (2)and (3)
C) (1), (2),and (3)
D) (2), (3),and (4)
E) (1), (2), (3),and (4)

Unlock Deck
Unlock for access to all 59 flashcards in this deck.
Unlock Deck
k this deck
18
Which of the following are correct resonance structures of SO3? 
A) (1)and (5)
B) (2)and (4)
C) (1), (2),and (4)
D) (2), (3),and (4)
E) (1), (2), (4),and (5)

A) (1)and (5)
B) (2)and (4)
C) (1), (2),and (4)
D) (2), (3),and (4)
E) (1), (2), (4),and (5)

Unlock Deck
Unlock for access to all 59 flashcards in this deck.
Unlock Deck
k this deck
19
Which of the following species has a Lewis structure with a molecular geometry similar to SO2?
A) H2S
B) NO2−
C) NO2
D) N2O
E) ClO2−
A) H2S
B) NO2−
C) NO2
D) N2O
E) ClO2−

Unlock Deck
Unlock for access to all 59 flashcards in this deck.
Unlock Deck
k this deck
20
Which of the following species has a Lewis structure with a molecular geometry similar to SF4?
A) BrF4+
B) ICl4−
C) NH4+
D) SO42−
E) CCl4
A) BrF4+
B) ICl4−
C) NH4+
D) SO42−
E) CCl4

Unlock Deck
Unlock for access to all 59 flashcards in this deck.
Unlock Deck
k this deck
21
Which of the following species have the same molecular geometry: PO43−,SF4,PF5,and XeF4?
A) PO43− and SF4
B) SF4 and PF5
C) PO43−,SF4,and XeF4
D) SF4 and XeF4
E) None of the species have the same molecular geometry.
A) PO43− and SF4
B) SF4 and PF5
C) PO43−,SF4,and XeF4
D) SF4 and XeF4
E) None of the species have the same molecular geometry.

Unlock Deck
Unlock for access to all 59 flashcards in this deck.
Unlock Deck
k this deck
22
Use VSEPR theory to predict the molecular geometry around each carbon atom in acetylene,C2H2.
A) linear
B) bent
C) trigonal planar
D) tetrahedron
E) octahedron
A) linear
B) bent
C) trigonal planar
D) tetrahedron
E) octahedron

Unlock Deck
Unlock for access to all 59 flashcards in this deck.
Unlock Deck
k this deck
23
How many lone pairs of electrons are on the sulfur atom in sulfite ion,SO32−?
A) 0
B) 1
C) 2
D) 3
E) 4
A) 0
B) 1
C) 2
D) 3
E) 4

Unlock Deck
Unlock for access to all 59 flashcards in this deck.
Unlock Deck
k this deck
24
Which of the following species have the same molecular geometry: CO2,H2O,BeCl2,and N2O?
A) CO2 and N2O only
B) H2O and N2O only
C) H2O and BeCl2 only
D) CO2 and BeCl2 only
E) CO2,BeCl2,and N2O
A) CO2 and N2O only
B) H2O and N2O only
C) H2O and BeCl2 only
D) CO2 and BeCl2 only
E) CO2,BeCl2,and N2O

Unlock Deck
Unlock for access to all 59 flashcards in this deck.
Unlock Deck
k this deck
25
Use VSEPR theory to predict the molecular geometry of IF5.
A) octahedron
B) square planar
C) tetrahedron
D) see-saw
E) square pyramid
A) octahedron
B) square planar
C) tetrahedron
D) see-saw
E) square pyramid

Unlock Deck
Unlock for access to all 59 flashcards in this deck.
Unlock Deck
k this deck
26
Use VSEPR theory to predict the molecular geometry of nitrogen trichloride,NCl3.
A) linear
B) trigonal planar
C) bent
D) tetrahedron
E) trigonal pyramid
A) linear
B) trigonal planar
C) bent
D) tetrahedron
E) trigonal pyramid

Unlock Deck
Unlock for access to all 59 flashcards in this deck.
Unlock Deck
k this deck
27
The nitrogen atom in cyanide ion,CN-,is surrounded by:
A) one single bond and three lone pairs of electrons.
B) one double bond and one lone pair of electrons.
C) one double bond and two lone pairs of electrons.
D) one triple bond and one lone pair of electrons.
E) one triple bond and no lone pairs of electrons.
A) one single bond and three lone pairs of electrons.
B) one double bond and one lone pair of electrons.
C) one double bond and two lone pairs of electrons.
D) one triple bond and one lone pair of electrons.
E) one triple bond and no lone pairs of electrons.

Unlock Deck
Unlock for access to all 59 flashcards in this deck.
Unlock Deck
k this deck
28
Use VSEPR theory to predict the molecular geometry of I3-.
A) bent
B) linear
C) trigonal planar
D) t-shaped
E) octahedron
A) bent
B) linear
C) trigonal planar
D) t-shaped
E) octahedron

Unlock Deck
Unlock for access to all 59 flashcards in this deck.
Unlock Deck
k this deck
29
Use VSEPR theory to predict the molecular geometry of H2Se.
A) bent
B) linear
C) tetrahedron
D) trigonal planar
E) trigonal pyramid
A) bent
B) linear
C) tetrahedron
D) trigonal planar
E) trigonal pyramid

Unlock Deck
Unlock for access to all 59 flashcards in this deck.
Unlock Deck
k this deck
30
Which of the following species have the same molecular geometry: H3O+,H2CO,NH3,and ICl3?
A) H3O+ and H2CO
B) H2CO and ICl3
C) H3O+ and NH3
D) H3O+,NH3,and ICl3
E) None of the species have the same molecular geometry.
A) H3O+ and H2CO
B) H2CO and ICl3
C) H3O+ and NH3
D) H3O+,NH3,and ICl3
E) None of the species have the same molecular geometry.

Unlock Deck
Unlock for access to all 59 flashcards in this deck.
Unlock Deck
k this deck
31
The central atom in SCl2 is surrounded by:
A) two single bonds and no lone pairs of electrons.
B) two single bonds and one lone pair of electrons.
C) two single bonds and two lone pairs of electrons.
D) one single bond,one double bond,and no lone pairs of electrons.
E) one single bond,one double bond,and one lone pair of electrons.
A) two single bonds and no lone pairs of electrons.
B) two single bonds and one lone pair of electrons.
C) two single bonds and two lone pairs of electrons.
D) one single bond,one double bond,and no lone pairs of electrons.
E) one single bond,one double bond,and one lone pair of electrons.

Unlock Deck
Unlock for access to all 59 flashcards in this deck.
Unlock Deck
k this deck
32
Use VSEPR theory to predict the molecular geometry of SO2.
A) bent
B) linear
C) trigonal planar
D) tetrahedron
E) trigonal pyramid
A) bent
B) linear
C) trigonal planar
D) tetrahedron
E) trigonal pyramid

Unlock Deck
Unlock for access to all 59 flashcards in this deck.
Unlock Deck
k this deck
33
Formal charge is:
A) the absolute value of the charge on a polyatomic anion or cation.
B) the difference between the number of lone pairs of electrons and shared pairs of electrons on any atom in a Lewis structure.
C) the difference between the number of valence electrons and the number of protons in any given atom.
D) equal to the number of valence electrons in a free atom minus the number of shared in covalent bonds.
E) the difference between the number of valence electrons in a free atom and the number of electrons assigned to the atom in a Lewis structure.
A) the absolute value of the charge on a polyatomic anion or cation.
B) the difference between the number of lone pairs of electrons and shared pairs of electrons on any atom in a Lewis structure.
C) the difference between the number of valence electrons and the number of protons in any given atom.
D) equal to the number of valence electrons in a free atom minus the number of shared in covalent bonds.
E) the difference between the number of valence electrons in a free atom and the number of electrons assigned to the atom in a Lewis structure.

Unlock Deck
Unlock for access to all 59 flashcards in this deck.
Unlock Deck
k this deck
34
What is the formal charge on each atom in CN−?
A) C = 0,N = 0
B) C = +1,N = −1
C) C = −1,N = 0
D) C = +2,N = −3
E) C = +4,N = −5
A) C = 0,N = 0
B) C = +1,N = −1
C) C = −1,N = 0
D) C = +2,N = −3
E) C = +4,N = −5

Unlock Deck
Unlock for access to all 59 flashcards in this deck.
Unlock Deck
k this deck
35
Use VSEPR theory to predict the molecular geometry of NH4+.
A) trigonal pyramid
B) square pyramid
C) see-saw
D) tetrahedron
E) trigonal planar
A) trigonal pyramid
B) square pyramid
C) see-saw
D) tetrahedron
E) trigonal planar

Unlock Deck
Unlock for access to all 59 flashcards in this deck.
Unlock Deck
k this deck
36
Use VSEPR theory to predict the molecular geometry of ICl3.
A) trigonal planar
B) trigonal pyramid
C) trigonal bipyramid
D) t-shaped
E) octahedron
A) trigonal planar
B) trigonal pyramid
C) trigonal bipyramid
D) t-shaped
E) octahedron

Unlock Deck
Unlock for access to all 59 flashcards in this deck.
Unlock Deck
k this deck
37
Use VSEPR theory to predict the molecular geometry of phosgene,Cl2CO.
A) t-shaped
B) trigonal planar
C) trigonal bipyramid
D) square planar
E) tetrahedron
A) t-shaped
B) trigonal planar
C) trigonal bipyramid
D) square planar
E) tetrahedron

Unlock Deck
Unlock for access to all 59 flashcards in this deck.
Unlock Deck
k this deck
38
Using formal charges and the octet rule,determine which Lewis structure of OCN− is most stable.
A)
B)
C)
D)
E)
A)

B)

C)

D)

E)


Unlock Deck
Unlock for access to all 59 flashcards in this deck.
Unlock Deck
k this deck
39
A Lewis structure of OCl- ion is drawn below.What is the formal charge on each atom? 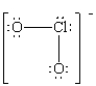
A) Cl atom = +1 and each O atom = -1
B) Cl atom = 0 and each O atom = -1
C) Cl atom = -1 and each O atom = 0
D) Cl atom = +3 and each O atom = -2
E) Cl atom = 0,one O atom = 0,one O atom = -1

A) Cl atom = +1 and each O atom = -1
B) Cl atom = 0 and each O atom = -1
C) Cl atom = -1 and each O atom = 0
D) Cl atom = +3 and each O atom = -2
E) Cl atom = 0,one O atom = 0,one O atom = -1

Unlock Deck
Unlock for access to all 59 flashcards in this deck.
Unlock Deck
k this deck
40
Use VSEPR theory to predict the molecular geometry of ClO3−.
A) bent
B) tetrahedron
C) square planar
D) trigonal planar
E) trigonal pyramid
A) bent
B) tetrahedron
C) square planar
D) trigonal planar
E) trigonal pyramid

Unlock Deck
Unlock for access to all 59 flashcards in this deck.
Unlock Deck
k this deck
41
How many sigma and pi bonds are present in the following molecule? 
A) 8 sigma bonds and 1 pi bond
B) 8 sigma bonds and 2 pi bonds
C) 10 sigma bonds and 2 pi bonds
D) 11 sigma bonds and 2 pi bonds
E) 11 sigma bonds and 1 pi bond

A) 8 sigma bonds and 1 pi bond
B) 8 sigma bonds and 2 pi bonds
C) 10 sigma bonds and 2 pi bonds
D) 11 sigma bonds and 2 pi bonds
E) 11 sigma bonds and 1 pi bond

Unlock Deck
Unlock for access to all 59 flashcards in this deck.
Unlock Deck
k this deck
42
What is the hybridization of the carbon atoms in benzene,C6H6?
A) sp
B) sp2
C) sp3
D) sp3d
E) sp3d2
A) sp
B) sp2
C) sp3
D) sp3d
E) sp3d2

Unlock Deck
Unlock for access to all 59 flashcards in this deck.
Unlock Deck
k this deck
43
G.N.Lewis is well known for multiple contributions to the study of chemistry.Which of these below is attributed to Lewis?
A) covalent bonding
B) ionic bonding
C) VSEPR
D) hybridization
E) paramagnetism
A) covalent bonding
B) ionic bonding
C) VSEPR
D) hybridization
E) paramagnetism

Unlock Deck
Unlock for access to all 59 flashcards in this deck.
Unlock Deck
k this deck
44
What are the approximate bond angles in SF4?
A) 90°
B) 109.5°
C) 120°
D) 90°,120°,and 180°
E) 180°
A) 90°
B) 109.5°
C) 120°
D) 90°,120°,and 180°
E) 180°

Unlock Deck
Unlock for access to all 59 flashcards in this deck.
Unlock Deck
k this deck
45
What are the O-Cl-O bond angles in ClO4−?
A) 90°
B) 109.5°
C) 120°
D) 90° and 120°
E) 180°
A) 90°
B) 109.5°
C) 120°
D) 90° and 120°
E) 180°

Unlock Deck
Unlock for access to all 59 flashcards in this deck.
Unlock Deck
k this deck
46
Which of the following molecules has the smallest bond angle between any two hydrogen atoms?
A) CH4
B) H2O
C) BH3
D) PH3
E) SiH4
A) CH4
B) H2O
C) BH3
D) PH3
E) SiH4

Unlock Deck
Unlock for access to all 59 flashcards in this deck.
Unlock Deck
k this deck
47
What are the approximate O-N-O bond angles in a nitrate ion?
A) 90°
B) 109.5°
C) 120°
D) 90° and 120°
E) 180°
A) 90°
B) 109.5°
C) 120°
D) 90° and 120°
E) 180°

Unlock Deck
Unlock for access to all 59 flashcards in this deck.
Unlock Deck
k this deck
48
What is the hybridization of the central atom in SO2?
A) sp
B) sp2
C) sp3
D) sp3d
E) sp3d2
A) sp
B) sp2
C) sp3
D) sp3d
E) sp3d2

Unlock Deck
Unlock for access to all 59 flashcards in this deck.
Unlock Deck
k this deck
49
How many sigma and pi bonds are present in H2CO?
A) 1 sigma bond and 3 pi bonds
B) 2 sigma bonds and 2 pi bonds
C) 2 sigma bonds and 1 pi bond
D) 3 sigma bonds and 1 pi bond
E) none of the above
A) 1 sigma bond and 3 pi bonds
B) 2 sigma bonds and 2 pi bonds
C) 2 sigma bonds and 1 pi bond
D) 3 sigma bonds and 1 pi bond
E) none of the above

Unlock Deck
Unlock for access to all 59 flashcards in this deck.
Unlock Deck
k this deck
50
Write the singly bonded Lewis dot structure for SF6.Which of the following statements best describes this structure?
A) It obeys the octet rule on all atoms.
B) It has less than an octet on at least one atom.
C) It has a lone pair of electrons on the sulfur atom.
D) It has less than an octet of electrons on all atoms.
E) It exceeds the octet rule.
A) It obeys the octet rule on all atoms.
B) It has less than an octet on at least one atom.
C) It has a lone pair of electrons on the sulfur atom.
D) It has less than an octet of electrons on all atoms.
E) It exceeds the octet rule.

Unlock Deck
Unlock for access to all 59 flashcards in this deck.
Unlock Deck
k this deck
51
Which of the following molecules are polar: H2S,CO2,NH3,BH3,and CCl4?
A) BH3
B) H2S and NH3
C) H2S,CO2,and CCl4
D) CO2,NH3,and CCl4
E) NH3,BH3,and CCl4
A) BH3
B) H2S and NH3
C) H2S,CO2,and CCl4
D) CO2,NH3,and CCl4
E) NH3,BH3,and CCl4

Unlock Deck
Unlock for access to all 59 flashcards in this deck.
Unlock Deck
k this deck
52
Which of the following species have the same molecular geometry: XeF4,ClF4+,SF4,and PO43−?
A) XeF4 and SF4
B) ClF4+,and SF4
C) ClF4+ and PO43−
D) XeF4 and ClF4+
E) XeF4,SF4,and PO43−
A) XeF4 and SF4
B) ClF4+,and SF4
C) ClF4+ and PO43−
D) XeF4 and ClF4+
E) XeF4,SF4,and PO43−

Unlock Deck
Unlock for access to all 59 flashcards in this deck.
Unlock Deck
k this deck
53
What is the hybridization of the phosphorus atom in PCl3?
A) sp
B) sp2
C) sp3
D) sp3d
E) sp3d2
A) sp
B) sp2
C) sp3
D) sp3d
E) sp3d2

Unlock Deck
Unlock for access to all 59 flashcards in this deck.
Unlock Deck
k this deck
54
What are the F-Xe-F bond angles in XeF4?
A) 90° and 180°
B) 109.5°
C) 120°
D) 60° and 120°
E) 180°
A) 90° and 180°
B) 109.5°
C) 120°
D) 60° and 120°
E) 180°

Unlock Deck
Unlock for access to all 59 flashcards in this deck.
Unlock Deck
k this deck
55
What hybridization change does the carbon atom undergo in the combustion of methane? CH4(g)+ 2O2(g)→ CO2(g)+ 2H2O(g)
A) sp → sp2
B) sp2 → sp3
C) sp3 → sp
D) sp2 → sp
E) none
A) sp → sp2
B) sp2 → sp3
C) sp3 → sp
D) sp2 → sp
E) none

Unlock Deck
Unlock for access to all 59 flashcards in this deck.
Unlock Deck
k this deck
56
This gas changed the perception of the reactivity of this group.
A) oxygen
B) xenon
C) helium
D) hydrogen
E) chlorine
A) oxygen
B) xenon
C) helium
D) hydrogen
E) chlorine

Unlock Deck
Unlock for access to all 59 flashcards in this deck.
Unlock Deck
k this deck
57
Which of the following compounds has polar covalent bonds: CCl4,Cl2,HCl,and KCl?
A) CCl4 only
B) Cl2 only
C) HCl and KCl
D) Cl2 and KCl
E) CCl4 and HCl
A) CCl4 only
B) Cl2 only
C) HCl and KCl
D) Cl2 and KCl
E) CCl4 and HCl

Unlock Deck
Unlock for access to all 59 flashcards in this deck.
Unlock Deck
k this deck
58
What is the hybridization of the central atom in I3−?
A) sp
B) sp2
C) sp3
D) sp3d
E) sp3d2
A) sp
B) sp2
C) sp3
D) sp3d
E) sp3d2

Unlock Deck
Unlock for access to all 59 flashcards in this deck.
Unlock Deck
k this deck
59
Write the singly bonded Lewis dot structure for BF3.Which of the following statements best describes this structure?
A) It obeys the octet rule on all atoms.
B) It has less than an octet on at least one atom.
C) It has a lone pair of electrons on the boron atom.
D) It has less than an octet of electrons on all atoms.
E) It exceeds the octet rule.
A) It obeys the octet rule on all atoms.
B) It has less than an octet on at least one atom.
C) It has a lone pair of electrons on the boron atom.
D) It has less than an octet of electrons on all atoms.
E) It exceeds the octet rule.

Unlock Deck
Unlock for access to all 59 flashcards in this deck.
Unlock Deck
k this deck


