Deck 3: Mass Relations in Chemistry;stoichiometry
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/56
Play
Full screen (f)
Deck 3: Mass Relations in Chemistry;stoichiometry
1
Which is a correct method for determining the mass in grams of 0.190 mol silver?
A)
B)
C)
D)
E) none of the above
A)
B)
C)
D)
E) none of the above
2
Which of the following samples contains the largest number of hydrogen atoms?
A) 2.0 moles of C6H16
B) 3.0 moles of C3H8
C) 4.0 moles of C3H6
D) 6.0 moles of C2H4
E) 8.0 moles of C2H2
A) 2.0 moles of C6H16
B) 3.0 moles of C3H8
C) 4.0 moles of C3H6
D) 6.0 moles of C2H4
E) 8.0 moles of C2H2
2.0 moles of C6H16
3
What is the mass in grams of 0.362 moles barium chloride (BaCl2)?
A) 0.362 g
B) 0.00174 g
C) 75.4 g
D) 208 g
E) 575 g
A) 0.362 g
B) 0.00174 g
C) 75.4 g
D) 208 g
E) 575 g
75.4 g
4
Which is a correct method for determining the total number of atoms in 123 grams of sulfur trioxide (SO3)?
A)
B)
C)
D)
E)
A)
B)
C)
D)
E)

Unlock Deck
Unlock for access to all 56 flashcards in this deck.
Unlock Deck
k this deck
5
How many hydrogen atoms are present in 1.0 g of NH3?
A) 0.059 atoms
B) 0.18 atoms
C) 3.5 × 1022 atoms
D) 1.1 × 1023 atoms
E) 1.2 × 1022 atoms
A) 0.059 atoms
B) 0.18 atoms
C) 3.5 × 1022 atoms
D) 1.1 × 1023 atoms
E) 1.2 × 1022 atoms

Unlock Deck
Unlock for access to all 56 flashcards in this deck.
Unlock Deck
k this deck
6
The molarity of a solution is defined as
A) the moles of solute per liter of solution.
B) the moles of solute per kilogram of solution.
C) the moles of solute per kilogram of solvent.
D) the mass of solute (in grams)per liter of solution.
E) the mass of solute (in grams)per liter of solvent.
A) the moles of solute per liter of solution.
B) the moles of solute per kilogram of solution.
C) the moles of solute per kilogram of solvent.
D) the mass of solute (in grams)per liter of solution.
E) the mass of solute (in grams)per liter of solvent.

Unlock Deck
Unlock for access to all 56 flashcards in this deck.
Unlock Deck
k this deck
7
You have a 5.0 g sample of each of the following elements: Ra,Rb,Rh,Rn,Ru.Which sample contains the most atoms?
A) Ra
B) Rb
C) Rh
D) Rn
E) Ru
A) Ra
B) Rb
C) Rh
D) Rn
E) Ru

Unlock Deck
Unlock for access to all 56 flashcards in this deck.
Unlock Deck
k this deck
8
The molar mass of nitrogen (N2)is 28.0 g/mole.What is the mass of a single nitrogen atom?
A) 2.32 × 10−23 g
B) 4.65 × 10−23 g
C) 9.30 × 10−23 g
D) 4.30 × 10−22 g
E) 8.43 × 10−22 g
A) 2.32 × 10−23 g
B) 4.65 × 10−23 g
C) 9.30 × 10−23 g
D) 4.30 × 10−22 g
E) 8.43 × 10−22 g

Unlock Deck
Unlock for access to all 56 flashcards in this deck.
Unlock Deck
k this deck
9
What mass of chlorine is present in 5.00 grams of carbon tetrachloride (CCl4)?
A) 0.130 g
B) 1.15 g
C) 0.564 g
D) 4.61 g
E) 0.922 g
A) 0.130 g
B) 1.15 g
C) 0.564 g
D) 4.61 g
E) 0.922 g

Unlock Deck
Unlock for access to all 56 flashcards in this deck.
Unlock Deck
k this deck
10
The molar mass of platinum is 195.08 g/mol.What is the mass of 1.00 × 102 Pt atoms?
A) 8.51 × 10-25 g
B) 3.24 × 10-24 g
C) 1.67 × 10-22 g
D) 3.24 × 10-22 g
E) 3.24 × 10-20 g
A) 8.51 × 10-25 g
B) 3.24 × 10-24 g
C) 1.67 × 10-22 g
D) 3.24 × 10-22 g
E) 3.24 × 10-20 g

Unlock Deck
Unlock for access to all 56 flashcards in this deck.
Unlock Deck
k this deck
11
The molar mass of boron is 10.81 g/mole.What is the mass of a single boron atom?
A) 1.661 × 10−24 g
B) 1.795 × 10−23 g
C) 6.510 × 1024 g
D) 1.536 × 10−25 g
E) 1.081 × 101 g
A) 1.661 × 10−24 g
B) 1.795 × 10−23 g
C) 6.510 × 1024 g
D) 1.536 × 10−25 g
E) 1.081 × 101 g

Unlock Deck
Unlock for access to all 56 flashcards in this deck.
Unlock Deck
k this deck
12
How many moles of HCl are present in 0.098 grams of HCl?
A) 0.00083 mol
B) 0.0027 mol
C) 0.28 mol
D) 3.6 mol
E) 380 mol
A) 0.00083 mol
B) 0.0027 mol
C) 0.28 mol
D) 3.6 mol
E) 380 mol

Unlock Deck
Unlock for access to all 56 flashcards in this deck.
Unlock Deck
k this deck
13
Which is a correct method for calculating the moles of calcium carbonate present in 132 grams of calcium carbonate?
A)
B)
C)
D)
E) none of the above
A)
B)
C)
D)
E) none of the above

Unlock Deck
Unlock for access to all 56 flashcards in this deck.
Unlock Deck
k this deck
14
What is the mass in grams of 0.338 mol of glucose (C6H12O6)?
A) 0.00188 g
B) 0.0164 g
C) 1.88 g
D) 53.3 g
E) 60.9 g
A) 0.00188 g
B) 0.0164 g
C) 1.88 g
D) 53.3 g
E) 60.9 g

Unlock Deck
Unlock for access to all 56 flashcards in this deck.
Unlock Deck
k this deck
15
A 1.45 g sample of chromium contains ____ atoms.
A) 1.25 × 1022
B) 1.68 × 1022
C) 8.73 × 1023
D) 2.16 × 1025
E) 4.54 × 1025
A) 1.25 × 1022
B) 1.68 × 1022
C) 8.73 × 1023
D) 2.16 × 1025
E) 4.54 × 1025

Unlock Deck
Unlock for access to all 56 flashcards in this deck.
Unlock Deck
k this deck
16
What is the mass of 0.71 mol Na?
A) 1.2 × 10-24 g
B) 12 g
C) 16 g
D) 0.031 g
E) 32 g
A) 1.2 × 10-24 g
B) 12 g
C) 16 g
D) 0.031 g
E) 32 g

Unlock Deck
Unlock for access to all 56 flashcards in this deck.
Unlock Deck
k this deck
17
Which of the following samples contains the largest number of atoms?
A) 2.0 moles of H3PO4
B) 3.0 moles of H2SO3
C) 4.0 moles of HNO3
D) 6.0 moles of HClO
E) 8.0 moles of HBr
A) 2.0 moles of H3PO4
B) 3.0 moles of H2SO3
C) 4.0 moles of HNO3
D) 6.0 moles of HClO
E) 8.0 moles of HBr

Unlock Deck
Unlock for access to all 56 flashcards in this deck.
Unlock Deck
k this deck
18
What mass of oxygen is present in 10.0 grams of potassium nitrate (KNO3)?
A) 4.75 g
B) 5.43 g
C) 6.39 g
D) 8.00 g
E) 9.17 g
A) 4.75 g
B) 5.43 g
C) 6.39 g
D) 8.00 g
E) 9.17 g

Unlock Deck
Unlock for access to all 56 flashcards in this deck.
Unlock Deck
k this deck
19
How many atoms are present in 5.00 grams of iron?
A) 8.95 × 10−23 atoms
B) 3.36 × 1018 atoms
C) 1.88 × 1021 atoms
D) 5.39 × 1022 atoms
E) 3.36 × 1026 atoms
A) 8.95 × 10−23 atoms
B) 3.36 × 1018 atoms
C) 1.88 × 1021 atoms
D) 5.39 × 1022 atoms
E) 3.36 × 1026 atoms

Unlock Deck
Unlock for access to all 56 flashcards in this deck.
Unlock Deck
k this deck
20
The mass of a single atom of chlorine atom is 5.887 × 10?23 grams.Which is a correct method for determining the molar mass of elemental chlorine,Cl2?
A)
B)
C)
D)
E)
A)
B)
C)
D)
E)

Unlock Deck
Unlock for access to all 56 flashcards in this deck.
Unlock Deck
k this deck
21
An oxide of nitrogen contains 63.1% oxygen and has a molar mass of 76.0 g/mol.What is the molecular formula for this compound?
A) N2O
B) NO
C) NO2
D) N2O3
E) N2O5
A) N2O
B) NO
C) NO2
D) N2O3
E) N2O5

Unlock Deck
Unlock for access to all 56 flashcards in this deck.
Unlock Deck
k this deck
22
Combustion analysis of 0.800 grams of an unknown hydrocarbon yields 2.613 g CO2 and 0.778 g H2O.What is the percent composition of the hydrocarbon?
A) 66.6% C;33.4% H
B) 82.3% C;17.7% H
C) 89.1% C;10.9% H
D) 92.4% C;7.60% H
E) not enough information given to solve the problem
A) 66.6% C;33.4% H
B) 82.3% C;17.7% H
C) 89.1% C;10.9% H
D) 92.4% C;7.60% H
E) not enough information given to solve the problem

Unlock Deck
Unlock for access to all 56 flashcards in this deck.
Unlock Deck
k this deck
23
What is the mass percent of each element in sulfuric acid,H2SO4?
A) 2.055% H,32.69% S,65.25% O
B) 1.028% H,32.69% S,66.28% O
C) 28.57% H,14.29% S,57.17% O
D) 1.028% H,33.72% S,65.25% O
E) 2.016% H,32.07% S,65.91% O
A) 2.055% H,32.69% S,65.25% O
B) 1.028% H,32.69% S,66.28% O
C) 28.57% H,14.29% S,57.17% O
D) 1.028% H,33.72% S,65.25% O
E) 2.016% H,32.07% S,65.91% O

Unlock Deck
Unlock for access to all 56 flashcards in this deck.
Unlock Deck
k this deck
24
A mass of 12.0 g of calcium chloride is diluted to a volume of 250 mL in a volumetric flask.Which of the equations below is a correct method for determining the chloride ion concentration?
A)
B)
C)
D)
E)
A)
B)
C)
D)
E)

Unlock Deck
Unlock for access to all 56 flashcards in this deck.
Unlock Deck
k this deck
25
If 5.15 g Fe(NO3)3 is dissolved in enough water to make exactly 150.0 mL of solution,what is the molar concentration of nitrate ion?
A) 0.00319 M
B) 0.0343 M
C) 0.142 M
D) 0.313 M
E) 0.426 M
A) 0.00319 M
B) 0.0343 M
C) 0.142 M
D) 0.313 M
E) 0.426 M

Unlock Deck
Unlock for access to all 56 flashcards in this deck.
Unlock Deck
k this deck
26
What is the mass of sodium iodide in 50.0 mL of 2.63 × 10-2 M NaI(aq)?
A) 0.00132 g
B) 0.00877 g
C) 0.0788 g
D) 0.197 g
E) 78.8 g
A) 0.00132 g
B) 0.00877 g
C) 0.0788 g
D) 0.197 g
E) 78.8 g

Unlock Deck
Unlock for access to all 56 flashcards in this deck.
Unlock Deck
k this deck
27
Hydrazine,a fuel used to power rocket engines,is a product of the reaction between ammonia and bleach.Balance the equation for the reaction. aNH3(aq)+ bOCl−(aq)→ cN2H4(l)+ dCl−(aq)+ eH2O(l)
A) a = 2,b = 1,c = 1,d = 1,e = 1
B) a = 2,b = 2,c = 1,d = 2,e = 2
C) a = 2,b = 1,c = 2,d = 1,e = 2
D) a = 4,b = 3,c = 1,d = 3,e = 1
E) a = 4,b = 3,c = 2,d = 3,e = 2
A) a = 2,b = 1,c = 1,d = 1,e = 1
B) a = 2,b = 2,c = 1,d = 2,e = 2
C) a = 2,b = 1,c = 2,d = 1,e = 2
D) a = 4,b = 3,c = 1,d = 3,e = 1
E) a = 4,b = 3,c = 2,d = 3,e = 2

Unlock Deck
Unlock for access to all 56 flashcards in this deck.
Unlock Deck
k this deck
28
Isopentyl acetate,a molecule composed of C,H,and O,smells like bananas.Combustion analysis of 1.750 grams of this molecule yields 1.695 g H2O and 4.142 g CO2.What is the simplest formula for isopentyl acetate?
A) C7H14O2
B) C7H7O4
C) C8H10O3
D) C8H16O
E) C9H6O
A) C7H14O2
B) C7H7O4
C) C8H10O3
D) C8H16O
E) C9H6O

Unlock Deck
Unlock for access to all 56 flashcards in this deck.
Unlock Deck
k this deck
29
Nitrogen and oxygen form an extensive series of oxides with the general formula NxOy.What is the empirical formula for an oxide that contains 30.44% by mass nitrogen?
A) N2O
B) NO
C) NO2
D) N2O3
E) N2O5
A) N2O
B) NO
C) NO2
D) N2O3
E) N2O5

Unlock Deck
Unlock for access to all 56 flashcards in this deck.
Unlock Deck
k this deck
30
What volume of 0.15 M HCl(aq)must be diluted to make 2.0 L of 0.050 M HCl(aq)?
A) 0.015 L
B) 0.10 L
C) 0.30 L
D) 0.67 L
E) 6.0 L
A) 0.015 L
B) 0.10 L
C) 0.30 L
D) 0.67 L
E) 6.0 L

Unlock Deck
Unlock for access to all 56 flashcards in this deck.
Unlock Deck
k this deck
31
What is the percent composition of iron(II)sulfate hexahydrate?
A) 4.2% Fe;4.2% S;41.6% O;50.0% H
B) 16.7% Fe;16.7%S;66.6% O
C) 21.5% Fe;12.3%S;24.6% O;41.6% H
D) 21.5% Fe;12.3%S;61.5% O;4.7% H
E) 36.8% Fe;21.1%S;42.1% O
A) 4.2% Fe;4.2% S;41.6% O;50.0% H
B) 16.7% Fe;16.7%S;66.6% O
C) 21.5% Fe;12.3%S;24.6% O;41.6% H
D) 21.5% Fe;12.3%S;61.5% O;4.7% H
E) 36.8% Fe;21.1%S;42.1% O

Unlock Deck
Unlock for access to all 56 flashcards in this deck.
Unlock Deck
k this deck
32
What is the maximum volume of 0.25 M KCl(aq)that can be prepared from 75 g KCl(s)?
A) 0.33 L
B) 1.0 L
C) 3.0 L
D) 4.0 L
E) 19 L
A) 0.33 L
B) 1.0 L
C) 3.0 L
D) 4.0 L
E) 19 L

Unlock Deck
Unlock for access to all 56 flashcards in this deck.
Unlock Deck
k this deck
33
If 8.19 g KIO3 is dissolved in enough water to make 500.0 mL of solution,what is the molarity of the potassium iodate solution? The molar mass of KIO3 is 214 g/mol.
A) 1.64 × 10-2 M
B) 1.91 × 10-2 M
C) 7.65 × 10-2 M
D) 3.51 M
E) 16.4 M
A) 1.64 × 10-2 M
B) 1.91 × 10-2 M
C) 7.65 × 10-2 M
D) 3.51 M
E) 16.4 M

Unlock Deck
Unlock for access to all 56 flashcards in this deck.
Unlock Deck
k this deck
34
Which is a correct method for determining the mass of carbon present in 0.132 grams of propane (C3H8)?
A)
B)
C)
D)
E)
A)
B)
C)
D)
E)

Unlock Deck
Unlock for access to all 56 flashcards in this deck.
Unlock Deck
k this deck
35
Soft drink bottles are made of polyethylene terephthalate (PET),a polymer composed of carbon,hydrogen,and oxygen.If 2.8880 g PET is burned in oxygen it produces 1.0000 g H2O and 6.1058 g CO2.What is the empirical formula of PET?
A) CHO
B) CH7O5
C) C5H7O
D) C8H10O
E) C10H8O5
A) CHO
B) CH7O5
C) C5H7O
D) C8H10O
E) C10H8O5

Unlock Deck
Unlock for access to all 56 flashcards in this deck.
Unlock Deck
k this deck
36
A molecule is found to contain 47.35% by mass C,10.60% by mass H,and 42.05% by mass O.What is the empirical formula for this molecule?
A) C2H6O
B) C3H4O
C) C3H8O2
D) C4H6O2
E) C4H8O3
A) C2H6O
B) C3H4O
C) C3H8O2
D) C4H6O2
E) C4H8O3

Unlock Deck
Unlock for access to all 56 flashcards in this deck.
Unlock Deck
k this deck
37
How many liters of 0.2805 M C6H12O6(aq)contain 1.000 g of C6H12O6?
A) 0.001557 L
B) 0.01979 L
C) 0.2805 L
D) 3.565 L
E) 50.5 L
A) 0.001557 L
B) 0.01979 L
C) 0.2805 L
D) 3.565 L
E) 50.5 L

Unlock Deck
Unlock for access to all 56 flashcards in this deck.
Unlock Deck
k this deck
38
Beryl is a mineral which contains 5.03% Be,10.04% Al,31.35% Si,and 53.58% O.What is the simplest formula for beryl?
A) BeAl(SiO3)2
B) BeAl(SiO3)3
C) Be3(AlSiO3)2
D) Be3Al2(SiO3)6
E) Be4Al(SiO3)8
A) BeAl(SiO3)2
B) BeAl(SiO3)3
C) Be3(AlSiO3)2
D) Be3Al2(SiO3)6
E) Be4Al(SiO3)8

Unlock Deck
Unlock for access to all 56 flashcards in this deck.
Unlock Deck
k this deck
39
Polyethylene is a polymer consisting of only carbon and hydrogen.If 2.300 g of the polymer is burned in oxygen it produces 2.955 g H2O and 7.217 g CO2.What is the empirical formula of polyethylene?
A) CH
B) CH2
C) C2H3
D) C5H8
E) C7H8
A) CH
B) CH2
C) C2H3
D) C5H8
E) C7H8

Unlock Deck
Unlock for access to all 56 flashcards in this deck.
Unlock Deck
k this deck
40
If 25.00 mL of 4.50 M NaOH(aq)is diluted with water to a volume of 750.0 mL,what is the molarity of the diluted NaOH(aq)?
A) 0.0333 M
B) 0.150 M
C) 0.155 M
D) 6.67 M
E) 1.35 × 103 M
A) 0.0333 M
B) 0.150 M
C) 0.155 M
D) 6.67 M
E) 1.35 × 103 M

Unlock Deck
Unlock for access to all 56 flashcards in this deck.
Unlock Deck
k this deck
41
If 16.4 g of oxygen gas react with excess hydrogen,what mass of water is produced? 2H2(g)+ O2(g)→ 2H2O(g)
A) 9.23 g
B) 18.5 g
C) 20.4 g
D) 23.9 g
E) 36.9 g
A) 9.23 g
B) 18.5 g
C) 20.4 g
D) 23.9 g
E) 36.9 g

Unlock Deck
Unlock for access to all 56 flashcards in this deck.
Unlock Deck
k this deck
42
What hydrate is sometimes referred to as "the ice that burns"?
A) copper(II)sulfate pentahydrate
B) carbon dioxide hydrate
C) methane hydrate
D) cobalt(II)chloride pentahydrate
E) none of the above
A) copper(II)sulfate pentahydrate
B) carbon dioxide hydrate
C) methane hydrate
D) cobalt(II)chloride pentahydrate
E) none of the above

Unlock Deck
Unlock for access to all 56 flashcards in this deck.
Unlock Deck
k this deck
43
Iron reacts with hydrochloric acid to produce iron(II)chloride and hydrogen gas. Fe(s)+ 2 HCl(aq)→ FeCl2(aq)+ H2(g)
What mass of H2(g)is produced from the reaction of 5.2 g Fe(s)with excess hydrochloric acid?
A) 0.094 g
B) 0.19 g
C) 5.2 g
D) 6.8 g
E) 1.4 × 102 g
What mass of H2(g)is produced from the reaction of 5.2 g Fe(s)with excess hydrochloric acid?
A) 0.094 g
B) 0.19 g
C) 5.2 g
D) 6.8 g
E) 1.4 × 102 g

Unlock Deck
Unlock for access to all 56 flashcards in this deck.
Unlock Deck
k this deck
44
A mass of 8.15 g C2H4(g)reacts with excess oxygen.If 16.2 g CO2(g)is collected,what is the percent yield of the reaction? C2H4(g)+ 3O2(g)→ 2CO2(g)+ 2H2O(g)
A) 25.6%
B) 31.7%
C) 41.0%
D) 57.1%
E) 63.3%
A) 25.6%
B) 31.7%
C) 41.0%
D) 57.1%
E) 63.3%

Unlock Deck
Unlock for access to all 56 flashcards in this deck.
Unlock Deck
k this deck
45
Under certain conditions the reaction of ammonia with excess oxygen will produce a 24.8% yield of NO.What mass of NH3 must react with excess oxygen to yield 12.5 g NO? 4NH3(g)+ 5O2(g)→ 4NO(g)+ 6H2O(g)
A) 1.76 g
B) 7.10 g
C) 28.6 g
D) 50.4 g
E) 88.8 g
A) 1.76 g
B) 7.10 g
C) 28.6 g
D) 50.4 g
E) 88.8 g

Unlock Deck
Unlock for access to all 56 flashcards in this deck.
Unlock Deck
k this deck
46
The compound P4S3 is used in matches.It reacts with oxygen to produce P4O10 and SO2.The unbalanced chemical equation is shown below. P4S3(s)+ O2(g)→ P4O10(s)+ SO2(g)
What mass of SO2 is produced from the combustion of 0.331 g P4S3?
A) 0.00150 g
B) 0.00451 g
C) 0.0321g
D) 0.0964 g
E) 0.289 g
What mass of SO2 is produced from the combustion of 0.331 g P4S3?
A) 0.00150 g
B) 0.00451 g
C) 0.0321g
D) 0.0964 g
E) 0.289 g

Unlock Deck
Unlock for access to all 56 flashcards in this deck.
Unlock Deck
k this deck
47
What mass of carbon dioxide can be made by reacting 1.56 grams of sodium bicarbonate with 0.687 grams of hydrochloric acid? NaHCO3(s)+ H+(aq)→ CO2(g)+ H2O(l)+ Na+(aq)
A) 2.25 g
B) 2.98 g
C) 0.817 g
D) 0.829 g
E) 11.4 g
A) 2.25 g
B) 2.98 g
C) 0.817 g
D) 0.829 g
E) 11.4 g

Unlock Deck
Unlock for access to all 56 flashcards in this deck.
Unlock Deck
k this deck
48
A mass of 4.00 g of H2(g)reacts with 2.00 g of O2(g).If 1.94 g of H2O(l)is collected,what is the percent yield of the reaction? 2H2(g)+ O2(g)→ 2H2O(l)
A) 5.4 %
B) 49 %
C) 32 %
D) 86 %
E) 97 %
A) 5.4 %
B) 49 %
C) 32 %
D) 86 %
E) 97 %

Unlock Deck
Unlock for access to all 56 flashcards in this deck.
Unlock Deck
k this deck
49
What mass of oxygen will react with 2.64 g of magnesium? 2Mg(s)+ O2(g)→ MgO(s)
A) 0.487 g
B) 1.00 g
C) 1.26 g
D) 1.74 g
E) 3.47 g
A) 0.487 g
B) 1.00 g
C) 1.26 g
D) 1.74 g
E) 3.47 g

Unlock Deck
Unlock for access to all 56 flashcards in this deck.
Unlock Deck
k this deck
50
The reaction of coal and water at a high temperature produces a mixture of hydrogen and carbon monoxide gases.This mixture is known as synthesis gas (or syngas).What mass of carbon monoxide can be formed from the reaction of 71.3 g of carbon with excess water? C(s)+ H2O(g)→ H2(g)+ CO(g)
A) 5.94 g
B) 12.0 g
C) 71.3 g
D) 107 g
E) 166 g
A) 5.94 g
B) 12.0 g
C) 71.3 g
D) 107 g
E) 166 g

Unlock Deck
Unlock for access to all 56 flashcards in this deck.
Unlock Deck
k this deck
51
Which of the balanced chemical equations is consistent with the following pictorial representation of a chemical reaction? 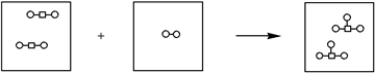
A) 2SO2 + O2 → 2SO3
B) H2 + I2 → 2HI
C) 2H2 + O2 → 2H2O
D) 2N2 + 3H2 → 2NH3
E) none of the above

A) 2SO2 + O2 → 2SO3
B) H2 + I2 → 2HI
C) 2H2 + O2 → 2H2O
D) 2N2 + 3H2 → 2NH3
E) none of the above

Unlock Deck
Unlock for access to all 56 flashcards in this deck.
Unlock Deck
k this deck
52
How many moles of ammonia can be made by reacting 7.0 mol of N2 with 4.0 mol of H2? N2(g)+ 3H2(g)→ 2NH3(g)
A) 2.7 mol
B) 4.0 mol
C) 7.0 mol
D) 11 mol
E) 14 mol
A) 2.7 mol
B) 4.0 mol
C) 7.0 mol
D) 11 mol
E) 14 mol

Unlock Deck
Unlock for access to all 56 flashcards in this deck.
Unlock Deck
k this deck
53
What is the balanced chemical equation for the complete combustion of benzoic acid,C6H5CO2H,to form carbon dioxide and water?
A) C6H5CO2H(s)→ 6C(s)+ CO2(g)+ 3H2(g)
B) C6H5CO2H(s)→ 7CO2(g)+ 3H2O(g)
C) C6H5CO2H(s)+ O2(g)→ CO2(g)+ H2O(g)
D) C6H5CO2H(s)+ 8O2(g)→ 7CO2(g)+ 3H2O(g)
E) 2C6H5CO2H(s)+ 15O2(g)→ 14CO2(g)+ 6H2O(g)
A) C6H5CO2H(s)→ 6C(s)+ CO2(g)+ 3H2(g)
B) C6H5CO2H(s)→ 7CO2(g)+ 3H2O(g)
C) C6H5CO2H(s)+ O2(g)→ CO2(g)+ H2O(g)
D) C6H5CO2H(s)+ 8O2(g)→ 7CO2(g)+ 3H2O(g)
E) 2C6H5CO2H(s)+ 15O2(g)→ 14CO2(g)+ 6H2O(g)

Unlock Deck
Unlock for access to all 56 flashcards in this deck.
Unlock Deck
k this deck
54
Chlorophyll,the substance responsible for the green color of leaves,has one magnesium atom per chlorophyll molecule and contains 2.72% magnesium by mass.What is the molar mass of chlorophyll?
A) 24.3 g/mol
B) 20.2 g/mol
C) 2020 g/mol
D) 8.94 g/mol
E) 894 g/mol
A) 24.3 g/mol
B) 20.2 g/mol
C) 2020 g/mol
D) 8.94 g/mol
E) 894 g/mol

Unlock Deck
Unlock for access to all 56 flashcards in this deck.
Unlock Deck
k this deck
55
Aluminum reacts in air with oxygen to form aluminum oxide.Which of the reactions below is correct and properly balanced?
A) Al(s)+ O(g)→ AlO(s)
B) 2Al(s)+ O2(g)→ 2AlO(s)
C) 3Al(s)+ O2(g)→ Al3O2(s)
D) 4Al(s)+ O2(g)→ 2Al2O(s)
E) 4Al(s)+ 3O2(g)→ 2Al2O3(s)
A) Al(s)+ O(g)→ AlO(s)
B) 2Al(s)+ O2(g)→ 2AlO(s)
C) 3Al(s)+ O2(g)→ Al3O2(s)
D) 4Al(s)+ O2(g)→ 2Al2O(s)
E) 4Al(s)+ 3O2(g)→ 2Al2O3(s)

Unlock Deck
Unlock for access to all 56 flashcards in this deck.
Unlock Deck
k this deck
56
Nitric oxide is made from the oxidation of ammonia.What mass of nitric oxide can be made from the reaction of 8.00 g NH3 with 17.0 g O2? 4NH3(g)+ 5O2(g)→ 4NO(g)+ 6H2O(g)
A) 4.54 g
B) 12.8 g
C) 14.1 g
D) 15.9 g
E) 25.0 g
A) 4.54 g
B) 12.8 g
C) 14.1 g
D) 15.9 g
E) 25.0 g

Unlock Deck
Unlock for access to all 56 flashcards in this deck.
Unlock Deck
k this deck


