Deck 2: Atoms, molecules, and Ions
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/59
Play
Full screen (f)
Deck 2: Atoms, molecules, and Ions
1
Rubidium has two naturally occurring isotopes.The average mass of Rb is 85.4678 amu.If 72.15% of Rb is found as Rb-85 (84.9117 amu),what is the mass of the other isotope?
A) 0.56 amu
B) 85.68 amu
C) 86.91 amu
D) 86.02 amu
E) 83.47 amu
A) 0.56 amu
B) 85.68 amu
C) 86.91 amu
D) 86.02 amu
E) 83.47 amu
86.91 amu
2
What is the identity of ?
A) zinc
B) silver
C) iridium
D) cesium
E) manganese
A) zinc
B) silver
C) iridium
D) cesium
E) manganese
manganese
3
Two isotopes of chlorine are found in nature,Cl-35 and Cl-37.The average mass of chlorine is 35.45 amu.The more abundant isotope of Cl has
A) 17 protons,17 electrons,and 18 neutrons.
B) 17 protons,17 electrons,and 18.45 neutrons.
C) 17 protons,17 electrons,and 20 neutrons.
D) 18 protons,18 electrons,and 17 neutrons.
E) 19 protons,19 electrons,and 16 neutrons.
A) 17 protons,17 electrons,and 18 neutrons.
B) 17 protons,17 electrons,and 18.45 neutrons.
C) 17 protons,17 electrons,and 20 neutrons.
D) 18 protons,18 electrons,and 17 neutrons.
E) 19 protons,19 electrons,and 16 neutrons.
17 protons,17 electrons,and 18 neutrons.
4
All atoms of the same element have the same number of ____.
A) neutrons
B) protons
C) protons and neutrons
D) electrons and neutrons
E) protons,neutrons,and electrons
A) neutrons
B) protons
C) protons and neutrons
D) electrons and neutrons
E) protons,neutrons,and electrons

Unlock Deck
Unlock for access to all 59 flashcards in this deck.
Unlock Deck
k this deck
5
Which species has 63 neutrons?
A)
B)
C)
D)
E) none of the above
A)
B)
C)
D)
E) none of the above

Unlock Deck
Unlock for access to all 59 flashcards in this deck.
Unlock Deck
k this deck
6
F-20,a radioactive isotope of fluorine,has
A) 9 protons,10 neutrons,and 1 electron.
B) 9 protons,10 neutrons,and 9 electrons.
C) 9 protons,11 neutrons,and 9 electrons.
D) 10 protons,9 neutrons,and 1 electron.
E) 10 protons,10 neutrons,and 10 electrons.
A) 9 protons,10 neutrons,and 1 electron.
B) 9 protons,10 neutrons,and 9 electrons.
C) 9 protons,11 neutrons,and 9 electrons.
D) 10 protons,9 neutrons,and 1 electron.
E) 10 protons,10 neutrons,and 10 electrons.

Unlock Deck
Unlock for access to all 59 flashcards in this deck.
Unlock Deck
k this deck
7
Which two atoms below have the same number of neutrons?
, , , ,
A) and
B) and
C) and
D) and
E) and
, , , ,
A) and
B) and
C) and
D) and
E) and

Unlock Deck
Unlock for access to all 59 flashcards in this deck.
Unlock Deck
k this deck
8
Which of the following atoms contains the fewest protons?
A) (232Th)
B) (231Pa)
C) (245Pu)
D) (238U)
E) (232Pa)
A) (232Th)
B) (231Pa)
C) (245Pu)
D) (238U)
E) (232Pa)

Unlock Deck
Unlock for access to all 59 flashcards in this deck.
Unlock Deck
k this deck
9
Silver has two stable isotopes with masses of 106.90509 amu and 108.9047 amu.The average molar mass of silver is 107.868 amu.What is the percent abundance of each isotope?
A) 50.0% Ag-107 and 50.0% Ag-109
B) 51.8% Ag-107 and 48.2% Ag-109
C) 55.4% Ag-107 and 44.6% Ag-109
D) 48.2% Ag-107 and 51.8% Ag-109
E) 44.6% Ag-107 and 55.4% Ag-109
A) 50.0% Ag-107 and 50.0% Ag-109
B) 51.8% Ag-107 and 48.2% Ag-109
C) 55.4% Ag-107 and 44.6% Ag-109
D) 48.2% Ag-107 and 51.8% Ag-109
E) 44.6% Ag-107 and 55.4% Ag-109

Unlock Deck
Unlock for access to all 59 flashcards in this deck.
Unlock Deck
k this deck
10
What is the mass number of an argon atom with 22 neutrons?
A) 2
B) 18
C) 22
D) 40
E) 39.95
A) 2
B) 18
C) 22
D) 40
E) 39.95

Unlock Deck
Unlock for access to all 59 flashcards in this deck.
Unlock Deck
k this deck
11
Which two of the ions below have the same number of electrons?
, , , ,
A) and
B) and
C) and
D) and
E) and
, , , ,
A) and
B) and
C) and
D) and
E) and

Unlock Deck
Unlock for access to all 59 flashcards in this deck.
Unlock Deck
k this deck
12
All of the following statements are true EXCEPT
A) all atoms of a given element have the same mass number.
B) for any neutral element,the number of electrons is equal to the number of protons.
C) the mass number is the sum of the number of protons and neutrons.
D) isotopes of atoms contain the same number of protons but a different number of neutrons.
E) the atomic number equals the number of protons in an atom.
A) all atoms of a given element have the same mass number.
B) for any neutral element,the number of electrons is equal to the number of protons.
C) the mass number is the sum of the number of protons and neutrons.
D) isotopes of atoms contain the same number of protons but a different number of neutrons.
E) the atomic number equals the number of protons in an atom.

Unlock Deck
Unlock for access to all 59 flashcards in this deck.
Unlock Deck
k this deck
13
Gallium has an average atomic mass of 69.7 amu.In a typical sample,60.4% of Ga exists as Ga-69 (68.9257 amu).What is the identity and the atomic mass of the other isotope?
A) ;70.9 amu
B) ;70.9 amu
C) ;70.9 amu
D) ;71.9 amu
E) ;71.9 amu
A) ;70.9 amu
B) ;70.9 amu
C) ;70.9 amu
D) ;71.9 amu
E) ;71.9 amu

Unlock Deck
Unlock for access to all 59 flashcards in this deck.
Unlock Deck
k this deck
14
What is the symbol for an element which contains 57 neutrons and has a mass number of 101?
A) Er
B) Ru
C) Md
D) La
E) Os
A) Er
B) Ru
C) Md
D) La
E) Os

Unlock Deck
Unlock for access to all 59 flashcards in this deck.
Unlock Deck
k this deck
15
Two isotopes of a given element will have the same number of ____,but a different number of ____ in their nucleus.
A) protons,electrons
B) electrons,protons
C) protons,neutrons
D) neutrons,protons
E) electrons,neutrons
A) protons,electrons
B) electrons,protons
C) protons,neutrons
D) neutrons,protons
E) electrons,neutrons

Unlock Deck
Unlock for access to all 59 flashcards in this deck.
Unlock Deck
k this deck
16
The average molar mass of lithium is 6.941.A sample of lithium consists of two isotopes with masses of 6.01512 amu and 7.01600 amu.Determine the percent abundance of each isotope.
A) 7.49% Li-6 and 92.51% Li-7
B) 8.45% Li-6 and 91.55% Li-7
C) 12.49% Li-6 and 87.51% Li-7
D) 91.55% Li-6 and 8.45% Li-7
E) 92.51% Li-6 and 7.49% Li-7
A) 7.49% Li-6 and 92.51% Li-7
B) 8.45% Li-6 and 91.55% Li-7
C) 12.49% Li-6 and 87.51% Li-7
D) 91.55% Li-6 and 8.45% Li-7
E) 92.51% Li-6 and 7.49% Li-7

Unlock Deck
Unlock for access to all 59 flashcards in this deck.
Unlock Deck
k this deck
17
How many protons,neutrons,and electrons are in a silver atom with a mass number of 108?
A) 47 protons,47 neutrons,61 electrons
B) 47 protons,61 neutrons,47 electrons
C) 61 protons,47 neutrons,47 electrons
D) 47 protons,108 neutrons,47 electrons
E) 61 protons,108 neutrons,61 electrons
A) 47 protons,47 neutrons,61 electrons
B) 47 protons,61 neutrons,47 electrons
C) 61 protons,47 neutrons,47 electrons
D) 47 protons,108 neutrons,47 electrons
E) 61 protons,108 neutrons,61 electrons

Unlock Deck
Unlock for access to all 59 flashcards in this deck.
Unlock Deck
k this deck
18
Which of the following are a pair of isotopes?
A) and
B) and
C) and
D) and
E) and
A) and
B) and
C) and
D) and
E) and

Unlock Deck
Unlock for access to all 59 flashcards in this deck.
Unlock Deck
k this deck
19
Rank the subatomic particles from least to greatest mass.
A) electrons = neutrons = protons
B) electrons = protons < neutrons
C) electrons < neutrons = protons
D) electrons < protons < neutrons
E) electrons < neutrons < protons
A) electrons = neutrons = protons
B) electrons = protons < neutrons
C) electrons < neutrons = protons
D) electrons < protons < neutrons
E) electrons < neutrons < protons

Unlock Deck
Unlock for access to all 59 flashcards in this deck.
Unlock Deck
k this deck
20
What is the atomic symbol for an element with 39 protons and 50 neutrons?
A)
B)
C)
D)
E)
A)
B)
C)
D)
E)

Unlock Deck
Unlock for access to all 59 flashcards in this deck.
Unlock Deck
k this deck
21
Identify the ions and their charges in Na2SO4.
A) Na+,SO4−
B) Na+,SO42−
C) Na+,SO4−
D) Na2+,SO4−
E) Na2+,SO42−
A) Na+,SO4−
B) Na+,SO42−
C) Na+,SO4−
D) Na2+,SO4−
E) Na2+,SO42−

Unlock Deck
Unlock for access to all 59 flashcards in this deck.
Unlock Deck
k this deck
22
How many elements are contained in period 2?
A) 3
B) 8
C) 10
D) 18
E) 32
A) 3
B) 8
C) 10
D) 18
E) 32

Unlock Deck
Unlock for access to all 59 flashcards in this deck.
Unlock Deck
k this deck
23
A strontium ion has ____ electrons.
A) 35
B) 36
C) 37
D) 38
E) 39
A) 35
B) 36
C) 37
D) 38
E) 39

Unlock Deck
Unlock for access to all 59 flashcards in this deck.
Unlock Deck
k this deck
24
Which two of the following elements are abundant in the Earth's crust,but missing from the human body: O,Al,Si,Fe,C,N?
A) O and Fe
B) Si and C
C) Al and Si
D) O and N
E) Fe and N
A) O and Fe
B) Si and C
C) Al and Si
D) O and N
E) Fe and N

Unlock Deck
Unlock for access to all 59 flashcards in this deck.
Unlock Deck
k this deck
25
The formula of ethanol,CH3CH2OH,is an example of a(n)
A) condensed formula.
B) empirical formula.
C) structural formula.
D) ionic compound formula.
E) molecular formula.
A) condensed formula.
B) empirical formula.
C) structural formula.
D) ionic compound formula.
E) molecular formula.

Unlock Deck
Unlock for access to all 59 flashcards in this deck.
Unlock Deck
k this deck
26
What is the correct formula for an ionic compound that contains magnesium ions and phosphide ions?
A) MgP
B) MgP2
C) Mg3P2
D) Mg3(PO4)2
E) Mg2P3
A) MgP
B) MgP2
C) Mg3P2
D) Mg3(PO4)2
E) Mg2P3

Unlock Deck
Unlock for access to all 59 flashcards in this deck.
Unlock Deck
k this deck
27
Which atom is most likely to form an ion with a +2 charge?
A) scandium
B) calcium
C) aluminum
D) oxygen
E) fluorine
A) scandium
B) calcium
C) aluminum
D) oxygen
E) fluorine

Unlock Deck
Unlock for access to all 59 flashcards in this deck.
Unlock Deck
k this deck
28
Which three elements are likely to have similar chemical and physical properties?
A) boron,silicon,and germanium
B) sodium,magnesium,and aluminum
C) sodium,potassium,and rubidium
D) oxygen,sulfur,and chlorine
E) carbon,nitrogen,and oxygen
A) boron,silicon,and germanium
B) sodium,magnesium,and aluminum
C) sodium,potassium,and rubidium
D) oxygen,sulfur,and chlorine
E) carbon,nitrogen,and oxygen

Unlock Deck
Unlock for access to all 59 flashcards in this deck.
Unlock Deck
k this deck
29
Which group of three elements contains a nonmetal,a metal,and a metalloid?
A) Li,Al,Si
B) Na,Hg,I
C) I,Hg,Si
D) K,O,Br
E) H,Al,N
A) Li,Al,Si
B) Na,Hg,I
C) I,Hg,Si
D) K,O,Br
E) H,Al,N

Unlock Deck
Unlock for access to all 59 flashcards in this deck.
Unlock Deck
k this deck
30
How many nonmetallic elements are there in group 13?
A) 0
B) 1
C) 2
D) 3
E) 4
A) 0
B) 1
C) 2
D) 3
E) 4

Unlock Deck
Unlock for access to all 59 flashcards in this deck.
Unlock Deck
k this deck
31
For a nonmetal in Group 16 of the periodic table,the most common monatomic ion will have a charge of ____.
A) -3
B) -2
C) -1
D) +1
E) +3
A) -3
B) -2
C) -1
D) +1
E) +3

Unlock Deck
Unlock for access to all 59 flashcards in this deck.
Unlock Deck
k this deck
32
Which group of three elements contains a transition metal,a halogen,and a noble gas?
A) S,I,Cu
B) Br,Kr,Ba
C) Ar,Hg,Rn
D) Ce,N,He
E) Cu,I,Xe
A) S,I,Cu
B) Br,Kr,Ba
C) Ar,Hg,Rn
D) Ce,N,He
E) Cu,I,Xe

Unlock Deck
Unlock for access to all 59 flashcards in this deck.
Unlock Deck
k this deck
33
Identify the ions and their charges in KH2PO4.
A) K+,H+,P3−,O2−
B) K+,H2+,P3−,O8−
C) K+,H22+,P−1,O4−2
D) K+,H2PO4−
E) K+,H2+,PO43−
A) K+,H+,P3−,O2−
B) K+,H2+,P3−,O8−
C) K+,H22+,P−1,O4−2
D) K+,H2PO4−
E) K+,H2+,PO43−

Unlock Deck
Unlock for access to all 59 flashcards in this deck.
Unlock Deck
k this deck
34
What is the mass (in grams)of a boron atom?
A) 10.8 g
B) 1.80 × 10-23 g
C) 1.66 × 10-24 g
D) 5.57 × 1022 g
E) 1.54 × 10-25 g
A) 10.8 g
B) 1.80 × 10-23 g
C) 1.66 × 10-24 g
D) 5.57 × 1022 g
E) 1.54 × 10-25 g

Unlock Deck
Unlock for access to all 59 flashcards in this deck.
Unlock Deck
k this deck
35
What element is in the fourth period in Group 3A?
A) Sb
B) Ga
C) In
D) Si
E) Tl
A) Sb
B) Ga
C) In
D) Si
E) Tl

Unlock Deck
Unlock for access to all 59 flashcards in this deck.
Unlock Deck
k this deck
36
What is the correct formula for an ionic compound that contains aluminum ions and chloride ions?
A) AlCl
B) AlCl2
C) AlCl3
D) Al2Cl3
E) Al3Cl2
A) AlCl
B) AlCl2
C) AlCl3
D) Al2Cl3
E) Al3Cl2

Unlock Deck
Unlock for access to all 59 flashcards in this deck.
Unlock Deck
k this deck
37
Group 1 elements are also known as
A) alkaline earth metals.
B) alkali metals.
C) chalcogens.
D) halogens.
E) noble gases.
A) alkaline earth metals.
B) alkali metals.
C) chalcogens.
D) halogens.
E) noble gases.

Unlock Deck
Unlock for access to all 59 flashcards in this deck.
Unlock Deck
k this deck
38
Which element is most likely to form an ion with a −2 charge?
A) K
B) Mg
C) P
D) Br
E) S
A) K
B) Mg
C) P
D) Br
E) S

Unlock Deck
Unlock for access to all 59 flashcards in this deck.
Unlock Deck
k this deck
39
Identify the halogen from period 4.
A) Br
B) I
C) Kr
D) Ar
E) K
A) Br
B) I
C) Kr
D) Ar
E) K

Unlock Deck
Unlock for access to all 59 flashcards in this deck.
Unlock Deck
k this deck
40
Of the naturally occurring elements in group 14,how many are nonmetals,metalloids,and metals?
A) 0 nonmetals,3 metalloids,and 2 metals
B) 1 nonmetal,2 metalloids,and 2 metals
C) 2 nonmetals,2 metalloids,and 1 metal
D) 2 nonmetals,1 metalloid,and 2 metals
E) 3 nonmetals,0 metalloids,and 2 metals
A) 0 nonmetals,3 metalloids,and 2 metals
B) 1 nonmetal,2 metalloids,and 2 metals
C) 2 nonmetals,2 metalloids,and 1 metal
D) 2 nonmetals,1 metalloid,and 2 metals
E) 3 nonmetals,0 metalloids,and 2 metals

Unlock Deck
Unlock for access to all 59 flashcards in this deck.
Unlock Deck
k this deck
41
What is the correct formula for sulfur dichloride?
A) SCl
B) SCl2
C) S2Cl
D) S2Cl2
E) S4Cl2
A) SCl
B) SCl2
C) S2Cl
D) S2Cl2
E) S4Cl2

Unlock Deck
Unlock for access to all 59 flashcards in this deck.
Unlock Deck
k this deck
42
What is the correct name for K3PO4?
A) tripotassium phosphate
B) potassium(I)monophosphorus tetraoxide
C) potassium(I)phosphate
D) potassium phosphate
E) potassium phosphide
A) tripotassium phosphate
B) potassium(I)monophosphorus tetraoxide
C) potassium(I)phosphate
D) potassium phosphate
E) potassium phosphide

Unlock Deck
Unlock for access to all 59 flashcards in this deck.
Unlock Deck
k this deck
43
What is the formula for hypochlorous acid?
A) HCl
B) HClO
C) HClO2
D) HClO3
E) HClO4
A) HCl
B) HClO
C) HClO2
D) HClO3
E) HClO4

Unlock Deck
Unlock for access to all 59 flashcards in this deck.
Unlock Deck
k this deck
44
What are the values for x and y,respectively,in CaxHyPO4?
A) 1 and 2
B) 2 and 1
C) 1 and 3
D) 2 and 2
E) 1 and 1
A) 1 and 2
B) 2 and 1
C) 1 and 3
D) 2 and 2
E) 1 and 1

Unlock Deck
Unlock for access to all 59 flashcards in this deck.
Unlock Deck
k this deck
45
Using the laws of constant composition and the conservation of mass,complete the molecular picture of hydrogen molecules (circles)reacting with chlorine molecules (squares)to give hydrogen chloride (HCl). 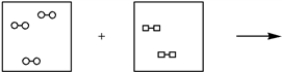
A)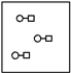
B)
C)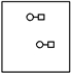
D)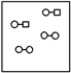
E) None of these are correct.

A)

B)

C)

D)

E) None of these are correct.

Unlock Deck
Unlock for access to all 59 flashcards in this deck.
Unlock Deck
k this deck
46
What is the correct name for H2SO4(aq)?
A) sulfuric acid
B) sulfide acid
C) sulfurous acid
D) hydrogen sulfate acid
E) hydrogen sulfide acid
A) sulfuric acid
B) sulfide acid
C) sulfurous acid
D) hydrogen sulfate acid
E) hydrogen sulfide acid

Unlock Deck
Unlock for access to all 59 flashcards in this deck.
Unlock Deck
k this deck
47
What is the correct formula for potassium dichromate?
A) K2Cr2O7
B) K2(Cr2O7)2
C) K2CrO4
D) K2(CrO4)2
E) KCrO4
A) K2Cr2O7
B) K2(Cr2O7)2
C) K2CrO4
D) K2(CrO4)2
E) KCrO4

Unlock Deck
Unlock for access to all 59 flashcards in this deck.
Unlock Deck
k this deck
48
What is the correct name for PF5?
A) phosphorus pentafluoride
B) phosphorus(V)fluorine
C) phosphorofluoride
D) pentafluorophosphorus
E) pentafluorophosphate
A) phosphorus pentafluoride
B) phosphorus(V)fluorine
C) phosphorofluoride
D) pentafluorophosphorus
E) pentafluorophosphate

Unlock Deck
Unlock for access to all 59 flashcards in this deck.
Unlock Deck
k this deck
49
What is the correct formula for iron(II)nitrate?
A) Fe2(NO3)2
B) Fe2NO3
C) Fe(NO3)2
D) Fe3N2
E) FeNO3
A) Fe2(NO3)2
B) Fe2NO3
C) Fe(NO3)2
D) Fe3N2
E) FeNO3

Unlock Deck
Unlock for access to all 59 flashcards in this deck.
Unlock Deck
k this deck
50
What is the correct name for TiCl4?
A) monotitanium tetrachloride
B) tetrachlorine titanate
C) titanium tetrachlorine
D) titanium(IV)tetrachloride
E) titanium(IV)chloride
A) monotitanium tetrachloride
B) tetrachlorine titanate
C) titanium tetrachlorine
D) titanium(IV)tetrachloride
E) titanium(IV)chloride

Unlock Deck
Unlock for access to all 59 flashcards in this deck.
Unlock Deck
k this deck
51
What is the correct name for CCl4?
A) carbon chlorine
B) tetracarbon chloride
C) carbon tetrachloride
D) carbon(IV)chloride
E) tetrachlorocarbide
A) carbon chlorine
B) tetracarbon chloride
C) carbon tetrachloride
D) carbon(IV)chloride
E) tetrachlorocarbide

Unlock Deck
Unlock for access to all 59 flashcards in this deck.
Unlock Deck
k this deck
52
Using the laws of constant composition and the conservation of mass,complete the molecular picture of hydrogen molecules (circles)reacting with oxygen molecules (squares)to give water. 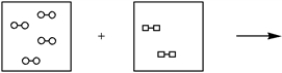
A)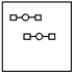
B)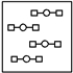
C)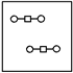
D)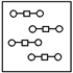
E)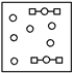

A)

B)

C)

D)

E)


Unlock Deck
Unlock for access to all 59 flashcards in this deck.
Unlock Deck
k this deck
53
What is the correct name for N2O3?
A) nitrogen oxide
B) nitrogen(II)oxide
C) nitrogen(III)oxide
D) trioxygen dinitride
E) dinitrogen trioxide
A) nitrogen oxide
B) nitrogen(II)oxide
C) nitrogen(III)oxide
D) trioxygen dinitride
E) dinitrogen trioxide

Unlock Deck
Unlock for access to all 59 flashcards in this deck.
Unlock Deck
k this deck
54
What is the correct formula for barium perchlorate?
A) BaClO4
B) BaClO3
C) Ba(ClO4)2
D) Ba(ClO3)2
E) Ba(ClO3)3
A) BaClO4
B) BaClO3
C) Ba(ClO4)2
D) Ba(ClO3)2
E) Ba(ClO3)3

Unlock Deck
Unlock for access to all 59 flashcards in this deck.
Unlock Deck
k this deck
55
What is the correct name for MnS?
A) manganese sulfide
B) dimanganese sulfate
C) dimanganese sulfide
D) manganese(II)sulfate
E) manganese(II)sulfide
A) manganese sulfide
B) dimanganese sulfate
C) dimanganese sulfide
D) manganese(II)sulfate
E) manganese(II)sulfide

Unlock Deck
Unlock for access to all 59 flashcards in this deck.
Unlock Deck
k this deck
56
Which of the following is a nonelectrolyte in water?
A) NaCl
B) SF6
C) KNO3
D) MgS
E) NH4Cl
A) NaCl
B) SF6
C) KNO3
D) MgS
E) NH4Cl

Unlock Deck
Unlock for access to all 59 flashcards in this deck.
Unlock Deck
k this deck
57
Sodium sulfate has the chemical formula Na2SO4.Based on this information,the formula for chromium(III)sulfate is ____.
A) CrSO4
B) Cr(SO4)3
C) Cr2(SO4)3
D) Cr2SO4
E) Cr3(SO4)2
A) CrSO4
B) Cr(SO4)3
C) Cr2(SO4)3
D) Cr2SO4
E) Cr3(SO4)2

Unlock Deck
Unlock for access to all 59 flashcards in this deck.
Unlock Deck
k this deck
58
What is the correct name for Al2O3?
A) alum
B) aluminum trioxide
C) aluminum ozinide
D) aluminum oxide
E) dialuminum trioxide
A) alum
B) aluminum trioxide
C) aluminum ozinide
D) aluminum oxide
E) dialuminum trioxide

Unlock Deck
Unlock for access to all 59 flashcards in this deck.
Unlock Deck
k this deck
59
What is the correct formula for aluminum selenide?
A) AlSe
B) AlSe2
C) Al2Se
D) Al2Se3
E) Al3Se2
A) AlSe
B) AlSe2
C) Al2Se
D) Al2Se3
E) Al3Se2

Unlock Deck
Unlock for access to all 59 flashcards in this deck.
Unlock Deck
k this deck


