Deck 27: Amino Acids and Proteins
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/46
Play
Full screen (f)
Deck 27: Amino Acids and Proteins
1
Why is the s-trans form of an amide bond more stable?
A)There is no s-cis form in an amide bond.
B)The oxygen is hydrogen-bonded with the nitrogen atom.
C)The nitrogen is able to be sp2-hybridized.
D)The more sterically bulky groups are farther apart.
A)There is no s-cis form in an amide bond.
B)The oxygen is hydrogen-bonded with the nitrogen atom.
C)The nitrogen is able to be sp2-hybridized.
D)The more sterically bulky groups are farther apart.
The more sterically bulky groups are farther apart.
2
What are the basic steps in the Merrifield peptide synthesis?
A)Coupling and hydrolysis
B)Hydrolysis and deprotection
C)Deprotection and hydrogenation
D)Coupling and deprotection
A)Coupling and hydrolysis
B)Hydrolysis and deprotection
C)Deprotection and hydrogenation
D)Coupling and deprotection
Coupling and deprotection
3
What amino acid is at the C-terminus of the following peptide? 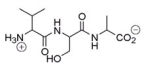
A)Valine
B)Serine
C)Alanine
D)Proline

A)Valine
B)Serine
C)Alanine
D)Proline
Alanine
4
What reagents would you use to convert acetaldehyde into alanine?
A)NH3
B)HCN
C)NaCN,NH4Cl
D)NaNH2
A)NH3
B)HCN
C)NaCN,NH4Cl
D)NaNH2

Unlock Deck
Unlock for access to all 46 flashcards in this deck.
Unlock Deck
k this deck
5
Which of the following aldehydes would be a good starting material in the Strecker synthesis of phenylalanine? 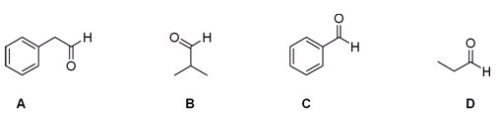
A)A
B)B
C)C
D)D

A)A
B)B
C)C
D)D

Unlock Deck
Unlock for access to all 46 flashcards in this deck.
Unlock Deck
k this deck
6
Which of the following compounds is not an amino acid? 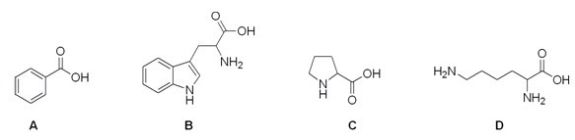
A)A
B)B
C)C
D)D

A)A
B)B
C)C
D)D

Unlock Deck
Unlock for access to all 46 flashcards in this deck.
Unlock Deck
k this deck
7
Which of the following is the correct structure for aspartic acid at pH = 1? 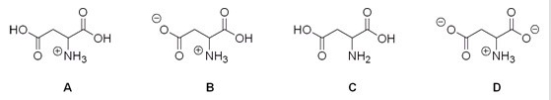
A)A
B)B
C)C
D)D

A)A
B)B
C)C
D)D

Unlock Deck
Unlock for access to all 46 flashcards in this deck.
Unlock Deck
k this deck
8
Which is the correct structure for the amino acid cysteine? 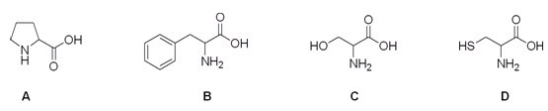
A)A
B)B
C)C
D)D

A)A
B)B
C)C
D)D

Unlock Deck
Unlock for access to all 46 flashcards in this deck.
Unlock Deck
k this deck
9
Why do amide bonds exist as reasonably stable s-cis or s-trans isomers at room temperature?
A)These amide bonds are not stable.
B)There is partial double bond character between the carbonyl carbon and the nitrogen atom.
C)The oxygen atom is hydrogen-bonded with the nitrogen atom.
D)The side-chains can form hydrogen bonds.
A)These amide bonds are not stable.
B)There is partial double bond character between the carbonyl carbon and the nitrogen atom.
C)The oxygen atom is hydrogen-bonded with the nitrogen atom.
D)The side-chains can form hydrogen bonds.

Unlock Deck
Unlock for access to all 46 flashcards in this deck.
Unlock Deck
k this deck
10
Which is the correct structure for serine? 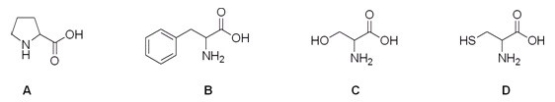
A)A
B)B
C)C
D)D

A)A
B)B
C)C
D)D

Unlock Deck
Unlock for access to all 46 flashcards in this deck.
Unlock Deck
k this deck
11
Which of the following is the major advantage of the Merrifield synthesis of peptides?
A)It allows for the formation of absolutely no by-products.
B)Impurities can be easily washed away.
C)Excess reagent can be used.
D)It prevents hydrogenation.
A)It allows for the formation of absolutely no by-products.
B)Impurities can be easily washed away.
C)Excess reagent can be used.
D)It prevents hydrogenation.

Unlock Deck
Unlock for access to all 46 flashcards in this deck.
Unlock Deck
k this deck
12
Which of the following is the structure of the amino acid lysine? 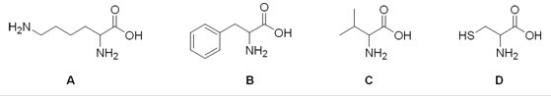
A)A
B)B
C)C
D)D

A)A
B)B
C)C
D)D

Unlock Deck
Unlock for access to all 46 flashcards in this deck.
Unlock Deck
k this deck
13
Which of the following is the correct structure for alanine at pH = 7? 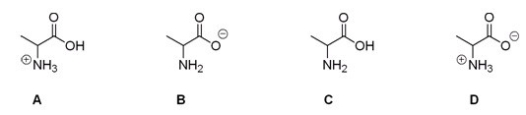
A)A
B)B
C)C
D)D

A)A
B)B
C)C
D)D

Unlock Deck
Unlock for access to all 46 flashcards in this deck.
Unlock Deck
k this deck
14
Which is the correct structure for the amino acid proline? 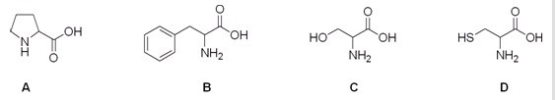
A)A
B)B
C)C
D)D

A)A
B)B
C)C
D)D

Unlock Deck
Unlock for access to all 46 flashcards in this deck.
Unlock Deck
k this deck
15
Which is the correct structure for the amino acid valine? 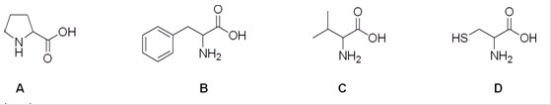
A)A
B)B
C)C
D)D

A)A
B)B
C)C
D)D

Unlock Deck
Unlock for access to all 46 flashcards in this deck.
Unlock Deck
k this deck
16
What is the separation of a racemic mixture into its component enantiomers called?
A)Diastereomers
B)Meso
C)Resolution
D)Neutralization
A)Diastereomers
B)Meso
C)Resolution
D)Neutralization

Unlock Deck
Unlock for access to all 46 flashcards in this deck.
Unlock Deck
k this deck
17
What amino acid is at the N-terminus of the following peptide? 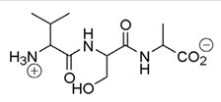
A)Valine
B)Serine
C)Alanine
D)Proline

A)Valine
B)Serine
C)Alanine
D)Proline

Unlock Deck
Unlock for access to all 46 flashcards in this deck.
Unlock Deck
k this deck
18
Why does the formation of salts of a racemic mixture of N-acetyl valine with a single enantiomer of a-methylbenzylamine enable the separation of the two enantiomers of N-acetyl valine?
A)They form amides.
B)They form diastereomers.
C)They form salts.
D)They form meso compounds.
A)They form amides.
B)They form diastereomers.
C)They form salts.
D)They form meso compounds.

Unlock Deck
Unlock for access to all 46 flashcards in this deck.
Unlock Deck
k this deck
19
Why can glycine not be resolved into two enantiomers?
A)It has no stereogenic centers.
B)It has no nitrogen atoms.
C)It has no aromatic rings.
D)It cannot be converted into a salt.
A)It has no stereogenic centers.
B)It has no nitrogen atoms.
C)It has no aromatic rings.
D)It cannot be converted into a salt.

Unlock Deck
Unlock for access to all 46 flashcards in this deck.
Unlock Deck
k this deck
20
What is the configuration of naturally occurring amino acids?
A)D
B)S
C)L
D)R
A)D
B)S
C)L
D)R

Unlock Deck
Unlock for access to all 46 flashcards in this deck.
Unlock Deck
k this deck
21
What are the only covalent bonds that stabilize the tertiary structure of a peptide?
A)Hydrogen bonds
B)Disulfide bonds
C)Van der Waal bonds
D)Ionic bonds
A)Hydrogen bonds
B)Disulfide bonds
C)Van der Waal bonds
D)Ionic bonds

Unlock Deck
Unlock for access to all 46 flashcards in this deck.
Unlock Deck
k this deck
22
What is the IUPAC or common name for the following compound? 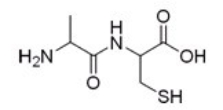
A)Cysteinylalanine
B)Alanylcysteine
C)Serinylalanine
D)Alanylserine

A)Cysteinylalanine
B)Alanylcysteine
C)Serinylalanine
D)Alanylserine

Unlock Deck
Unlock for access to all 46 flashcards in this deck.
Unlock Deck
k this deck
23
What is the major organic product for the following reaction? 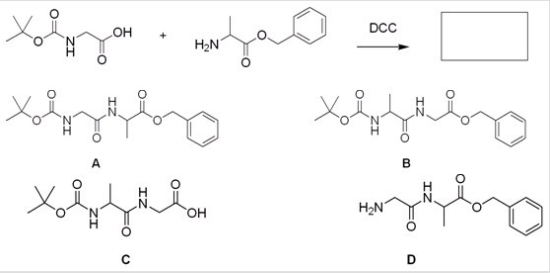
A)A
B)B
C)C
D)D

A)A
B)B
C)C
D)D

Unlock Deck
Unlock for access to all 46 flashcards in this deck.
Unlock Deck
k this deck
24
Give the order (1st to last)in which the following amino acids would be eluted with a buffer of pH = 4 from a column containing a cationic-exchange resin (Dowex 50): Arg (pI = 10.8),Ser (pI = 5.7),Asp (pI = 2.8)and His (pI = 7.6).
A)His,Ser,Asp,Arg
B)Arg,His,Ser,Asp
C)His,Ser,Arg,Asp
D)Asp,Ser,His,Arg
A)His,Ser,Asp,Arg
B)Arg,His,Ser,Asp
C)His,Ser,Arg,Asp
D)Asp,Ser,His,Arg

Unlock Deck
Unlock for access to all 46 flashcards in this deck.
Unlock Deck
k this deck
25
Which of the following amino acids does not have an L-isomer?
A)Alanine
B)Valine
C)Glycine
D)Leucine
A)Alanine
B)Valine
C)Glycine
D)Leucine

Unlock Deck
Unlock for access to all 46 flashcards in this deck.
Unlock Deck
k this deck
26
Which of the following correctly describes the tertiary structure of a peptide?
A)the three-dimensional conformations of localized regions of a protein
B)the particular sequence of amino acids that are joined together by peptide bonds
C)the structure is defined by the b-pleated sheet form
D)the three-dimensional shape adopted by the entire peptide
A)the three-dimensional conformations of localized regions of a protein
B)the particular sequence of amino acids that are joined together by peptide bonds
C)the structure is defined by the b-pleated sheet form
D)the three-dimensional shape adopted by the entire peptide

Unlock Deck
Unlock for access to all 46 flashcards in this deck.
Unlock Deck
k this deck
27
Which of the following is (are)globular protein(s)?
A)Hemoglobin
B)Keratins
C)Collagens
D)Elastins
A)Hemoglobin
B)Keratins
C)Collagens
D)Elastins

Unlock Deck
Unlock for access to all 46 flashcards in this deck.
Unlock Deck
k this deck
28
What is the appropriate sequence of reaction conditions used to synthesize valine from acetamidomalonic ester? 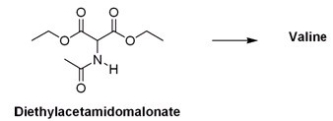
A)(1)NaOH; (2)(CH3)2CHBr; (3)Cl2,H2O,heat
B)(1)NaOCH2CH3; (2)(CH3)2CH2CHBr; (3)HCl,H2O,heat
C)(1)NaOCH2CH3; (2)(CH3)2CHBr; (3)HCl,H2O,heat
D)(1)NaOH; (2)CH3CH2CH2Br; (3)HCl,H2O,heat

A)(1)NaOH; (2)(CH3)2CHBr; (3)Cl2,H2O,heat
B)(1)NaOCH2CH3; (2)(CH3)2CH2CHBr; (3)HCl,H2O,heat
C)(1)NaOCH2CH3; (2)(CH3)2CHBr; (3)HCl,H2O,heat
D)(1)NaOH; (2)CH3CH2CH2Br; (3)HCl,H2O,heat

Unlock Deck
Unlock for access to all 46 flashcards in this deck.
Unlock Deck
k this deck
29
Which of the following amino acids is achiral?
A)Phenylalanine
B)Glycine
C)Valine
D)Alanine
A)Phenylalanine
B)Glycine
C)Valine
D)Alanine

Unlock Deck
Unlock for access to all 46 flashcards in this deck.
Unlock Deck
k this deck
30
Which of the following correctly describes a protein?
A)a dipeptide; two amino acids joined together by one amide bond
B)a tripeptide; three amino acids joined together by two amide bonds
C)a polymer of more than 40 amino acids joined together by amide bonds
D)a polypeptide of any number of amino acids joined together by amide bonds
A)a dipeptide; two amino acids joined together by one amide bond
B)a tripeptide; three amino acids joined together by two amide bonds
C)a polymer of more than 40 amino acids joined together by amide bonds
D)a polypeptide of any number of amino acids joined together by amide bonds

Unlock Deck
Unlock for access to all 46 flashcards in this deck.
Unlock Deck
k this deck
31
Which one of the following naturally occurring amino acids does not have an S configuration?
A)Cysteine
B)Alanine
C)Tyrosine
D)Isoleucine
A)Cysteine
B)Alanine
C)Tyrosine
D)Isoleucine

Unlock Deck
Unlock for access to all 46 flashcards in this deck.
Unlock Deck
k this deck
32
What is formed by the combination of four amino acids?
A)A protein
B)A polypeptide
C)A tripeptide
D)A tetrapeptide
A)A protein
B)A polypeptide
C)A tripeptide
D)A tetrapeptide

Unlock Deck
Unlock for access to all 46 flashcards in this deck.
Unlock Deck
k this deck
33
Reaction of a polypeptide,composed of 12 amino acids,with carboxypeptidase A releases Met (C-terminal amino acid).The polypeptide undergoes partial hydrolysis to give 12 groups of peptides.Use the groups of overlapping amino acids to determine the proper sequence of this polypeptide.Note: Since these lists of peptides are separated by commas,they are not necessarily in the proper sequence. Ser,Lys,Trp-5.Met,Ala,Gly- 9.Lys,Ser
Gly,His,Ala-6.Ser,Lys,Val -10.Glu,His,Val
Glu,Val,Ser-7.Glu,His-11.Trp,Leu,Glu
Leu,Glu,Ser-8.Leu,Lys,Trp-12.Ala,Met
A)Met-Ala-Gly-His-Glu-Val-Ser-Lys-Trp-Leu-Glu-Ser
B)Met-Ala-Gly-Glu-His-Ser-Val-Lys-Trp-Leu-Glu-Ser
C)Ser-Glu-Leu-Trp-Lys-Ser-Val-Glu-His-Gly-Ala-Met
D)Ser-Lys-Leu-Trp-Lys-Ser-Val-His-Glu-Gly-Ala-Met
Gly,His,Ala-6.Ser,Lys,Val -10.Glu,His,Val
Glu,Val,Ser-7.Glu,His-11.Trp,Leu,Glu
Leu,Glu,Ser-8.Leu,Lys,Trp-12.Ala,Met
A)Met-Ala-Gly-His-Glu-Val-Ser-Lys-Trp-Leu-Glu-Ser
B)Met-Ala-Gly-Glu-His-Ser-Val-Lys-Trp-Leu-Glu-Ser
C)Ser-Glu-Leu-Trp-Lys-Ser-Val-Glu-His-Gly-Ala-Met
D)Ser-Lys-Leu-Trp-Lys-Ser-Val-His-Glu-Gly-Ala-Met

Unlock Deck
Unlock for access to all 46 flashcards in this deck.
Unlock Deck
k this deck
34
What is (are)the missing reagent(s)in the reaction below? 
A)NH3
B)HCN
C)NaCN,NH4Cl
D)NaNH2

A)NH3
B)HCN
C)NaCN,NH4Cl
D)NaNH2

Unlock Deck
Unlock for access to all 46 flashcards in this deck.
Unlock Deck
k this deck
35
Below is a 2D chromatogram that shows the separation of five amino acids.In this technique,the amino acid mixture is first separated by chromatography using a polar solvent system.Then the plate is rotated 90°,and the amino acids are further separated by electrophoresis.Identify the spots obtained from a mixture of Trp,Glu,Lys,Ile and Thr.A table with isoelectric points is included to aid in solving this problem. 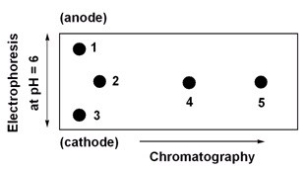 amino acid-pI value Trp-5.9
amino acid-pI value Trp-5.9
Glu-3.2
Lys-9.7
Ile-6.0
Thr-5.6
A)1=Lys,2=Thr,3=Glu,4=Trp,5=Ile
B)1=Glu,2=Thr,3=Lys,4=Trp,5=Ile
C)1=Lys,2=Ile,3=Glu,4=Trp,5=Thr
D)1=Glu,2=Ile,3=Lys,4=Trp,5=Thr
 amino acid-pI value Trp-5.9
amino acid-pI value Trp-5.9Glu-3.2
Lys-9.7
Ile-6.0
Thr-5.6
A)1=Lys,2=Thr,3=Glu,4=Trp,5=Ile
B)1=Glu,2=Thr,3=Lys,4=Trp,5=Ile
C)1=Lys,2=Ile,3=Glu,4=Trp,5=Thr
D)1=Glu,2=Ile,3=Lys,4=Trp,5=Thr

Unlock Deck
Unlock for access to all 46 flashcards in this deck.
Unlock Deck
k this deck
36
Amino acid synthesis is possible by all except one of the pathways listed.Which is not a synthetic pathway for amino acids?
A)Amination of malonic ester followed by hydrolysis and decarboxylation
B)Nucleophilic addition of NH3 to an aldehyde followed by addition of cyanide to the imine,and,finally,hydrolysis
C)SN2 reaction using an a-halo carboxylic acid with ammonia as the nucleophile
D)Reaction of NH4Cl and NaCN with an aldehyde followed by an acidic work-up
A)Amination of malonic ester followed by hydrolysis and decarboxylation
B)Nucleophilic addition of NH3 to an aldehyde followed by addition of cyanide to the imine,and,finally,hydrolysis
C)SN2 reaction using an a-halo carboxylic acid with ammonia as the nucleophile
D)Reaction of NH4Cl and NaCN with an aldehyde followed by an acidic work-up

Unlock Deck
Unlock for access to all 46 flashcards in this deck.
Unlock Deck
k this deck
37
Below is a proposed method for resolving a racemic mixture of (R)- and (S)-phenylalanine.Upon separation of the products,will this method produce (R)- or (S)-phenylalanine? 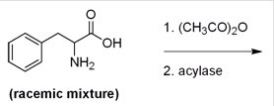
A)(R)-Phenylalanine is produced since the acylase only hydrolyzes the amides of L-amino acids.
B)(S)-Phenylalanine is produced since the acylase only hydrolyzes the amides of L-amino acids.
C)(R)-Phenylalanine is produced since the acylase only hydrolyzes the amides of D-amino acids.
D)(S)-Phenylalanine is produced since the acylase only hydrolyzes the amides of D-amino acids.

A)(R)-Phenylalanine is produced since the acylase only hydrolyzes the amides of L-amino acids.
B)(S)-Phenylalanine is produced since the acylase only hydrolyzes the amides of L-amino acids.
C)(R)-Phenylalanine is produced since the acylase only hydrolyzes the amides of D-amino acids.
D)(S)-Phenylalanine is produced since the acylase only hydrolyzes the amides of D-amino acids.

Unlock Deck
Unlock for access to all 46 flashcards in this deck.
Unlock Deck
k this deck
38
Which of the following is (are)fibrous protein(s)?
A)Keratins
B)Hormones
C)Enzymes
D)Antibodies
A)Keratins
B)Hormones
C)Enzymes
D)Antibodies

Unlock Deck
Unlock for access to all 46 flashcards in this deck.
Unlock Deck
k this deck
39
Which of the following correctly describes a peptide bond?
A)a bond between sp3-hybridized atoms only
B)a bond usually found in the s-cis conformation
C)an amide bond
D)the result of the formation of the carbonyl group of one amino acid with a R-group from the other amino acid
A)a bond between sp3-hybridized atoms only
B)a bond usually found in the s-cis conformation
C)an amide bond
D)the result of the formation of the carbonyl group of one amino acid with a R-group from the other amino acid

Unlock Deck
Unlock for access to all 46 flashcards in this deck.
Unlock Deck
k this deck
40
Which of the following correctly describes the Merrifield synthesis?
A)It uses a solid phase technique for the synthesis of peptides.
B)It uses DCC (dicyclohexylcarbodiimide)to form the amide bond in a peptide synthesis.
C)It uses BOC (tert-butoxycarbonyl)as a protecting group for peptide synthesis.
D)It uses aqueous phase techniques for the synthesis of large polypeptides.
A)It uses a solid phase technique for the synthesis of peptides.
B)It uses DCC (dicyclohexylcarbodiimide)to form the amide bond in a peptide synthesis.
C)It uses BOC (tert-butoxycarbonyl)as a protecting group for peptide synthesis.
D)It uses aqueous phase techniques for the synthesis of large polypeptides.

Unlock Deck
Unlock for access to all 46 flashcards in this deck.
Unlock Deck
k this deck
41
What is the product of the following reaction? (Assume workup to neutralize.) 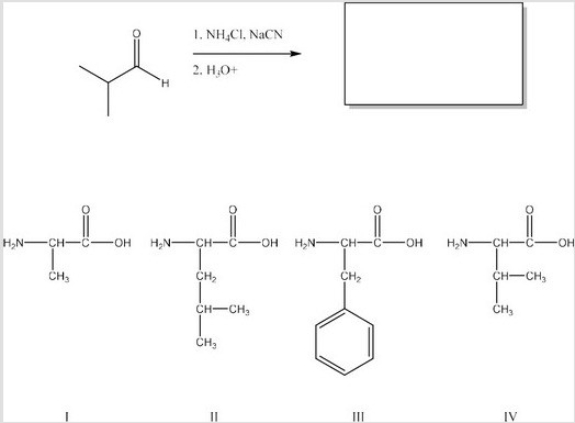
A)I
B)II
C)III
D)IV

A)I
B)II
C)III
D)IV

Unlock Deck
Unlock for access to all 46 flashcards in this deck.
Unlock Deck
k this deck
42
What is the product of the following reaction? (Assume workup to neutralize.) 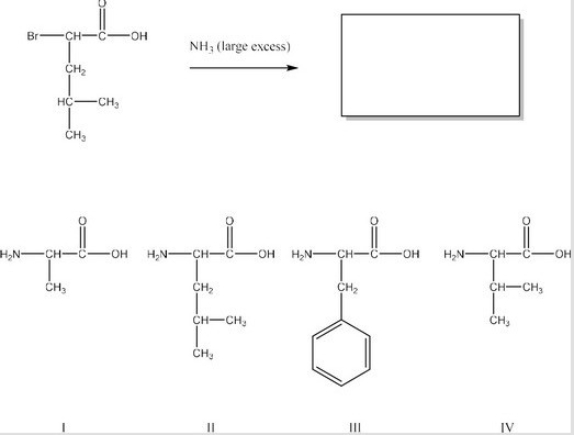
A)I
B)II
C)III
D)IV

A)I
B)II
C)III
D)IV

Unlock Deck
Unlock for access to all 46 flashcards in this deck.
Unlock Deck
k this deck
43
What is the product of the following reaction? (Assume workup to neutralize.) 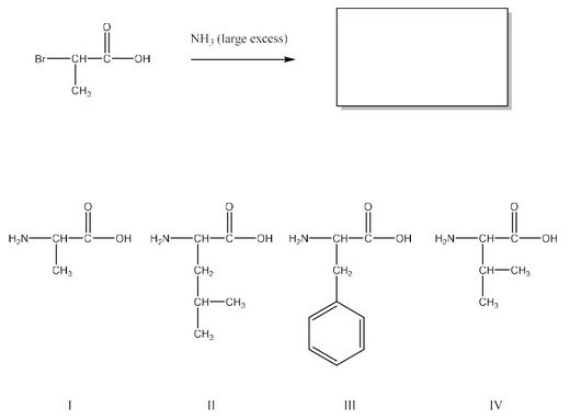
A)I
B)II
C)III
D)IV

A)I
B)II
C)III
D)IV

Unlock Deck
Unlock for access to all 46 flashcards in this deck.
Unlock Deck
k this deck
44
What is the major organic product for the following reaction? 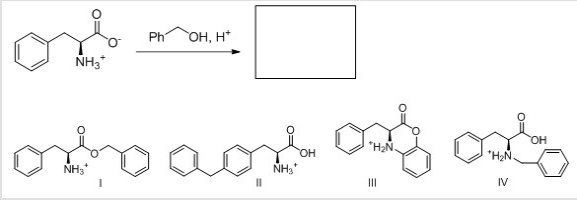
A)I
B)II
C)III
D)IV

A)I
B)II
C)III
D)IV

Unlock Deck
Unlock for access to all 46 flashcards in this deck.
Unlock Deck
k this deck
45
What is the product of the following reaction? (Assume workup to neutralize.) 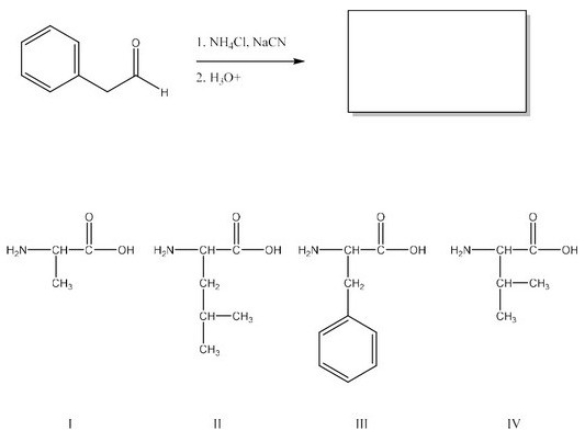
A)I
B)II
C)III
D)IV

A)I
B)II
C)III
D)IV

Unlock Deck
Unlock for access to all 46 flashcards in this deck.
Unlock Deck
k this deck
46
What is the name given to the pH at which the concentration of the zwitterionic form of an amino acid is at a maximum concentration?
A)Electric point
B)Dipolar point
C)Neutral point
D)Isoelectric point
A)Electric point
B)Dipolar point
C)Neutral point
D)Isoelectric point

Unlock Deck
Unlock for access to all 46 flashcards in this deck.
Unlock Deck
k this deck


