Deck 28: Synthetic Polymers
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/45
Play
Full screen (f)
Deck 28: Synthetic Polymers
1
The monomer used to make superglue is shown below.Which of the following methods of polymerization is most suitable for this type of monomer? 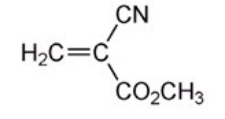
A)Free-radical chain-growth
B)Cationic chain-growth
C)Anionic chain-growth
D)Acid-catalyzed step-growth

A)Free-radical chain-growth
B)Cationic chain-growth
C)Anionic chain-growth
D)Acid-catalyzed step-growth
Anionic chain-growth
2
Which of the following best describes the polymer chain shown below? 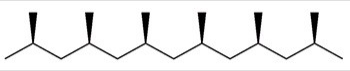
A)Atactic polypropylene
B)Isotactic polypropylene
C)Syndiotactic polypropylene
D)Cross-linked polypropylene

A)Atactic polypropylene
B)Isotactic polypropylene
C)Syndiotactic polypropylene
D)Cross-linked polypropylene
Isotactic polypropylene
3
Which type of polymerization process uses benzoyl peroxide (or other peroxides)as an initiator?
A)Free-radical chain-growth
B)Cationic chain-growth
C)Anionic chain-growth
D)Acid-catalyzed step-growth
A)Free-radical chain-growth
B)Cationic chain-growth
C)Anionic chain-growth
D)Acid-catalyzed step-growth
Free-radical chain-growth
4
The repeating unit of poly(methyl methacrylate)is shown below.Which one of the following is the monomer used to make poly(methyl methacrylate)? 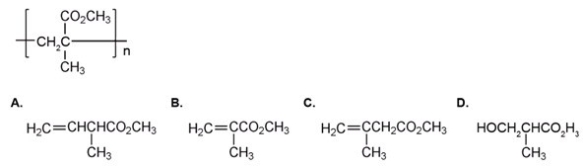
A)A
B)B
C)C
D)D

A)A
B)B
C)C
D)D

Unlock Deck
Unlock for access to all 45 flashcards in this deck.
Unlock Deck
k this deck
5
What modification occurs when a small amount of p-divinylbenzene is added to the polymerization reaction of styrene to form polystyrene? (Recall that the vinyl group is -CH=CH2.)
A)Cross-linking of the polystyrene
B)Isotactic stereochemistry of the polystyrene
C)Syndiotactic stereochemistry of the polystyrene
D)"Softening" of the polystyrene by a plasticizer
A)Cross-linking of the polystyrene
B)Isotactic stereochemistry of the polystyrene
C)Syndiotactic stereochemistry of the polystyrene
D)"Softening" of the polystyrene by a plasticizer

Unlock Deck
Unlock for access to all 45 flashcards in this deck.
Unlock Deck
k this deck
6
In which type of polymer are the chains packed less tightly together,resulting in lower melting points and a softer texture?
A)Isotactic polymers
B)Syndiotactic polymers
C)Atactic polymers
D)Cross-linked polymers
A)Isotactic polymers
B)Syndiotactic polymers
C)Atactic polymers
D)Cross-linked polymers

Unlock Deck
Unlock for access to all 45 flashcards in this deck.
Unlock Deck
k this deck
7
Identify the repeating unit in the polymer formed from the following reaction sequence. 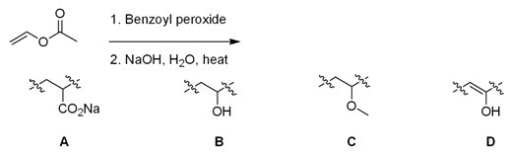
A)A
B)B
C)C
D)D

A)A
B)B
C)C
D)D

Unlock Deck
Unlock for access to all 45 flashcards in this deck.
Unlock Deck
k this deck
8
Which of the following is (are)condensation polymer(s)? I.polypropylene
II)Teflon
III)nylon
A)Only I
B)Only II
C)Only III
D)Both II and III
II)Teflon
III)nylon
A)Only I
B)Only II
C)Only III
D)Both II and III

Unlock Deck
Unlock for access to all 45 flashcards in this deck.
Unlock Deck
k this deck
9
Which of the following is (are)addition polymer(s)? I.polypropylene
II)Teflon
III)nylon
A)Only I
B)Only II
C)Only III
D)Both I and II
II)Teflon
III)nylon
A)Only I
B)Only II
C)Only III
D)Both I and II

Unlock Deck
Unlock for access to all 45 flashcards in this deck.
Unlock Deck
k this deck
10
Which of the following initiators can be used for anionic chain-growth polymerization?
A)Benzoyl peroxide
B)CH3CH2CH2CH2Li
C)BF3
D)Al(CH2CH3)3,TiCl4
A)Benzoyl peroxide
B)CH3CH2CH2CH2Li
C)BF3
D)Al(CH2CH3)3,TiCl4

Unlock Deck
Unlock for access to all 45 flashcards in this deck.
Unlock Deck
k this deck
11
Which one of the following monomers undergoes cationic polymerization most readily?
A)H2C=CH2
B)H2C=CHCH3
C)H2C=C(CH3)2
D)H2C=CHCN
A)H2C=CH2
B)H2C=CHCH3
C)H2C=C(CH3)2
D)H2C=CHCN

Unlock Deck
Unlock for access to all 45 flashcards in this deck.
Unlock Deck
k this deck
12
The acid-catalyzed dimerization of isobutylene gives a mixture of two isomeric alkenes,A and B.Hydrogenation of this mixture gives a single C8H18 hydrocarbon.What is the structure of this hydrocarbon? 
A)2,2,4-Trimethylpentane
B)2,3,4-Trimethylpentane
C)2,4-Dimethylhexane
D)2,5-Dimethyhexane

A)2,2,4-Trimethylpentane
B)2,3,4-Trimethylpentane
C)2,4-Dimethylhexane
D)2,5-Dimethyhexane

Unlock Deck
Unlock for access to all 45 flashcards in this deck.
Unlock Deck
k this deck
13
Which of the following initiators can be used for free radical chain-growth polymerization?
A)Benzoyl peroxide
B)CH3CH2CH2CH2Li
C)BF3
D)Al(CH2CH3)3,TiCl4
A)Benzoyl peroxide
B)CH3CH2CH2CH2Li
C)BF3
D)Al(CH2CH3)3,TiCl4

Unlock Deck
Unlock for access to all 45 flashcards in this deck.
Unlock Deck
k this deck
14
Which one of the following monomers undergoes anionic polymerization most readily?
A)H2C=CH2
B)H2C=CHCH3
C)H2C=C(CH3)2
D)H2C=CHCN
A)H2C=CH2
B)H2C=CHCH3
C)H2C=C(CH3)2
D)H2C=CHCN

Unlock Deck
Unlock for access to all 45 flashcards in this deck.
Unlock Deck
k this deck
15
Which of the following monomers yields the polymer shown below? 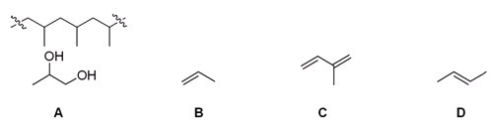
A)A
B)B
C)C
D)D

A)A
B)B
C)C
D)D

Unlock Deck
Unlock for access to all 45 flashcards in this deck.
Unlock Deck
k this deck
16
Which of the following is used to make Teflon?
A)Fluoroethene
B)1,1,4,4-Tetrafluorobutadiene
C)1,2-Difluoroethene
D)Tetrafluoroethylene
A)Fluoroethene
B)1,1,4,4-Tetrafluorobutadiene
C)1,2-Difluoroethene
D)Tetrafluoroethylene

Unlock Deck
Unlock for access to all 45 flashcards in this deck.
Unlock Deck
k this deck
17
Which of the following is the repeating unit in polyvinyl chloride (PVC)? 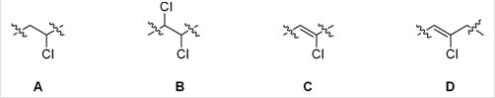
A)A
B)B
C)C
D)D
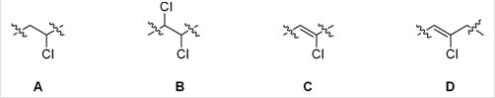
A)A
B)B
C)C
D)D

Unlock Deck
Unlock for access to all 45 flashcards in this deck.
Unlock Deck
k this deck
18
Which of the following is the monomer that gives the polymer shown below? 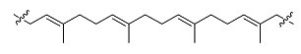
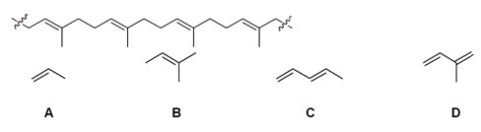
A)A
B)B
C)C
D)D


A)A
B)B
C)C
D)D

Unlock Deck
Unlock for access to all 45 flashcards in this deck.
Unlock Deck
k this deck
19
Which of the following monomers is used to make the polymer carbowax,shown below? 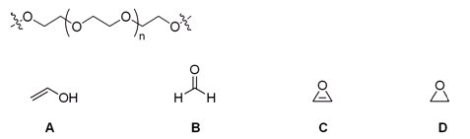
A)A
B)B
C)C
D)D

A)A
B)B
C)C
D)D

Unlock Deck
Unlock for access to all 45 flashcards in this deck.
Unlock Deck
k this deck
20
Which of the following best describes the polymer chain shown below? 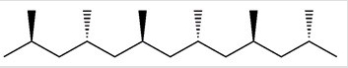
A)Atactic polypropylene
B)Isotactic polypropylene
C)Syndiotactic polypropylene
D)Cross-linked polypropylene

A)Atactic polypropylene
B)Isotactic polypropylene
C)Syndiotactic polypropylene
D)Cross-linked polypropylene

Unlock Deck
Unlock for access to all 45 flashcards in this deck.
Unlock Deck
k this deck
21
What is the purpose of plasticizers?
A)To harden plastics
B)To soften plastics
C)To initiate polymerizations
D)To cross-link polymer chains
A)To harden plastics
B)To soften plastics
C)To initiate polymerizations
D)To cross-link polymer chains

Unlock Deck
Unlock for access to all 45 flashcards in this deck.
Unlock Deck
k this deck
22
Bakelite is formed by the acid-catalyzed polymerization of phenol with formaldehyde.What is (are)the product(s)of the first step in this polymerization,shown below? (Note: in the answers below the hydroxymethyl group is -CH2OH.) 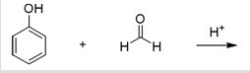
A)Ortho and para-hydroxybenzaldehyde
B)Meta-hydroxybenzaldehyde
C)Ortho and para-(hydroxymethyl)phenol
D)Meta-(hydroxymethyl)phenol

A)Ortho and para-hydroxybenzaldehyde
B)Meta-hydroxybenzaldehyde
C)Ortho and para-(hydroxymethyl)phenol
D)Meta-(hydroxymethyl)phenol

Unlock Deck
Unlock for access to all 45 flashcards in this deck.
Unlock Deck
k this deck
23
Which monomer and which type of initiator would you use to synthesize the following polymer? 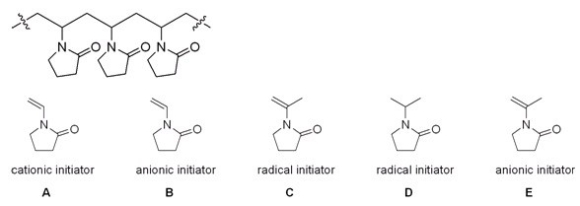
A)A
B)B
C)C
D)D
E)E

A)A
B)B
C)C
D)D
E)E

Unlock Deck
Unlock for access to all 45 flashcards in this deck.
Unlock Deck
k this deck
24
What is the name given to polymers that soften when heated and harden when cooled?
A)Cross-linked polymers
B)Copolymers
C)Thermosetting polymers
D)Thermoplastics
A)Cross-linked polymers
B)Copolymers
C)Thermosetting polymers
D)Thermoplastics

Unlock Deck
Unlock for access to all 45 flashcards in this deck.
Unlock Deck
k this deck
25
Melamine and formaldehyde combine to form the polymer,Melmac®.How is Melmac® classified,and what physical characteristics are consistent with its structure? 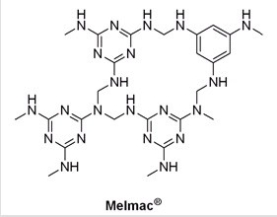
A)Polycarbonate,transparent
B)Polyurethane,elastic
C)Thermosetting,rigid
D)Thermoplastic,can be melted and molded into new shapes

A)Polycarbonate,transparent
B)Polyurethane,elastic
C)Thermosetting,rigid
D)Thermoplastic,can be melted and molded into new shapes

Unlock Deck
Unlock for access to all 45 flashcards in this deck.
Unlock Deck
k this deck
26
Vulcanization is the process of cross-linking polymer chains in rubber using what chemical?
A)Sulfur
B)Formaldehyde
C)Benzoyl peroxide
D)Ethylene glycol
A)Sulfur
B)Formaldehyde
C)Benzoyl peroxide
D)Ethylene glycol

Unlock Deck
Unlock for access to all 45 flashcards in this deck.
Unlock Deck
k this deck
27
Which of the following is the repeating unit of the polymer formed in the polymerization of p-hydroxybenzoic acid? 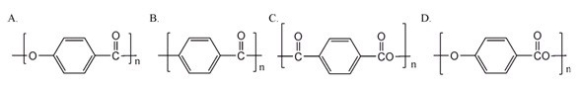
A)A
B)B
C)C
D)D

A)A
B)B
C)C
D)D

Unlock Deck
Unlock for access to all 45 flashcards in this deck.
Unlock Deck
k this deck
28
What is the classification of the following polymer in which X and Y represent repeating units? 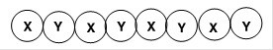
A)Block copolymer
B)Homopolymer
C)Alternating copolymer
D)Random copolymer

A)Block copolymer
B)Homopolymer
C)Alternating copolymer
D)Random copolymer

Unlock Deck
Unlock for access to all 45 flashcards in this deck.
Unlock Deck
k this deck
29
Which of the following sets of products results if nylon 66 is treated with sulfuric acid? 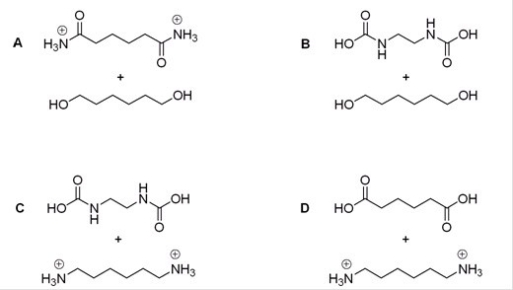
A)A
B)B
C)C
D)D

A)A
B)B
C)C
D)D

Unlock Deck
Unlock for access to all 45 flashcards in this deck.
Unlock Deck
k this deck
30
What monomer(s)would be used to make the polyester shown below? 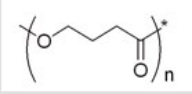
A)Butanedioic acid
B)4-Hydroxybutanal
C)4-Hydroxybutanoic acid
D)Butanedioic acid and 1,4-butanediol

A)Butanedioic acid
B)4-Hydroxybutanal
C)4-Hydroxybutanoic acid
D)Butanedioic acid and 1,4-butanediol

Unlock Deck
Unlock for access to all 45 flashcards in this deck.
Unlock Deck
k this deck
31
The repeating unit of a polymer is shown below.This polymer is formed by 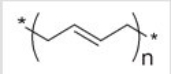
A)addition of ethylene.
B)addition of trans-2-butene.
C)1,2 addition of butadiene.
D)1,4 addition of butadiene.

A)addition of ethylene.
B)addition of trans-2-butene.
C)1,2 addition of butadiene.
D)1,4 addition of butadiene.

Unlock Deck
Unlock for access to all 45 flashcards in this deck.
Unlock Deck
k this deck
32
Rank the following monomers (X,Y and Z)in order of increasing ability to undergo anionic polymerization,starting with the least reactive monomer. 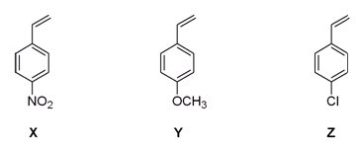
A)Y< X < Z
B)Z < Y < X
C)X < Y < Z
D)Y < Z < X

A)Y< X < Z
B)Z < Y < X
C)X < Y < Z
D)Y < Z < X

Unlock Deck
Unlock for access to all 45 flashcards in this deck.
Unlock Deck
k this deck
33
Which of the following is the repeating unit of the polymer formed in the polymerization reaction shown below? 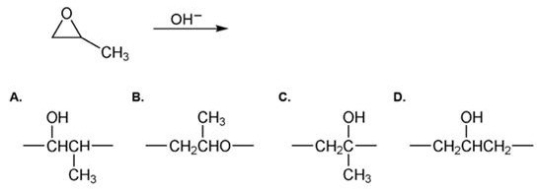
A)A
B)B
C)C
D)D

A)A
B)B
C)C
D)D

Unlock Deck
Unlock for access to all 45 flashcards in this deck.
Unlock Deck
k this deck
34
What is the classification of the following polymer in which X and Y represent repeating units? 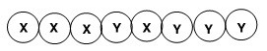
A)Block copolymer
B)Homopolymer
C)Alternating copolymer
D)Random copolymer

A)Block copolymer
B)Homopolymer
C)Alternating copolymer
D)Random copolymer

Unlock Deck
Unlock for access to all 45 flashcards in this deck.
Unlock Deck
k this deck
35
Which of the following is (are)repeating unit(s)of step-growth polymers? 
A)Only I
B)Only II
C)Only III
D)I and II

A)Only I
B)Only II
C)Only III
D)I and II

Unlock Deck
Unlock for access to all 45 flashcards in this deck.
Unlock Deck
k this deck
36
What is the structure of the polymer which results when styrene is reacted with BF3 and H2O? 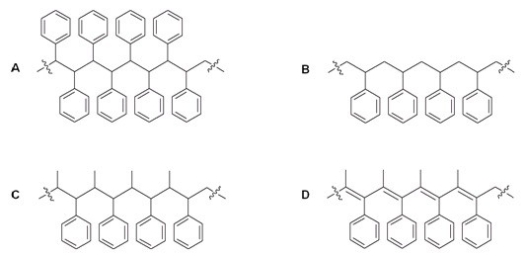
A)A
B)B
C)C
D)D

A)A
B)B
C)C
D)D

Unlock Deck
Unlock for access to all 45 flashcards in this deck.
Unlock Deck
k this deck
37
Which of the following initiators is used to make isotactic polypropylene?
A)Benzoyl peroxide
B)CH3CH2CH2CH2Li
C)BF3
D)Al(CH2CH3)3,TiCl4
A)Benzoyl peroxide
B)CH3CH2CH2CH2Li
C)BF3
D)Al(CH2CH3)3,TiCl4

Unlock Deck
Unlock for access to all 45 flashcards in this deck.
Unlock Deck
k this deck
38
What is the name given to polymers with single monomers?
A)Homopolymers
B)Copolymers
C)Block polymers
D)Random polymers
A)Homopolymers
B)Copolymers
C)Block polymers
D)Random polymers

Unlock Deck
Unlock for access to all 45 flashcards in this deck.
Unlock Deck
k this deck
39
Identify the monomer(s)needed to make the following polyester. 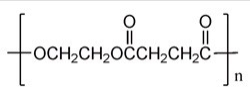
A)3-Hydroxybutanoic acid
B)Butanedioic acid
C)Butanedioic acid and ethylene glycol
D)Butanedioic acid and ethanol

A)3-Hydroxybutanoic acid
B)Butanedioic acid
C)Butanedioic acid and ethylene glycol
D)Butanedioic acid and ethanol

Unlock Deck
Unlock for access to all 45 flashcards in this deck.
Unlock Deck
k this deck
40
What is the classification of the following polymer in which X and Y represent repeating units? 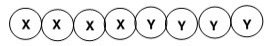
A)Block copolymer
B)Homopolymer
C)Alternating copolymer
D)Random copolymer
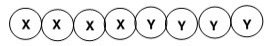
A)Block copolymer
B)Homopolymer
C)Alternating copolymer
D)Random copolymer

Unlock Deck
Unlock for access to all 45 flashcards in this deck.
Unlock Deck
k this deck
41
What type of polymer is Nylon?
A)Epoxy
B)Polyurethane
C)Polycarbonate
D)Polyamide
A)Epoxy
B)Polyurethane
C)Polycarbonate
D)Polyamide

Unlock Deck
Unlock for access to all 45 flashcards in this deck.
Unlock Deck
k this deck
42
What are the structures of the three monomers needed to synthesize the following polymer? 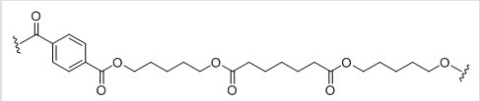
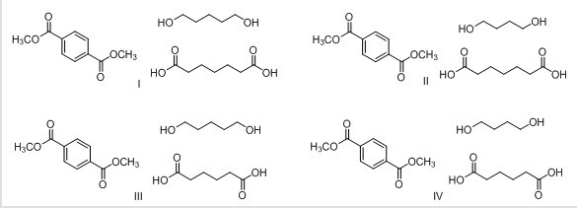
A)I
B)II
C)III
D)IV


A)I
B)II
C)III
D)IV

Unlock Deck
Unlock for access to all 45 flashcards in this deck.
Unlock Deck
k this deck
43
What is the name given to polymers with two or more different monomers?
A)Homopolymers
B)Copolymers
C)Block polymers
D)Random polymers
A)Homopolymers
B)Copolymers
C)Block polymers
D)Random polymers

Unlock Deck
Unlock for access to all 45 flashcards in this deck.
Unlock Deck
k this deck
44
What type of polymer is Lexan?
A)Epoxy
B)Polyurethane
C)Polycarbonate
D)Polyamide
A)Epoxy
B)Polyurethane
C)Polycarbonate
D)Polyamide

Unlock Deck
Unlock for access to all 45 flashcards in this deck.
Unlock Deck
k this deck
45
What type of polymer is Spandex?
A)HDPE
B)Polyurethane
C)Polycarbonate
D)Polyamide
A)HDPE
B)Polyurethane
C)Polycarbonate
D)Polyamide

Unlock Deck
Unlock for access to all 45 flashcards in this deck.
Unlock Deck
k this deck


