Deck 7: Alkyl Halides and Nucleophilic Substitution
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/73
Play
Full screen (f)
Deck 7: Alkyl Halides and Nucleophilic Substitution
1
Which of the following alkyl halides is a tertiary alkyl halide? 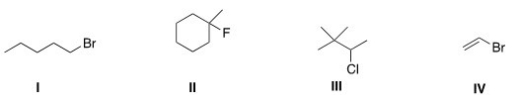
A)I
B)II
C)III
D)IV

A)I
B)II
C)III
D)IV
II
2
Which of the following alkyl halides is a secondary alkyl halide? 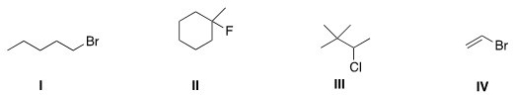
A)I
B)II
C)III
D)IV

A)I
B)II
C)III
D)IV
III
3
Rank the following halides in order of decreasing polarity,putting the most polar first. 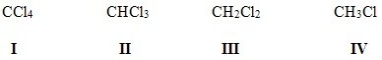
A)II > IV > I > III
B)IV > II > I > III
C)I > II > III > IV
D)IV > III > II > I
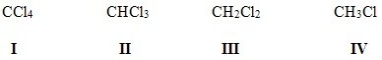
A)II > IV > I > III
B)IV > II > I > III
C)I > II > III > IV
D)IV > III > II > I
IV > III > II > I
4
Which of the following structures have the correct common name? 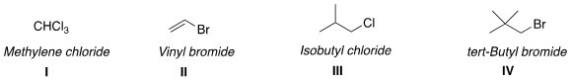
A)I and II
B)II and III
C)I and III
D)III and IV
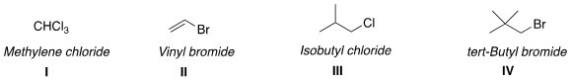
A)I and II
B)II and III
C)I and III
D)III and IV

Unlock Deck
Unlock for access to all 73 flashcards in this deck.
Unlock Deck
k this deck
5
Which of the following alkyl halides is a primary alkyl halide? 
A)I
B)II
C)III
D)IV

A)I
B)II
C)III
D)IV

Unlock Deck
Unlock for access to all 73 flashcards in this deck.
Unlock Deck
k this deck
6
What is the IUPAC name of the following compound? 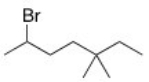
A)2-Bromo-5,5-dimethylheptane
B)3,3-Dimethyl-6-bromoheptane
C)6-Bromo-3,3-dimethylheptane
D)2-Bromo-5,5-dimethyloctane

A)2-Bromo-5,5-dimethylheptane
B)3,3-Dimethyl-6-bromoheptane
C)6-Bromo-3,3-dimethylheptane
D)2-Bromo-5,5-dimethyloctane

Unlock Deck
Unlock for access to all 73 flashcards in this deck.
Unlock Deck
k this deck
7
What is the IUPAC name of the following compound? 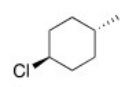
A)trans-4-Methylcyclohexyl chloride
B)trans-p-Chloromethylcyclohexane
C)trans-4-Methyl-1-chlorocyclohexane
D)trans-1-Chloro-4-methylcyclohexane

A)trans-4-Methylcyclohexyl chloride
B)trans-p-Chloromethylcyclohexane
C)trans-4-Methyl-1-chlorocyclohexane
D)trans-1-Chloro-4-methylcyclohexane

Unlock Deck
Unlock for access to all 73 flashcards in this deck.
Unlock Deck
k this deck
8
What is the IUPAC name of the following compound? 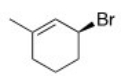
A)(R)-3-Bromo-1-methylcyclohexene
B)(S)-3-Bromo-1-methylcyclohexene
C)(S)-1-Bromo-3-methyl-2-cyclohexene
D)(R)-1-Bromo-3-methyl-2-cyclohexene

A)(R)-3-Bromo-1-methylcyclohexene
B)(S)-3-Bromo-1-methylcyclohexene
C)(S)-1-Bromo-3-methyl-2-cyclohexene
D)(R)-1-Bromo-3-methyl-2-cyclohexene

Unlock Deck
Unlock for access to all 73 flashcards in this deck.
Unlock Deck
k this deck
9
Which of the following statements is true?
A)All good leaving groups are strong bases with weak conjugate acids.
B)Left-to-right across a row of the periodic table,leaving group ability decreases.
C)Down a column of the periodic table,leaving group ability decreases.
D)The conjugate bases of strong acids are good leaving groups.
A)All good leaving groups are strong bases with weak conjugate acids.
B)Left-to-right across a row of the periodic table,leaving group ability decreases.
C)Down a column of the periodic table,leaving group ability decreases.
D)The conjugate bases of strong acids are good leaving groups.

Unlock Deck
Unlock for access to all 73 flashcards in this deck.
Unlock Deck
k this deck
10
Which of the following structures have the correct common name? 
A)I and II
B)II and III
C)II and IV
D)III and IV

A)I and II
B)II and III
C)II and IV
D)III and IV

Unlock Deck
Unlock for access to all 73 flashcards in this deck.
Unlock Deck
k this deck
11
What is the IUPAC name of the following compound? 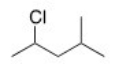
A)2-Methyl-4-chloropentane
B)2-Chloro-4-methylpentane
C)2-Chloro-1-isopropylpropane
D)2-Chloro-2-methylpentane

A)2-Methyl-4-chloropentane
B)2-Chloro-4-methylpentane
C)2-Chloro-1-isopropylpropane
D)2-Chloro-2-methylpentane

Unlock Deck
Unlock for access to all 73 flashcards in this deck.
Unlock Deck
k this deck
12
Which of the following statements about the reactions of alkyl halides is true?
A)The characteristic reactions of alkyl halides are addition and elimination.
B)The characteristic reactions of alkyl halides are addition and substitution.
C)The characteristic reactions of alkyl halides are elimination and substitution.
D)The characteristic reactions of alkyl halides are oxidation and reduction.
A)The characteristic reactions of alkyl halides are addition and elimination.
B)The characteristic reactions of alkyl halides are addition and substitution.
C)The characteristic reactions of alkyl halides are elimination and substitution.
D)The characteristic reactions of alkyl halides are oxidation and reduction.

Unlock Deck
Unlock for access to all 73 flashcards in this deck.
Unlock Deck
k this deck
13
What is the IUPAC name of the following compound? 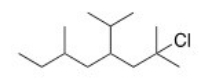
A)2-Chloro-4-isopropyl-2,6-dimethyloctane
B)2-Chloro-4-isopropyl-2,7-dimethylnonane
C)2,6-Dimethyl-2-chloro-4-isopropyloctane
D)7-Chloro-5-isopropyl-3,7-dimethyloctane

A)2-Chloro-4-isopropyl-2,6-dimethyloctane
B)2-Chloro-4-isopropyl-2,7-dimethylnonane
C)2,6-Dimethyl-2-chloro-4-isopropyloctane
D)7-Chloro-5-isopropyl-3,7-dimethyloctane

Unlock Deck
Unlock for access to all 73 flashcards in this deck.
Unlock Deck
k this deck
14
What is the IUPAC name of the following compound? 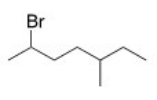
A)2-Bromo-5-methyloctane
B)2-Bromo-3-methylheptane
C)2-Bromo-5-methylheptane
D)6-Bromo-3-methylheptane

A)2-Bromo-5-methyloctane
B)2-Bromo-3-methylheptane
C)2-Bromo-5-methylheptane
D)6-Bromo-3-methylheptane

Unlock Deck
Unlock for access to all 73 flashcards in this deck.
Unlock Deck
k this deck
15
Rank the following in order of decreasing leaving group ability,putting the best first. 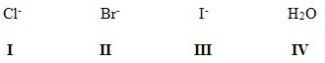
A)III > II > IV > I
B)III > II > I > IV
C)IV > I > II > III
D)II > III > I > IV
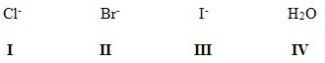
A)III > II > IV > I
B)III > II > I > IV
C)IV > I > II > III
D)II > III > I > IV

Unlock Deck
Unlock for access to all 73 flashcards in this deck.
Unlock Deck
k this deck
16
Which of the following alkyl halides is a vinyl alkyl halide? 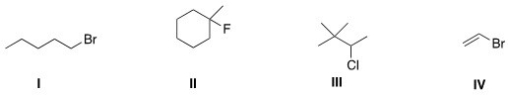
A)I
B)II
C)III
D)IV

A)I
B)II
C)III
D)IV

Unlock Deck
Unlock for access to all 73 flashcards in this deck.
Unlock Deck
k this deck
17
Which of the following structures have the correct common name? 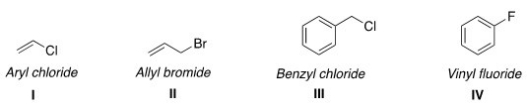
A)I and II
B)II and IV
C)II and III
D)III and IV

A)I and II
B)II and IV
C)II and III
D)III and IV

Unlock Deck
Unlock for access to all 73 flashcards in this deck.
Unlock Deck
k this deck
18
What is the IUPAC name of the following compound? 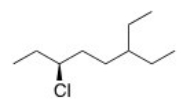
A)(R)-3-Chloro-6-ethyloctane
B)(S)-3-Chloro-6-ethyloctane
C)(S)-6-Chloro-3-ethyloctane
D)(R)-6-Chloro-3-ethyloctane

A)(R)-3-Chloro-6-ethyloctane
B)(S)-3-Chloro-6-ethyloctane
C)(S)-6-Chloro-3-ethyloctane
D)(R)-6-Chloro-3-ethyloctane

Unlock Deck
Unlock for access to all 73 flashcards in this deck.
Unlock Deck
k this deck
19
Rank the following molecules in order of increasing polarity,putting the least polar first. 
A)IV < III < II < I
B)I < IV < III < II
C)I < II < III < IV
D)II < I < III < IV
A)IV < III < II < I
B)I < IV < III < II
C)I < II < III < IV
D)II < I < III < IV

Unlock Deck
Unlock for access to all 73 flashcards in this deck.
Unlock Deck
k this deck
20
What is the IUPAC name of the following compound? 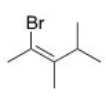
A)(E)-2-Bromo-3,4-dimethyl-2-pentene
B)(Z)-1-Bromo-1,2,3-trimethyl-1-butene
C)(Z)-2-Bromo-3,4-dimethyl-2-pentene
D)(E)-1-Bromo-1,2,3-trimethyl-1-butene

A)(E)-2-Bromo-3,4-dimethyl-2-pentene
B)(Z)-1-Bromo-1,2,3-trimethyl-1-butene
C)(Z)-2-Bromo-3,4-dimethyl-2-pentene
D)(E)-1-Bromo-1,2,3-trimethyl-1-butene

Unlock Deck
Unlock for access to all 73 flashcards in this deck.
Unlock Deck
k this deck
21
The reaction of 1-bromobutane with sodium hydroxide affords the substitution product 1-butanol.What would happen to the rate of the reaction if the concentration of both 1-bromobutane and sodium hydroxide were doubled?
A)The rate remains the same.
B)The rate decreases by a factor of 2.
C)The rate increases by a factor of 2.
D)The rate increases by a factor of 4.
A)The rate remains the same.
B)The rate decreases by a factor of 2.
C)The rate increases by a factor of 2.
D)The rate increases by a factor of 4.

Unlock Deck
Unlock for access to all 73 flashcards in this deck.
Unlock Deck
k this deck
22
The reaction of tert-butyl bromide,(CH3)3CBr,with ethanol affords the substitution product tert-butyl ethyl ether,(CH3)3COCH2CH3,in acidic conditions.What would happen to the rate of the reaction if the concentration of ethanol was doubled?
A)The rate remains the same.
B)The rate decreases by a factor of 2.
C)The rate increases by a factor of 2.
D)The rate increases by a factor of 4.
A)The rate remains the same.
B)The rate decreases by a factor of 2.
C)The rate increases by a factor of 2.
D)The rate increases by a factor of 4.

Unlock Deck
Unlock for access to all 73 flashcards in this deck.
Unlock Deck
k this deck
23
Which of the following statements is not true?
A)In polar protic solvents,nucleophilicity decreases down a column of the periodic table as the size of the anion increases.
B)Nucleophilicity is affected by the solvent used in a substitution reaction.
C)Polar protic solvents are capable of intermolecular hydrogen bonding.
D)Polar protic solvents solvate both cations and anions.
A)In polar protic solvents,nucleophilicity decreases down a column of the periodic table as the size of the anion increases.
B)Nucleophilicity is affected by the solvent used in a substitution reaction.
C)Polar protic solvents are capable of intermolecular hydrogen bonding.
D)Polar protic solvents solvate both cations and anions.

Unlock Deck
Unlock for access to all 73 flashcards in this deck.
Unlock Deck
k this deck
24
Which of the following solvents is not a polar protic solvent? 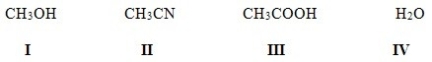
A)I
B)II
C)III
D)IV
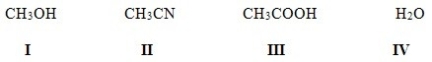
A)I
B)II
C)III
D)IV

Unlock Deck
Unlock for access to all 73 flashcards in this deck.
Unlock Deck
k this deck
25
Rank the alkyl halides in order of decreasing SN2 reactivity,putting the most reactive first. 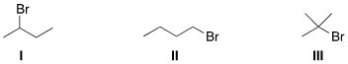
A)I > II > III
B)II > I > III
C)III > I > II
D)I > III > II

A)I > II > III
B)II > I > III
C)III > I > II
D)I > III > II

Unlock Deck
Unlock for access to all 73 flashcards in this deck.
Unlock Deck
k this deck
26
Which of the following is a polar aprotic solvent? 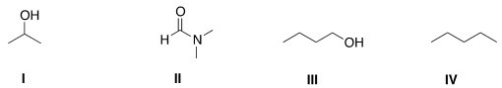
A)I
B)II
C)III
D)IV
A)I
B)II
C)III
D)IV

Unlock Deck
Unlock for access to all 73 flashcards in this deck.
Unlock Deck
k this deck
27
Which of the following statements is not true?
A)Sodium ethoxide is a better nucleophile than sodium tert-butoxide.
B)Sodium tert-butoxide and sodium ethoxide have similar strengths as bases.
C)Sterically hindered bases are also called nonnucleophilic bases.
D)Steric hindrance decreases basicity but not nucleophilicity.
A)Sodium ethoxide is a better nucleophile than sodium tert-butoxide.
B)Sodium tert-butoxide and sodium ethoxide have similar strengths as bases.
C)Sterically hindered bases are also called nonnucleophilic bases.
D)Steric hindrance decreases basicity but not nucleophilicity.

Unlock Deck
Unlock for access to all 73 flashcards in this deck.
Unlock Deck
k this deck
28
Which of the following is the most nucleophilic?
A)CH4
B)H2O
C)NH3
D)HF
A)CH4
B)H2O
C)NH3
D)HF

Unlock Deck
Unlock for access to all 73 flashcards in this deck.
Unlock Deck
k this deck
29
Which of the following alkyl halides undergoes the fastest SN2 reaction with sodium hydroxide?
A)1-Iodobutane
B)1-Chlorobutane
C)1-Fluorobutane
D)1-Bromobutane
A)1-Iodobutane
B)1-Chlorobutane
C)1-Fluorobutane
D)1-Bromobutane

Unlock Deck
Unlock for access to all 73 flashcards in this deck.
Unlock Deck
k this deck
30
Rank the following in order of increasing nucleophilicity,putting the least nucleophilic first. 
A)II < IV < I < III
B)IV < III < II < I
C)III < IV < I < II
D)II < I < IV < III

A)II < IV < I < III
B)IV < III < II < I
C)III < IV < I < II
D)II < I < IV < III

Unlock Deck
Unlock for access to all 73 flashcards in this deck.
Unlock Deck
k this deck
31
Given the following substitution reaction,what would be the effect of changing the solvent from CH3OH to (CH3)2S=O? CH3(CH2)5Br + NaOH ® CH3(CH2)5OH + Br-
A)The rate would decrease because SN1 reactions are favored by polar protic solvents.
B)The rate would increase because SN2 reactions are favored by polar aprotic solvents.
C)The rate would increase because SN1 reactions are favored by polar protic solvents.
D)The rate would decrease because SN2 reactions are favored by polar aprotic solvents.
A)The rate would decrease because SN1 reactions are favored by polar protic solvents.
B)The rate would increase because SN2 reactions are favored by polar aprotic solvents.
C)The rate would increase because SN1 reactions are favored by polar protic solvents.
D)The rate would decrease because SN2 reactions are favored by polar aprotic solvents.

Unlock Deck
Unlock for access to all 73 flashcards in this deck.
Unlock Deck
k this deck
32
Rank the following in order of decreasing nucleophilicity,putting the most nucleophilic first. 
A)II > III > I > IV
B)III > II > IV > I
C)II > III > IV > I
D)III > II > I > IV

A)II > III > I > IV
B)III > II > IV > I
C)II > III > IV > I
D)III > II > I > IV

Unlock Deck
Unlock for access to all 73 flashcards in this deck.
Unlock Deck
k this deck
33
Which compound is most likely to follow second-order kinetics in a substitution reaction?
A)CH3Br
B)(CH3)3CCH2Br
C)CH3CH2Br
D)(CH3)2CHBr
A)CH3Br
B)(CH3)3CCH2Br
C)CH3CH2Br
D)(CH3)2CHBr

Unlock Deck
Unlock for access to all 73 flashcards in this deck.
Unlock Deck
k this deck
34
Which of the following statements is not true?
A)All good leaving groups are weak bases with strong conjugate acids.
B)Left-to-right across a row of the periodic table,leaving group ability increases.
C)Down a column of the periodic table,leaving group ability increases.
D)Equilibrium favors the products of the nucleophilic substitution when the leaving group is a stronger base than the nucleophile.
A)All good leaving groups are weak bases with strong conjugate acids.
B)Left-to-right across a row of the periodic table,leaving group ability increases.
C)Down a column of the periodic table,leaving group ability increases.
D)Equilibrium favors the products of the nucleophilic substitution when the leaving group is a stronger base than the nucleophile.

Unlock Deck
Unlock for access to all 73 flashcards in this deck.
Unlock Deck
k this deck
35
Which of the following anions is the most nucleophilic in polar aprotic solvents?
A)F-
B)Cl-
C)Br-
D)I-
A)F-
B)Cl-
C)Br-
D)I-

Unlock Deck
Unlock for access to all 73 flashcards in this deck.
Unlock Deck
k this deck
36
Which of the following statements about SN2 reactions is true?
A)The rate of reaction is dependent on just the substrate.
B)The fastest reaction will occur with a tertiary alkyl halide.
C)The mechanism is a two-step process.
D)Displacement occurs with inversion of configuration.
A)The rate of reaction is dependent on just the substrate.
B)The fastest reaction will occur with a tertiary alkyl halide.
C)The mechanism is a two-step process.
D)Displacement occurs with inversion of configuration.

Unlock Deck
Unlock for access to all 73 flashcards in this deck.
Unlock Deck
k this deck
37
Which of the following statements about the SN2 mechanism for nucleophilic substitution reactions is true?
A)Involves one step and occurs with retention of configuration.
B)Involves two steps and occurs with inversion of configuration.
C)Involves one step and occurs with inversion of configuration.
D)Involves one step and occurs with racemization.
A)Involves one step and occurs with retention of configuration.
B)Involves two steps and occurs with inversion of configuration.
C)Involves one step and occurs with inversion of configuration.
D)Involves one step and occurs with racemization.

Unlock Deck
Unlock for access to all 73 flashcards in this deck.
Unlock Deck
k this deck
38
Rank the following ions in order of increasing nucleophilicity in polar protic solvents,starting with the least nucleophilic ion. 
A)I < II < III < IV
B)IV < III < II < I
C)I < II < IV < III
D)IV < III < I < II

A)I < II < III < IV
B)IV < III < II < I
C)I < II < IV < III
D)IV < III < I < II

Unlock Deck
Unlock for access to all 73 flashcards in this deck.
Unlock Deck
k this deck
39
The reaction of bromoethane with sodium acetate affords the substitution product methyl acetate.What is the effect of doubling the concentration of sodium acetate on the rate of the reaction?
A)The rate remains the same.
B)The rate decreases by a factor of 2.
C)The rate increases by a factor of 2.
D)The rate increases by a factor of 4.
A)The rate remains the same.
B)The rate decreases by a factor of 2.
C)The rate increases by a factor of 2.
D)The rate increases by a factor of 4.

Unlock Deck
Unlock for access to all 73 flashcards in this deck.
Unlock Deck
k this deck
40
Rank the following in order of increasing leaving group ability,putting the worst leaving group first. 
A)IV < II < III < I
B)III < IV < I < II
C)II < IV < I < III
D)I < III < II < IV

A)IV < II < III < I
B)III < IV < I < II
C)II < IV < I < III
D)I < III < II < IV

Unlock Deck
Unlock for access to all 73 flashcards in this deck.
Unlock Deck
k this deck
41
Which of the following statements about the Hammond postulate is true?
A)In an exothermic reaction,lowering the energy of the transition state increases the activation energy,Ea.
B)In an endothermic reaction,the more stable product forms faster.
C)In an endothermic reaction,the less stable product forms faster.
D)In an endothermic reaction,the activation energy,Ea,is similar for both products.
A)In an exothermic reaction,lowering the energy of the transition state increases the activation energy,Ea.
B)In an endothermic reaction,the more stable product forms faster.
C)In an endothermic reaction,the less stable product forms faster.
D)In an endothermic reaction,the activation energy,Ea,is similar for both products.

Unlock Deck
Unlock for access to all 73 flashcards in this deck.
Unlock Deck
k this deck
42
What is the product of the nucleophilic substitution reaction shown below? 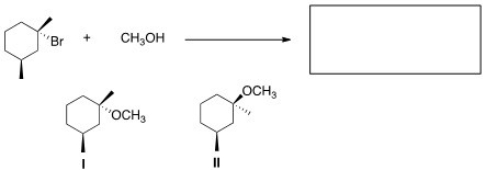
A)Only I
B)Only II
C)I and II
D)None

A)Only I
B)Only II
C)I and II
D)None

Unlock Deck
Unlock for access to all 73 flashcards in this deck.
Unlock Deck
k this deck
43
Which of the following alkyl halides will react fastest with CH3OH in an SN1 mechanism? 
A)I
B)II
C)III
D)IV

A)I
B)II
C)III
D)IV

Unlock Deck
Unlock for access to all 73 flashcards in this deck.
Unlock Deck
k this deck
44
What is the product of the nucleophilic substitution reaction shown below? 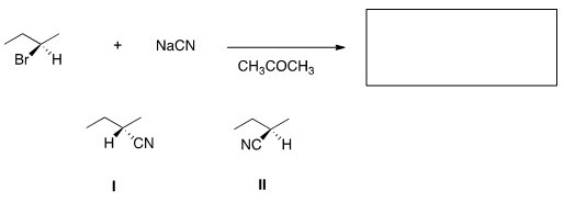
A)Only I
B)Only II
C)I and II
D)None

A)Only I
B)Only II
C)I and II
D)None

Unlock Deck
Unlock for access to all 73 flashcards in this deck.
Unlock Deck
k this deck
45
Which of the following statements about an SN1 reaction mechanism is true?
A)The reaction is fastest with primary alkyl halide.
B)The reaction exhibits a one-step mechanism.
C)The reaction rate increases as the leaving group ability increases.
D)The reaction rate increases as the strength of the nucleophile increases.
A)The reaction is fastest with primary alkyl halide.
B)The reaction exhibits a one-step mechanism.
C)The reaction rate increases as the leaving group ability increases.
D)The reaction rate increases as the strength of the nucleophile increases.

Unlock Deck
Unlock for access to all 73 flashcards in this deck.
Unlock Deck
k this deck
46
Which of the following carbocations is the most stable? 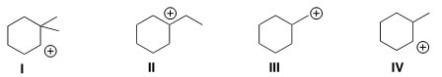
A)I
B)II
C)III
D)IV

A)I
B)II
C)III
D)IV

Unlock Deck
Unlock for access to all 73 flashcards in this deck.
Unlock Deck
k this deck
47
For the following reaction,use the identity of the alkyl halide and nucleophile to determine which substitution mechanism occurs.Then determine which solvent affords the faster reaction. 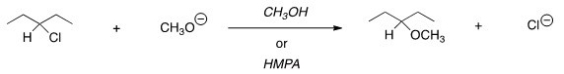
A)SN1,CH3OH
B)SN1,HMPA
C)SN2,CH3OH
D)SN2,HMPA

A)SN1,CH3OH
B)SN1,HMPA
C)SN2,CH3OH
D)SN2,HMPA

Unlock Deck
Unlock for access to all 73 flashcards in this deck.
Unlock Deck
k this deck
48
Which of the following is not a characteristic of an SN1 reaction?
A)The rate is proportional to the concentration of substrate.
B)The reaction is favored in polar protic solvents.
C)The rate is independent of the concentration of the nucleophile.
D)The electrophilic carbon undergoes inversion of stereochemistry.
A)The rate is proportional to the concentration of substrate.
B)The reaction is favored in polar protic solvents.
C)The rate is independent of the concentration of the nucleophile.
D)The electrophilic carbon undergoes inversion of stereochemistry.

Unlock Deck
Unlock for access to all 73 flashcards in this deck.
Unlock Deck
k this deck
49
What is the rate-determining step of an SN1 reaction mechanism?
A)Reaction of the nucleophile with the carbocation to form the product.
B)Breaking the bond between the carbon and the leaving group to generate a carbocation.
C)Attack of the nucleophile on the substrate to generate a pentavalent carbon.
D)None of these.
A)Reaction of the nucleophile with the carbocation to form the product.
B)Breaking the bond between the carbon and the leaving group to generate a carbocation.
C)Attack of the nucleophile on the substrate to generate a pentavalent carbon.
D)None of these.

Unlock Deck
Unlock for access to all 73 flashcards in this deck.
Unlock Deck
k this deck
50
Which of the following rate laws describes the kinetics of an SN1 reaction?
A)Rate = k[alkyl halide]
B)Rate = k[alkyl halide][nucleophile]
C)Rate = k[nucleophile]
D)Rate = k[solvent]
A)Rate = k[alkyl halide]
B)Rate = k[alkyl halide][nucleophile]
C)Rate = k[nucleophile]
D)Rate = k[solvent]

Unlock Deck
Unlock for access to all 73 flashcards in this deck.
Unlock Deck
k this deck
51
What is the product of the nucleophilic substitution reaction shown below? 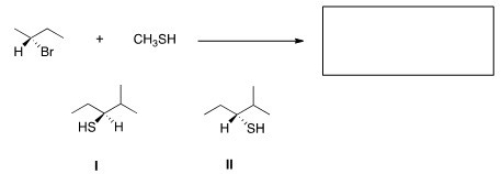
A)Only I
B)Only II
C)I and II
D)None

A)Only I
B)Only II
C)I and II
D)None

Unlock Deck
Unlock for access to all 73 flashcards in this deck.
Unlock Deck
k this deck
52
Which of the following statements about the Hammond postulate is not true?
A)The Hammond postulate provides a quantitative estimate of the energy of a transition state.
B)In endothermic reactions,the transition state is closer in energy to the products.
C)In exothermic reactions,the transition state is closer in energy to the reactants.
D)The Hammond postulate provides a qualitative estimate of the energy of a transition state.
A)The Hammond postulate provides a quantitative estimate of the energy of a transition state.
B)In endothermic reactions,the transition state is closer in energy to the products.
C)In exothermic reactions,the transition state is closer in energy to the reactants.
D)The Hammond postulate provides a qualitative estimate of the energy of a transition state.

Unlock Deck
Unlock for access to all 73 flashcards in this deck.
Unlock Deck
k this deck
53
What is the product of the nucleophilic substitution reaction shown below? 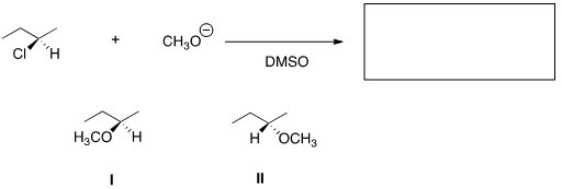
A)Only I
B)Only II
C)I and II
D)None

A)Only I
B)Only II
C)I and II
D)None

Unlock Deck
Unlock for access to all 73 flashcards in this deck.
Unlock Deck
k this deck
54
Rank the following carbocations in order of decreasing stability,putting the most stable first. 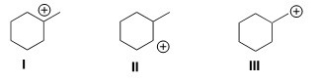
A)I > II > III
B)II > I > III
C)III > I > II
D)III > II > I

A)I > II > III
B)II > I > III
C)III > I > II
D)III > II > I

Unlock Deck
Unlock for access to all 73 flashcards in this deck.
Unlock Deck
k this deck
55
Which of the following statements about carbocation stability is true?
A)Carbocations are stabilized by electron-withdrawing inductive effects only.
B)Carbocations are stabilized by electron-withdrawing inductive effects and hyperconjugation.
C)Carbocations are stabilized by electron-donating inductive effects only.
D)Carbocations are stabilized by electro-donating inductive effects and hyperconjugation.
A)Carbocations are stabilized by electron-withdrawing inductive effects only.
B)Carbocations are stabilized by electron-withdrawing inductive effects and hyperconjugation.
C)Carbocations are stabilized by electron-donating inductive effects only.
D)Carbocations are stabilized by electro-donating inductive effects and hyperconjugation.

Unlock Deck
Unlock for access to all 73 flashcards in this deck.
Unlock Deck
k this deck
56
The reaction of what nucleophile and substrate is represented by the following transition state? 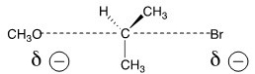
A)Methanol with 2-bromopropane
B)Methoxide with 2-bromopropane
C)Methoxide with 1-bromopropane
D)Methanol with 1-bromopropane

A)Methanol with 2-bromopropane
B)Methoxide with 2-bromopropane
C)Methoxide with 1-bromopropane
D)Methanol with 1-bromopropane

Unlock Deck
Unlock for access to all 73 flashcards in this deck.
Unlock Deck
k this deck
57
What is the product of the nucleophilic substitution reaction shown below? 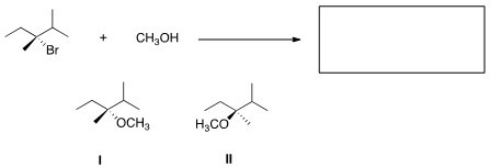
A)Only I
B)Only II
C)I and II
D)None

A)Only I
B)Only II
C)I and II
D)None

Unlock Deck
Unlock for access to all 73 flashcards in this deck.
Unlock Deck
k this deck
58
For the following reaction,use the identity of the alkyl halide and nucleophile to determine which substitution mechanism occurs.Then determine which solvent affords the faster reaction. 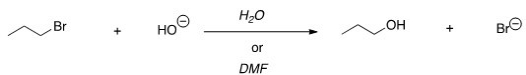
A)SN1,H2O
B)SN1,DMF
C)SN2,H2O
D)SN2,DMF

A)SN1,H2O
B)SN1,DMF
C)SN2,H2O
D)SN2,DMF

Unlock Deck
Unlock for access to all 73 flashcards in this deck.
Unlock Deck
k this deck
59
Which of the following statements about an SN1 reaction mechanism is true?
A)The reaction involves two steps and occurs fastest with primary alkyl halides.
B)The reaction involves one step and occurs fastest with primary alkyl halides.
C)The reaction involves one step and occurs fastest with tertiary alkyl halides.
D)The reaction involves two steps and occurs fastest with tertiary alkyl halides.
A)The reaction involves two steps and occurs fastest with primary alkyl halides.
B)The reaction involves one step and occurs fastest with primary alkyl halides.
C)The reaction involves one step and occurs fastest with tertiary alkyl halides.
D)The reaction involves two steps and occurs fastest with tertiary alkyl halides.

Unlock Deck
Unlock for access to all 73 flashcards in this deck.
Unlock Deck
k this deck
60
For the following reaction,use the identity of the alkyl halide and nucleophile to determine which substitution mechanism occurs.Then determine which solvent affords the faster reaction. 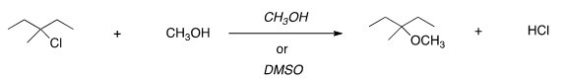
A)SN1,CH3OH
B)SN1,DMSO
C)SN2,CH3OH
D)SN2,DMSO

A)SN1,CH3OH
B)SN1,DMSO
C)SN2,CH3OH
D)SN2,DMSO

Unlock Deck
Unlock for access to all 73 flashcards in this deck.
Unlock Deck
k this deck
61
Which of the following alkyl halides would react the fastest with H2O in SN1 reaction? CH3CH2CH2CH2Br,(CH3)2CHCH2Br,CH3CH2CH(CH3)Br,(CH3)3CBr
A)CH3CH2CH2CH2Br
B)(CH3)2CHCH2Br
C)CH3CH2CH(CH3)Br
D)(CH3)3CBr
A)CH3CH2CH2CH2Br
B)(CH3)2CHCH2Br
C)CH3CH2CH(CH3)Br
D)(CH3)3CBr

Unlock Deck
Unlock for access to all 73 flashcards in this deck.
Unlock Deck
k this deck
62
Which alkyl halide will form the most stable carbocation? 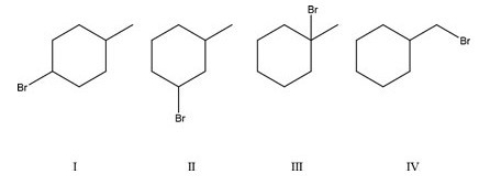
A)I
B)II
C)III
D)IV

A)I
B)II
C)III
D)IV

Unlock Deck
Unlock for access to all 73 flashcards in this deck.
Unlock Deck
k this deck
63
What is the product of the nucleophilic substitution reaction shown below? 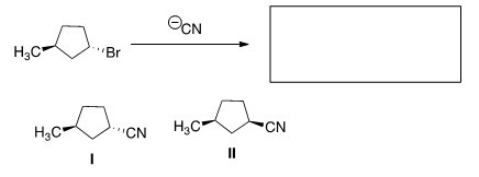
A)Only I
B)Only II
C)I and II
D)None

A)Only I
B)Only II
C)I and II
D)None

Unlock Deck
Unlock for access to all 73 flashcards in this deck.
Unlock Deck
k this deck
64
What is the IUPAC name of the following compound? 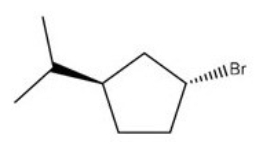
A)(1R,3R)-1-bromo-3-isopropylcyclopentane
B)(1R,3S)-1-bromo-3-isopropylcyclopentane
C)(1S,3R)-1-bromo-3-isopropylcyclopentane
D)(1S,3S)-1-bromo-3-isopropylcyclopentane

A)(1R,3R)-1-bromo-3-isopropylcyclopentane
B)(1R,3S)-1-bromo-3-isopropylcyclopentane
C)(1S,3R)-1-bromo-3-isopropylcyclopentane
D)(1S,3S)-1-bromo-3-isopropylcyclopentane

Unlock Deck
Unlock for access to all 73 flashcards in this deck.
Unlock Deck
k this deck
65
Identify the nucleophile and type of substitution reaction in the following: 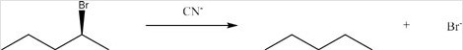
A)CN-,Sn1
B)CN-,Sn2
C)Br-,Sn1
D)Br-,Sn2

A)CN-,Sn1
B)CN-,Sn2
C)Br-,Sn1
D)Br-,Sn2

Unlock Deck
Unlock for access to all 73 flashcards in this deck.
Unlock Deck
k this deck
66
What is the starting material in the reaction shown below? 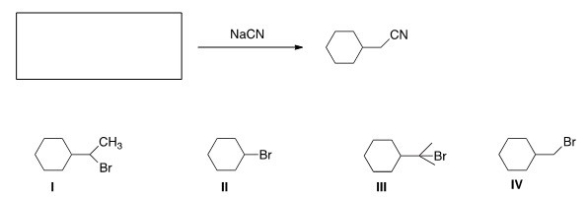
A)I
B)II
C)III
D)IV

A)I
B)II
C)III
D)IV

Unlock Deck
Unlock for access to all 73 flashcards in this deck.
Unlock Deck
k this deck
67
What is the starting material in the reaction shown below? 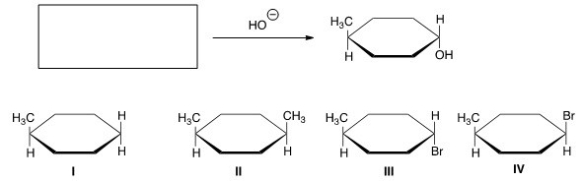
A)I
B)II
C)III
D)IV

A)I
B)II
C)III
D)IV

Unlock Deck
Unlock for access to all 73 flashcards in this deck.
Unlock Deck
k this deck
68
Rank the following compounds in order of increasing SN1 reactivity? 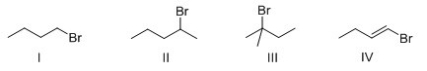
A)I > II > III > IV
B)IV > I > II > III
C)IV > III > II > I
D)III > II > I > IV

A)I > II > III > IV
B)IV > I > II > III
C)IV > III > II > I
D)III > II > I > IV

Unlock Deck
Unlock for access to all 73 flashcards in this deck.
Unlock Deck
k this deck
69
Which of the following alkyl halides would react the fastest with -OH in SN2 reaction? CH3CH2Br,CH3CH2Cl,CH3CH2F,CH3CH2I
A)CH3CH2Br
B)CH3CH2Cl
C)CH3CH2F
D)CH3CH2I
A)CH3CH2Br
B)CH3CH2Cl
C)CH3CH2F
D)CH3CH2I

Unlock Deck
Unlock for access to all 73 flashcards in this deck.
Unlock Deck
k this deck
70
Rank the following compounds in order of increasing SN2 reactivity? 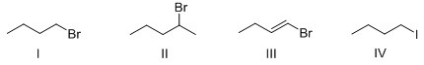
A)I > II > III > IV
B)IV > I > II > III
C)IV > III > II > I
D)III > II > I > IV

A)I > II > III > IV
B)IV > I > II > III
C)IV > III > II > I
D)III > II > I > IV

Unlock Deck
Unlock for access to all 73 flashcards in this deck.
Unlock Deck
k this deck
71
What is the IUPAC name of the following compound? 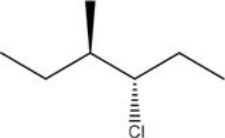
A)(3R,4R)-3-chloro-4-methylhexane
B)(3R,4S)-3-chloro-4-methylhexane
C)(3S,4R)-3-chloro-4-methylhexane
D)(3S,4S)-3-chloro-4-methylhexane

A)(3R,4R)-3-chloro-4-methylhexane
B)(3R,4S)-3-chloro-4-methylhexane
C)(3S,4R)-3-chloro-4-methylhexane
D)(3S,4S)-3-chloro-4-methylhexane

Unlock Deck
Unlock for access to all 73 flashcards in this deck.
Unlock Deck
k this deck
72
Pick the solvent that gives the fastest SN2 reaction between CH2CH2Br and -OCH3.
A)CH2CH2OH
B)CH3OH
C)DMSO
D)H2O
A)CH2CH2OH
B)CH3OH
C)DMSO
D)H2O

Unlock Deck
Unlock for access to all 73 flashcards in this deck.
Unlock Deck
k this deck
73
Which of the following statements explain why aryl halides and vinyl halides do not undergo nucleophilic substitution by either the SN1 or SN2 mechanism?
A)They don't undergo SN1 reactions because a higher percent s-character makes the bond longer and stronger.
B)They don't undergo SN2 reactions because a higher percent s-character makes the bond shorter and stronger.
C)They don't undergo SN2 reactions because heterolysis of the C-X bond forms a highly unstable carbocation.
D)They don't undergo SN1 reactions because the carbocation is highly electronegative.
A)They don't undergo SN1 reactions because a higher percent s-character makes the bond longer and stronger.
B)They don't undergo SN2 reactions because a higher percent s-character makes the bond shorter and stronger.
C)They don't undergo SN2 reactions because heterolysis of the C-X bond forms a highly unstable carbocation.
D)They don't undergo SN1 reactions because the carbocation is highly electronegative.

Unlock Deck
Unlock for access to all 73 flashcards in this deck.
Unlock Deck
k this deck


