Deck 8: Alkyl Halides and Elimination Reactions
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/52
Play
Full screen (f)
Deck 8: Alkyl Halides and Elimination Reactions
1
Which of the following statements about an E1 mechanism is not true?
A)The reaction is fastest with tertiary alkyl halides.
B)A better leaving group makes the reaction rate increase.
C)The reaction follows first-order kinetics.
D)Stronger bases favor the E1 reaction.
A)The reaction is fastest with tertiary alkyl halides.
B)A better leaving group makes the reaction rate increase.
C)The reaction follows first-order kinetics.
D)Stronger bases favor the E1 reaction.
Stronger bases favor the E1 reaction.
2
Which of the following is the major elimination product of the following reaction? 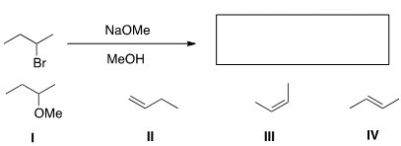
A)I
B)II
C)III
D)IV

A)I
B)II
C)III
D)IV
IV
3
What is the major product of the following reaction? 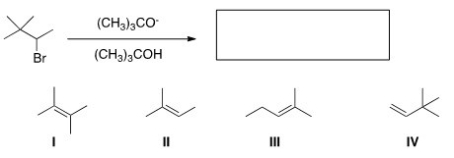
A)I
B)II
C)III
D)IV

A)I
B)II
C)III
D)IV
IV
4
Which of the following is the most reactive substrate in an E1 reaction? 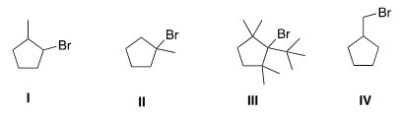
A)I
B)II
C)III
D)IV

A)I
B)II
C)III
D)IV

Unlock Deck
Unlock for access to all 52 flashcards in this deck.
Unlock Deck
k this deck
5
What is the major elimination product in the reaction of 1-bromobutane with potassium tert-butoxide in tert-butanol?
A)cis-2-Butene
B)1-Butene
C)trans-2-Butene
D)Butanol
A)cis-2-Butene
B)1-Butene
C)trans-2-Butene
D)Butanol

Unlock Deck
Unlock for access to all 52 flashcards in this deck.
Unlock Deck
k this deck
6
Which of the following represents the rate law for an E2 reaction?
A)Rate = k[alkyl halide]
B)Rate = k[alkyl halide][base]
C)Rate = k[alkyl halide]2
D)Rate = k[base]2
A)Rate = k[alkyl halide]
B)Rate = k[alkyl halide][base]
C)Rate = k[alkyl halide]2
D)Rate = k[base]2

Unlock Deck
Unlock for access to all 52 flashcards in this deck.
Unlock Deck
k this deck
7
How many unique β carbons are found in the alkyl halide below? 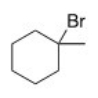
A)1
B)2
C)3
D)4

A)1
B)2
C)3
D)4

Unlock Deck
Unlock for access to all 52 flashcards in this deck.
Unlock Deck
k this deck
8
Which of the following is the most reactive substrate in an E2 reaction? 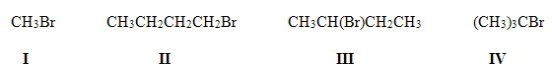
A)I
B)II
C)III
D)IV
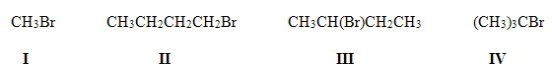
A)I
B)II
C)III
D)IV

Unlock Deck
Unlock for access to all 52 flashcards in this deck.
Unlock Deck
k this deck
9
Which of the following statements about the mechanism of an E2 reaction is true?
A)All bonds are broken and formed in multiple steps.
B)The reaction is not concerted.
C)Entropy favors the reactants of an E2 reaction.
D)Entropy favors the products of an E2 reaction.
A)All bonds are broken and formed in multiple steps.
B)The reaction is not concerted.
C)Entropy favors the reactants of an E2 reaction.
D)Entropy favors the products of an E2 reaction.

Unlock Deck
Unlock for access to all 52 flashcards in this deck.
Unlock Deck
k this deck
10
How many unique β carbons are found in the alkyl halide below? 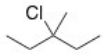
A)1
B)2
C)3
D)4

A)1
B)2
C)3
D)4

Unlock Deck
Unlock for access to all 52 flashcards in this deck.
Unlock Deck
k this deck
11
Which of the following is the least reactive substrate in an E2 reaction? 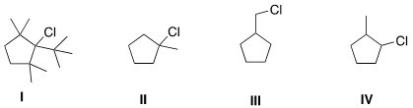
A)I
B)II
C)III
D)IV

A)I
B)II
C)III
D)IV

Unlock Deck
Unlock for access to all 52 flashcards in this deck.
Unlock Deck
k this deck
12
What is the major elimination product obtained from the following reaction? 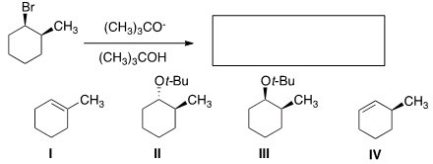
A)I
B)II
C)III
D)IV

A)I
B)II
C)III
D)IV

Unlock Deck
Unlock for access to all 52 flashcards in this deck.
Unlock Deck
k this deck
13
Which of the following statements about the mechanism of an E2 reaction is not true?
A)It is fastest with tertiary halides.
B)It exhibits first-order kinetics.
C)A better leaving group should make a faster reaction.
D)All bonds are broken and formed in a single step.
A)It is fastest with tertiary halides.
B)It exhibits first-order kinetics.
C)A better leaving group should make a faster reaction.
D)All bonds are broken and formed in a single step.

Unlock Deck
Unlock for access to all 52 flashcards in this deck.
Unlock Deck
k this deck
14
Which of the following statements about an E1 mechanism is true?
A)The identity of the leaving group affects the rate of reaction.
B)The reaction follows second-order kinetics.
C)The reaction is slowest with tertiary substrates.
D)Polar aprotic solvents favor the E1 mechanism.
A)The identity of the leaving group affects the rate of reaction.
B)The reaction follows second-order kinetics.
C)The reaction is slowest with tertiary substrates.
D)Polar aprotic solvents favor the E1 mechanism.

Unlock Deck
Unlock for access to all 52 flashcards in this deck.
Unlock Deck
k this deck
15
Which of the following statements about an E1 mechanism is not true?
A)It is a two-step process and has the same first step as an SN1 mechanism.
B)It involves the formation of a carbocation from eliminating a good leaving group.
C)A common competing reaction is rearrangement of a less stable carbocation to a more stable carbocation.
D)The loss of a proton by the carbocation is a slow step.
A)It is a two-step process and has the same first step as an SN1 mechanism.
B)It involves the formation of a carbocation from eliminating a good leaving group.
C)A common competing reaction is rearrangement of a less stable carbocation to a more stable carbocation.
D)The loss of a proton by the carbocation is a slow step.

Unlock Deck
Unlock for access to all 52 flashcards in this deck.
Unlock Deck
k this deck
16
How many different E2 products can form from the dehydrohalogenation of 2-bromobutane?
A)1
B)2
C)3
D)4
A)1
B)2
C)3
D)4

Unlock Deck
Unlock for access to all 52 flashcards in this deck.
Unlock Deck
k this deck
17
Classify each alkene in vitamin D3 labeled I,II,III by the number of carbon substituents bonded to the double bond. 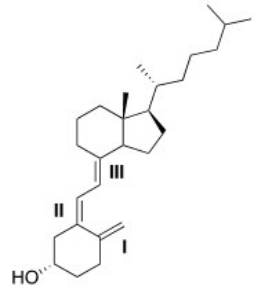
A)I = Disubstituted; II = trisubstituted; III = trisubstituted
B)I = Monosubstituted; II = disubstituted; III = trisubstituted
C)I = Disubstituted; II = disubstituted; III = trisubstituted
D)I = Disubstituted; II = trisubstituted; III = disubstituted

A)I = Disubstituted; II = trisubstituted; III = trisubstituted
B)I = Monosubstituted; II = disubstituted; III = trisubstituted
C)I = Disubstituted; II = disubstituted; III = trisubstituted
D)I = Disubstituted; II = trisubstituted; III = disubstituted

Unlock Deck
Unlock for access to all 52 flashcards in this deck.
Unlock Deck
k this deck
18
What is the major elimination product obtained from the following reaction? 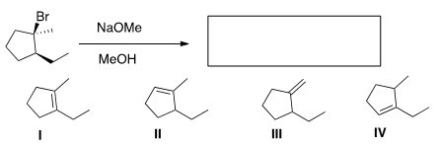
A)I
B)II
C)III
D)IV

A)I
B)II
C)III
D)IV

Unlock Deck
Unlock for access to all 52 flashcards in this deck.
Unlock Deck
k this deck
19
What is the major elimination product obtained from the following reaction? 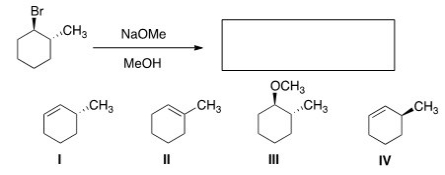
A)I
B)II
C)III
D)IV

A)I
B)II
C)III
D)IV

Unlock Deck
Unlock for access to all 52 flashcards in this deck.
Unlock Deck
k this deck
20
What is the major elimination product obtained from the following reaction? 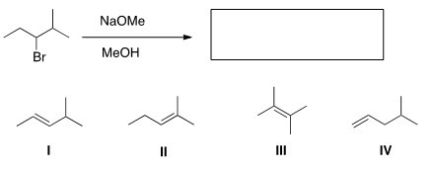
A)I
B)II
C)III
D)IV

A)I
B)II
C)III
D)IV

Unlock Deck
Unlock for access to all 52 flashcards in this deck.
Unlock Deck
k this deck
21
What is (are)the elimination product(s)of the following reaction? 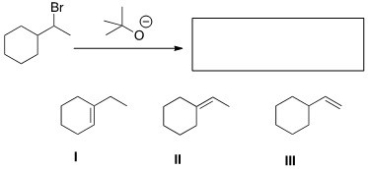
A)Only I
B)Only II
C)Only III
D)II and III

A)Only I
B)Only II
C)Only III
D)II and III

Unlock Deck
Unlock for access to all 52 flashcards in this deck.
Unlock Deck
k this deck
22
What is (are)the elimination product(s)of the following reaction? 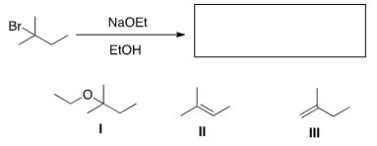
A)Only I
B)Only II
C)Only III
D)II and III

A)Only I
B)Only II
C)Only III
D)II and III

Unlock Deck
Unlock for access to all 52 flashcards in this deck.
Unlock Deck
k this deck
23
Which of the following is the dihalide that can be used to prepare the alkyne below? 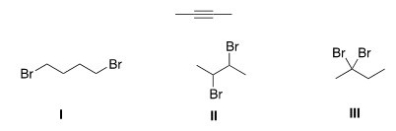
A)Only I
B)Only II
C)Only III
D)II and III

A)Only I
B)Only II
C)Only III
D)II and III

Unlock Deck
Unlock for access to all 52 flashcards in this deck.
Unlock Deck
k this deck
24
What is the product of the following reaction? 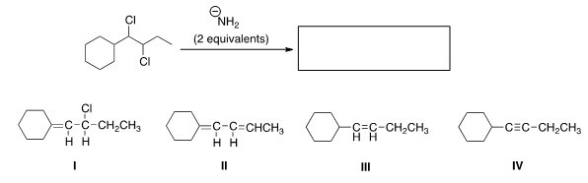
A)I
B)II
C)III
D)IV

A)I
B)II
C)III
D)IV

Unlock Deck
Unlock for access to all 52 flashcards in this deck.
Unlock Deck
k this deck
25
What is the product of the following reaction? 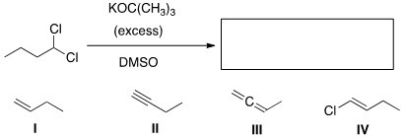
A)I
B)II
C)III
D)IV

A)I
B)II
C)III
D)IV

Unlock Deck
Unlock for access to all 52 flashcards in this deck.
Unlock Deck
k this deck
26
Select the elimination product(s)formed by treating the indicated alkyl halide with a base. 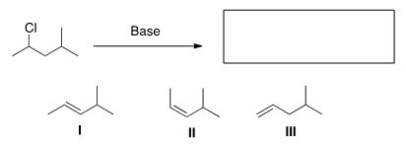
A)Only I
B)Only II
C)Only III
D)I,II,and III

A)Only I
B)Only II
C)Only III
D)I,II,and III

Unlock Deck
Unlock for access to all 52 flashcards in this deck.
Unlock Deck
k this deck
27
Which of the following is most likely to react as a nucleophile rather than a base? 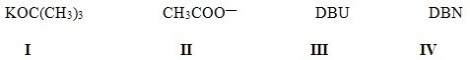
A)I
B)II
C)III
D)IV
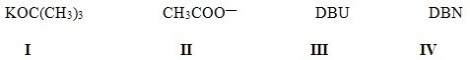
A)I
B)II
C)III
D)IV

Unlock Deck
Unlock for access to all 52 flashcards in this deck.
Unlock Deck
k this deck
28
Which of the following is the dihalide that can be used to prepare the alkyne below? 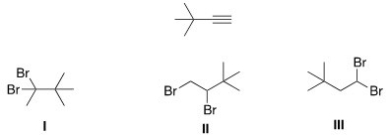
A)I and II
B)II and III
C)I and III
D)I,II,and III

A)I and II
B)II and III
C)I and III
D)I,II,and III

Unlock Deck
Unlock for access to all 52 flashcards in this deck.
Unlock Deck
k this deck
29
What is (are)the product(s)of the following reaction? 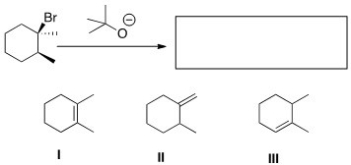
A)I and II
B)II and III
C)I and III
D)I,II,and III

A)I and II
B)II and III
C)I and III
D)I,II,and III

Unlock Deck
Unlock for access to all 52 flashcards in this deck.
Unlock Deck
k this deck
30
What is the product of the following reaction? 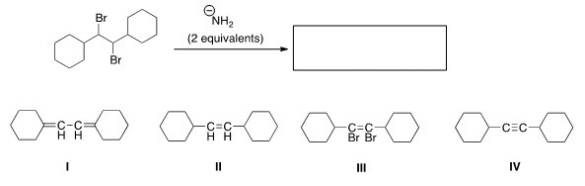
A)I
B)II
C)III
D)IV

A)I
B)II
C)III
D)IV

Unlock Deck
Unlock for access to all 52 flashcards in this deck.
Unlock Deck
k this deck
31
What is (are)the product(s)of the following reaction? 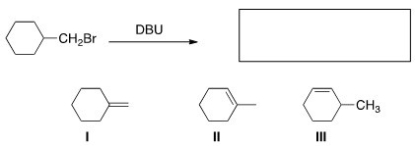
A)Only I
B)Only II
C)Only III
D)I and II

A)Only I
B)Only II
C)Only III
D)I and II

Unlock Deck
Unlock for access to all 52 flashcards in this deck.
Unlock Deck
k this deck
32
What is (are)the product(s)of the following reaction? 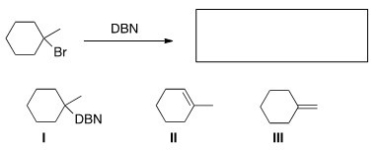
A)Only I
B)Only II
C)Only III
D)II and III

A)Only I
B)Only II
C)Only III
D)II and III

Unlock Deck
Unlock for access to all 52 flashcards in this deck.
Unlock Deck
k this deck
33
Which of the following alkyl halides would afford the indicated product upon reaction with sodium methoxide? 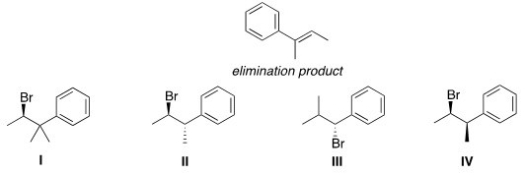
A)I
B)II
C)III
D)IV

A)I
B)II
C)III
D)IV

Unlock Deck
Unlock for access to all 52 flashcards in this deck.
Unlock Deck
k this deck
34
Which of the labeled protons in the compound below is most readily abstracted under E2 conditions? 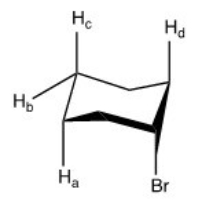
A)Ha
B)Hb
C)Hc
D)Hd

A)Ha
B)Hb
C)Hc
D)Hd

Unlock Deck
Unlock for access to all 52 flashcards in this deck.
Unlock Deck
k this deck
35
What is the product of the following reaction? 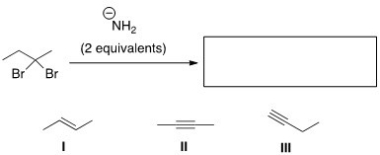
A)Only I
B)Only II
C)Only III
D)II and III

A)Only I
B)Only II
C)Only III
D)II and III

Unlock Deck
Unlock for access to all 52 flashcards in this deck.
Unlock Deck
k this deck
36
What is (are)the starting material(s)in the reaction below? 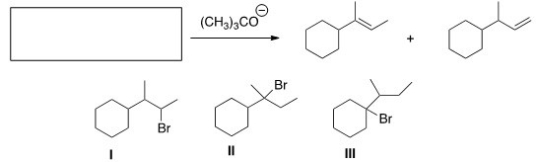
A)Only I
B)Only II
C)Only III
D)II and III

A)Only I
B)Only II
C)Only III
D)II and III

Unlock Deck
Unlock for access to all 52 flashcards in this deck.
Unlock Deck
k this deck
37
Which of the following is most likely to react as a base rather than a nucleophile? 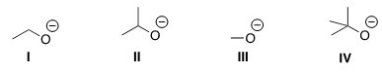
A)I
B)II
C)III
D)IV
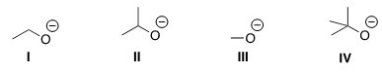
A)I
B)II
C)III
D)IV

Unlock Deck
Unlock for access to all 52 flashcards in this deck.
Unlock Deck
k this deck
38
Which of the following alkyl halides will afford the product below as the major product in an E2 reaction? 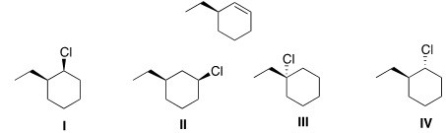
A)I
B)II
C)III
D)IV

A)I
B)II
C)III
D)IV

Unlock Deck
Unlock for access to all 52 flashcards in this deck.
Unlock Deck
k this deck
39
What is (are)the product(s)of the following reaction? 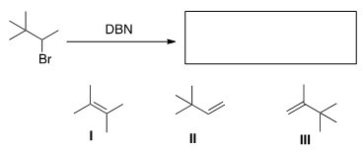
A)Only I
B)Only II
C)Only III
D)I and II

A)Only I
B)Only II
C)Only III
D)I and II

Unlock Deck
Unlock for access to all 52 flashcards in this deck.
Unlock Deck
k this deck
40
Which of the following is the major E2 product formed from the following alkyl halide? 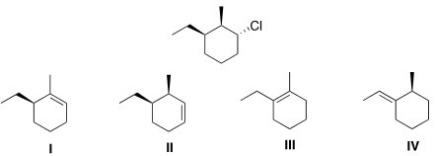
A)I
B)II
C)III
D)IV

A)I
B)II
C)III
D)IV

Unlock Deck
Unlock for access to all 52 flashcards in this deck.
Unlock Deck
k this deck
41
Consider the following E2 reaction.What rate equation would be observed for this reaction? ![<strong>Consider the following E2 reaction.What rate equation would be observed for this reaction? </strong> A)Rate = k[CH<sub>3</sub>CH<sub>2</sub>CH<sub>2</sub>Br] B)Rate = k[CH<sub>3</sub>CH<sub>2</sub>CH<sub>2</sub>Br][KOC(CH<sub>3</sub>)<sub>3</sub>] C)Rate = k[CH<sub>3</sub>CH<sub>2</sub>CH<sub>2</sub>Br][KOC(CH<sub>3</sub>)<sub>3</sub>]<sup>2</sup> D)Rate = k[CH<sub>3</sub>CH<sub>2</sub>CH<sub>2</sub>Br]<sup>2</sup>[KOC(CH<sub>3</sub>)<sub>3</sub>]](https://storage.examlex.com/TB7814/11eac680_9c11_89c3_a448_551695386186_TB7814_00.jpg)
A)Rate = k[CH3CH2CH2Br]
B)Rate = k[CH3CH2CH2Br][KOC(CH3)3]
C)Rate = k[CH3CH2CH2Br][KOC(CH3)3]2
D)Rate = k[CH3CH2CH2Br]2[KOC(CH3)3]
![<strong>Consider the following E2 reaction.What rate equation would be observed for this reaction? </strong> A)Rate = k[CH<sub>3</sub>CH<sub>2</sub>CH<sub>2</sub>Br] B)Rate = k[CH<sub>3</sub>CH<sub>2</sub>CH<sub>2</sub>Br][KOC(CH<sub>3</sub>)<sub>3</sub>] C)Rate = k[CH<sub>3</sub>CH<sub>2</sub>CH<sub>2</sub>Br][KOC(CH<sub>3</sub>)<sub>3</sub>]<sup>2</sup> D)Rate = k[CH<sub>3</sub>CH<sub>2</sub>CH<sub>2</sub>Br]<sup>2</sup>[KOC(CH<sub>3</sub>)<sub>3</sub>]](https://storage.examlex.com/TB7814/11eac680_9c11_89c3_a448_551695386186_TB7814_00.jpg)
A)Rate = k[CH3CH2CH2Br]
B)Rate = k[CH3CH2CH2Br][KOC(CH3)3]
C)Rate = k[CH3CH2CH2Br][KOC(CH3)3]2
D)Rate = k[CH3CH2CH2Br]2[KOC(CH3)3]

Unlock Deck
Unlock for access to all 52 flashcards in this deck.
Unlock Deck
k this deck
42
Which alkyl halide(s)would give the following alkene as the only product in an elimination reaction? 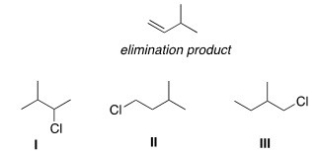
A)Only I
B)Only II
C)Only III
D)I and II

A)Only I
B)Only II
C)Only III
D)I and II

Unlock Deck
Unlock for access to all 52 flashcards in this deck.
Unlock Deck
k this deck
43
Which of the following is the most stable alkene? 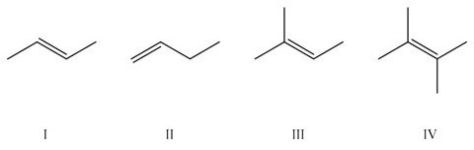
A)I
B)II
C)III
D)IV

A)I
B)II
C)III
D)IV

Unlock Deck
Unlock for access to all 52 flashcards in this deck.
Unlock Deck
k this deck
44
What is the most likely mechanism for the reaction below? 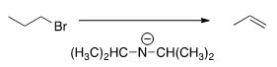
A)SN1
B)SN2
C)E1
D)E2

A)SN1
B)SN2
C)E1
D)E2

Unlock Deck
Unlock for access to all 52 flashcards in this deck.
Unlock Deck
k this deck
45
Which of the following alkyl halide reacts the fastest in an E2 reaction? 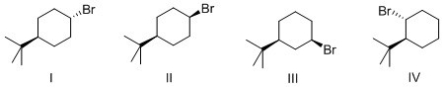
A)I
B)II
C)III
D)IV

A)I
B)II
C)III
D)IV

Unlock Deck
Unlock for access to all 52 flashcards in this deck.
Unlock Deck
k this deck
46
Which of the following alkenes is the most stable? 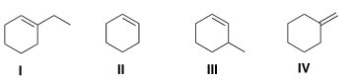
A)I
B)II
C)III
D)IV

A)I
B)II
C)III
D)IV

Unlock Deck
Unlock for access to all 52 flashcards in this deck.
Unlock Deck
k this deck
47
What is the major product and likely mechanism for the following reaction? 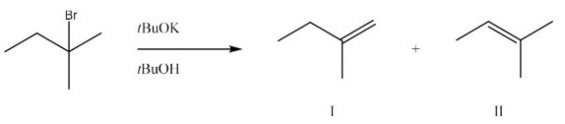
A)I,E1
B)I,E2
C)II,E1
D)II,E2

A)I,E1
B)I,E2
C)II,E1
D)II,E2

Unlock Deck
Unlock for access to all 52 flashcards in this deck.
Unlock Deck
k this deck
48
What is the most likely mechanism for the reaction below? 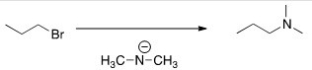
A)SN1
B)SN2
C)E1
D)E2

A)SN1
B)SN2
C)E1
D)E2

Unlock Deck
Unlock for access to all 52 flashcards in this deck.
Unlock Deck
k this deck
49
Which is the most likely mechanism for the following reaction? 
A)SN1
B)SN2
C)E1
D)E2

A)SN1
B)SN2
C)E1
D)E2

Unlock Deck
Unlock for access to all 52 flashcards in this deck.
Unlock Deck
k this deck
50
Which of the following alkyl chloride affords alkene A exclusively under E2 reaction condition? 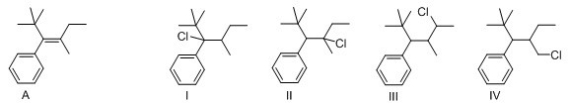
A)I
B)II
C)III
D)IV

A)I
B)II
C)III
D)IV

Unlock Deck
Unlock for access to all 52 flashcards in this deck.
Unlock Deck
k this deck
51
Which of the following alkyl halide would react the fastest with -OH in E2 reaction? 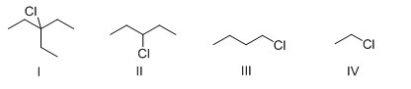
A)I
B)II
C)III
D)IV

A)I
B)II
C)III
D)IV

Unlock Deck
Unlock for access to all 52 flashcards in this deck.
Unlock Deck
k this deck
52
What is the most likely mechanism for the following reaction? 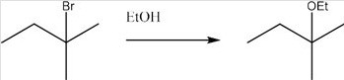
A)SN1
B)SN2
C)E1
D)E2

A)SN1
B)SN2
C)E1
D)E2

Unlock Deck
Unlock for access to all 52 flashcards in this deck.
Unlock Deck
k this deck


