Deck 6: Understanding Organic Reactions
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/53
Play
Full screen (f)
Deck 6: Understanding Organic Reactions
1
What kind of reaction does the conversion of A to B represent? 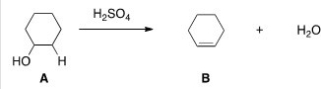
A)Addition reaction
B)Elimination reaction
C)Substitution reaction
D)Oxidation-reduction reaction

A)Addition reaction
B)Elimination reaction
C)Substitution reaction
D)Oxidation-reduction reaction
Elimination reaction
2
Which of the following statements is true?
A)Bond dissociation energies increase down a column of the periodic table.
B)When DH° is positive,more energy is released in forming bonds than is needed to break bonds.
C)When DH° is negative,more energy is needed to break bonds than is released in forming bonds.
D)Bond dissociation energies decrease down a column of the periodic table.
A)Bond dissociation energies increase down a column of the periodic table.
B)When DH° is positive,more energy is released in forming bonds than is needed to break bonds.
C)When DH° is negative,more energy is needed to break bonds than is released in forming bonds.
D)Bond dissociation energies decrease down a column of the periodic table.
Bond dissociation energies decrease down a column of the periodic table.
3
Which of the Keq corresponds to the lowest value of DG°?
A)Keq = 10-3
B)Keq = 10-2
C)Keq = 10-1
D)DG° cannot be determined.
A)Keq = 10-3
B)Keq = 10-2
C)Keq = 10-1
D)DG° cannot be determined.
Keq = 10-1
4
Using the bond dissociation energies given,calculate DH° for the following reaction. 
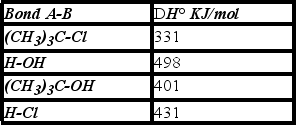
A)+3 KJ/mol
B)-3 KJ/mol
C)-67 KJ/mol
D)+70 KJ/mol


A)+3 KJ/mol
B)-3 KJ/mol
C)-67 KJ/mol
D)+70 KJ/mol

Unlock Deck
Unlock for access to all 53 flashcards in this deck.
Unlock Deck
k this deck
5
Which of the following statements about equilibrium is true?
A)Equilibrium favors the products when the energy of the products is higher than the energy of the reactants.
B)Equilibrium favors the reactants when the energy of the product is lower than the energy of the reactants.
C)Equilibrium favors the products when they are less stable than the starting material of a reaction.
D)Equilibrium favors the products when they are more stable than the starting material of a reaction.
A)Equilibrium favors the products when the energy of the products is higher than the energy of the reactants.
B)Equilibrium favors the reactants when the energy of the product is lower than the energy of the reactants.
C)Equilibrium favors the products when they are less stable than the starting material of a reaction.
D)Equilibrium favors the products when they are more stable than the starting material of a reaction.

Unlock Deck
Unlock for access to all 53 flashcards in this deck.
Unlock Deck
k this deck
6
Which of the following statements is not true?
A)Bond breaking is endothermic.
B)The bond dissociation energy for bond breaking is always negative.
C)Bond making is exothermic.
D)The bond dissociation energy for bond formation is always negative.
A)Bond breaking is endothermic.
B)The bond dissociation energy for bond breaking is always negative.
C)Bond making is exothermic.
D)The bond dissociation energy for bond formation is always negative.

Unlock Deck
Unlock for access to all 53 flashcards in this deck.
Unlock Deck
k this deck
7
Which of the following statements about substitution reactions is true?
A)Substitution reactions involve π bonds.
B)Substitution reactions involve σ bonds.
C)One σ bond breaks and another forms at a different carbon atom.
D)One π bond breaks and another forms at the same carbon atom.
A)Substitution reactions involve π bonds.
B)Substitution reactions involve σ bonds.
C)One σ bond breaks and another forms at a different carbon atom.
D)One π bond breaks and another forms at the same carbon atom.

Unlock Deck
Unlock for access to all 53 flashcards in this deck.
Unlock Deck
k this deck
8
Using the bond dissociation energies given,calculate DH° for the following reaction. 
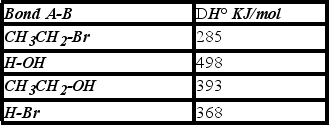
A)+108 KJ/mol
B)-130 KJ/mol
C)-22 KJ/mol
D)+22 KJ/mol


A)+108 KJ/mol
B)-130 KJ/mol
C)-22 KJ/mol
D)+22 KJ/mol

Unlock Deck
Unlock for access to all 53 flashcards in this deck.
Unlock Deck
k this deck
9
Which of the following expressions summarizes the correct relationship between the free energy change,DG°,and the equilibrium constant,Keq?
A)Keq > 1 when DG° > 0
B)Keq > 1 when DG° < 0
C)Keq < 1 when DG° < 0
D)Keq < 1 when DG° = 0
A)Keq > 1 when DG° > 0
B)Keq > 1 when DG° < 0
C)Keq < 1 when DG° < 0
D)Keq < 1 when DG° = 0

Unlock Deck
Unlock for access to all 53 flashcards in this deck.
Unlock Deck
k this deck
10
Which of the following statements about bond breaking is true?
A)Homolysis and heterolysis require energy.
B)In homolysis,the electrons in the bond are divided unequally.
C)In heterolysis,the electrons in the bond are divided equally.
D)Homolysis generates charged intermediates.
A)Homolysis and heterolysis require energy.
B)In homolysis,the electrons in the bond are divided unequally.
C)In heterolysis,the electrons in the bond are divided equally.
D)Homolysis generates charged intermediates.

Unlock Deck
Unlock for access to all 53 flashcards in this deck.
Unlock Deck
k this deck
11
Which of the following statements about elimination reactions is true?
A)Two σ bonds are broken.
B)Two σ bonds are formed.
C)Two π bonds are broken.
D)Two π bonds are formed.
A)Two σ bonds are broken.
B)Two σ bonds are formed.
C)Two π bonds are broken.
D)Two π bonds are formed.

Unlock Deck
Unlock for access to all 53 flashcards in this deck.
Unlock Deck
k this deck
12
Which of the Keq corresponds to the highest value of DG°?
A)Keq = 10-1
B)Keq = 10-2
C)Keq = 10-3
D)Keq = 10-5
A)Keq = 10-1
B)Keq = 10-2
C)Keq = 10-3
D)Keq = 10-5

Unlock Deck
Unlock for access to all 53 flashcards in this deck.
Unlock Deck
k this deck
13
Which of the following statements about addition reactions is true?
A)Two π bonds are formed.
B)Two π bonds are broken.
C)Two σ bonds are formed.
D)One π bond is formed.
A)Two π bonds are formed.
B)Two π bonds are broken.
C)Two σ bonds are formed.
D)One π bond is formed.

Unlock Deck
Unlock for access to all 53 flashcards in this deck.
Unlock Deck
k this deck
14
Which of the following statements is not true?
A)In polar reactions,a nucleophile reacts with an electrophile.
B)Carbocations are electrophiles.
C)Carbanions are nucleophiles.
D)A half-headed curved arrow shows the movement of an electron pair.
A)In polar reactions,a nucleophile reacts with an electrophile.
B)Carbocations are electrophiles.
C)Carbanions are nucleophiles.
D)A half-headed curved arrow shows the movement of an electron pair.

Unlock Deck
Unlock for access to all 53 flashcards in this deck.
Unlock Deck
k this deck
15
Which of the following statements is true?
A)Ionic intermediates are formed in radical reactions.
B)Radicals are intermediates in polar reactions.
C)Carbocations are electrophiles.
D)Radicals are nucleophiles.
A)Ionic intermediates are formed in radical reactions.
B)Radicals are intermediates in polar reactions.
C)Carbocations are electrophiles.
D)Radicals are nucleophiles.

Unlock Deck
Unlock for access to all 53 flashcards in this deck.
Unlock Deck
k this deck
16
Which of the Keq corresponds to the most negative value of DG°?
A)Keq = 1
B)Keq = 101
C)Keq = 102
D)Keq = 103
A)Keq = 1
B)Keq = 101
C)Keq = 102
D)Keq = 103

Unlock Deck
Unlock for access to all 53 flashcards in this deck.
Unlock Deck
k this deck
17
What kind of reaction does the conversion of A to B represent? 
A)Addition reaction
B)Substitution reaction
C)Elimination reaction
D)Acid-base reaction

A)Addition reaction
B)Substitution reaction
C)Elimination reaction
D)Acid-base reaction

Unlock Deck
Unlock for access to all 53 flashcards in this deck.
Unlock Deck
k this deck
18
What kind of reaction does the conversion of A to B represent? 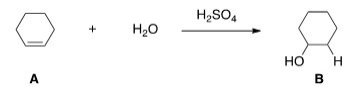
A)Acid-base reaction
B)Elimination reaction
C)Substitution reaction
D)Addition reaction

A)Acid-base reaction
B)Elimination reaction
C)Substitution reaction
D)Addition reaction

Unlock Deck
Unlock for access to all 53 flashcards in this deck.
Unlock Deck
k this deck
19
Which of the following statements about bond breaking is not true?
A)Homolysis generates uncharged reactive intermediates with unpaired electrons.
B)Homolysis require energy but heterolysis does not require energy.
C)Heterolysis generates charged intermediates.
D)Heterolysis involves unequal sharing of bonding electrons by atoms.
A)Homolysis generates uncharged reactive intermediates with unpaired electrons.
B)Homolysis require energy but heterolysis does not require energy.
C)Heterolysis generates charged intermediates.
D)Heterolysis involves unequal sharing of bonding electrons by atoms.

Unlock Deck
Unlock for access to all 53 flashcards in this deck.
Unlock Deck
k this deck
20
Which of the following statements about the equilibrium constant,Keq,is true?
A)When Keq > 1,the equilibrium favors the reactants.
B)When Keq < 1,the equilibrium favors the products.
C)The size of Keq tells about the position of equilibrium.
D)For a reaction to be useful,the equilibrium must favor the reactants.
A)When Keq > 1,the equilibrium favors the reactants.
B)When Keq < 1,the equilibrium favors the products.
C)The size of Keq tells about the position of equilibrium.
D)For a reaction to be useful,the equilibrium must favor the reactants.

Unlock Deck
Unlock for access to all 53 flashcards in this deck.
Unlock Deck
k this deck
21
Which of the following statements is true?
A)The size of the activation energy tells us about the reaction mechanism.
B)The size of the activation energy tells us about the reaction rate.
C)A slow reaction has low activation energy.
D)A fast reaction has high activation energy.
A)The size of the activation energy tells us about the reaction mechanism.
B)The size of the activation energy tells us about the reaction rate.
C)A slow reaction has low activation energy.
D)A fast reaction has high activation energy.

Unlock Deck
Unlock for access to all 53 flashcards in this deck.
Unlock Deck
k this deck
22
A decrease in which of the following results in an increase in the rate of a chemical reaction?
A)Energy of activation
B)Concentration
C)Temperature
D)Kinetic energy
A)Energy of activation
B)Concentration
C)Temperature
D)Kinetic energy

Unlock Deck
Unlock for access to all 53 flashcards in this deck.
Unlock Deck
k this deck
23
How many transition states and intermediates would the reaction profile have for the reaction shown below? 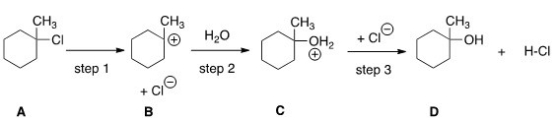
A)Three transition states and three intermediates
B)Two transition states and two intermediates
C)Three transition states and two intermediates
D)Two transition states and three intermediates

A)Three transition states and three intermediates
B)Two transition states and two intermediates
C)Three transition states and two intermediates
D)Two transition states and three intermediates

Unlock Deck
Unlock for access to all 53 flashcards in this deck.
Unlock Deck
k this deck
24
The equilibrium constant for the conversion of A to D is predicted to be which of the following? 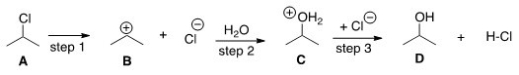
A)Keq = 1
B)Keq < 1
C)Keq > 1
D)Cannot be determined from the information provided

A)Keq = 1
B)Keq < 1
C)Keq > 1
D)Cannot be determined from the information provided

Unlock Deck
Unlock for access to all 53 flashcards in this deck.
Unlock Deck
k this deck
25
Which of the following statements about a two-step reaction mechanism is true?
A)The transition states are located at energy minima.
B)Each step is characterized by its own value of DH° and Ea.
C)The rate-determining step has the lower energy transition state.
D)The reactive intermediate is located at an energy maximum.
A)The transition states are located at energy minima.
B)Each step is characterized by its own value of DH° and Ea.
C)The rate-determining step has the lower energy transition state.
D)The reactive intermediate is located at an energy maximum.

Unlock Deck
Unlock for access to all 53 flashcards in this deck.
Unlock Deck
k this deck
26
In which reaction is Keq > 1? 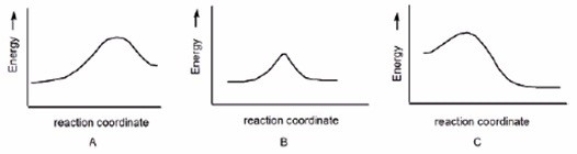
A)A
B)B
C)C

A)A
B)B
C)C

Unlock Deck
Unlock for access to all 53 flashcards in this deck.
Unlock Deck
k this deck
27
Which reaction has a positive DG°,assuming that entropy changes are negligible compared to enthalpy changes? 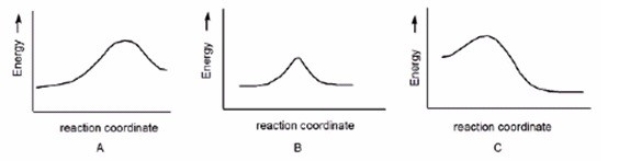
A)A
B)B
C)C

A)A
B)B
C)C

Unlock Deck
Unlock for access to all 53 flashcards in this deck.
Unlock Deck
k this deck
28
Which of the following statements is true?
A)The product is favored in reaction in which DH° is a positive value.
B)Entropy decreases when an acyclic compound forms a ring.
C)In homolytic bond cleavage,entropy decreases and favors formation of products.
D)The starting material is favored in a reaction in which DH° is a negative value.
A)The product is favored in reaction in which DH° is a positive value.
B)Entropy decreases when an acyclic compound forms a ring.
C)In homolytic bond cleavage,entropy decreases and favors formation of products.
D)The starting material is favored in a reaction in which DH° is a negative value.

Unlock Deck
Unlock for access to all 53 flashcards in this deck.
Unlock Deck
k this deck
29
Which of the following reaction quantities will have an effect on reaction rate?
A)DG°
B)DH°
C)Keq
D)Ea
A)DG°
B)DH°
C)Keq
D)Ea

Unlock Deck
Unlock for access to all 53 flashcards in this deck.
Unlock Deck
k this deck
30
Which reaction is slowest? 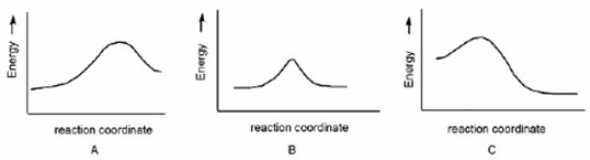
A)A
B)B
C)C

A)A
B)B
C)C

Unlock Deck
Unlock for access to all 53 flashcards in this deck.
Unlock Deck
k this deck
31
Which step would most likely have the largest energy of activation? 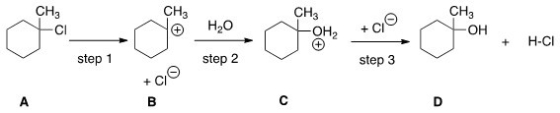
A)Step one
B)Step two
C)Step three
D)It cannot be determined from the information provided

A)Step one
B)Step two
C)Step three
D)It cannot be determined from the information provided

Unlock Deck
Unlock for access to all 53 flashcards in this deck.
Unlock Deck
k this deck
32
What kind of reaction does the conversion of A to D represent? 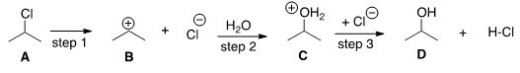
A)Addition reaction
B)Substitution reaction
C)Elimination reaction
D)Oxidation-reduction reaction

A)Addition reaction
B)Substitution reaction
C)Elimination reaction
D)Oxidation-reduction reaction

Unlock Deck
Unlock for access to all 53 flashcards in this deck.
Unlock Deck
k this deck
33
Which reaction is fast and has Keq = 1? 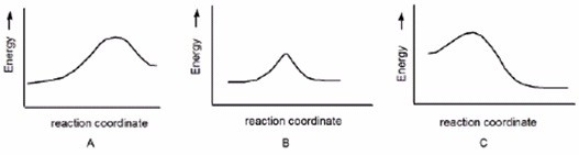
A)A
B)B
C)C

A)A
B)B
C)C

Unlock Deck
Unlock for access to all 53 flashcards in this deck.
Unlock Deck
k this deck
34
If the conversion of A to B is slow and B to C is fast,what is the rate equation for this reaction? ![<strong>If the conversion of A to B is slow and B to C is fast,what is the rate equation for this reaction? </strong> A)Rate = k[(CH<sub>3</sub>)<sub>2</sub>CHCl][H<sub>2</sub>O] B)Rate = k[(CH<sub>3</sub>)<sub>2</sub>CHCl] C)Rate = k[(CH<sub>3</sub>)<sub>2</sub>CH]<sup>+</sup>[H<sub>2</sub>O] D)Rate = k[(CH<sub>3</sub>)<sub>2</sub>CH]<sup>+</sup>](https://storage.examlex.com/TB7814/11eac680_9c17_7d7c_a448_a35f1e83c0c2_TB7814_00.jpg)
A)Rate = k[(CH3)2CHCl][H2O]
B)Rate = k[(CH3)2CHCl]
C)Rate = k[(CH3)2CH]+[H2O]
D)Rate = k[(CH3)2CH]+
![<strong>If the conversion of A to B is slow and B to C is fast,what is the rate equation for this reaction? </strong> A)Rate = k[(CH<sub>3</sub>)<sub>2</sub>CHCl][H<sub>2</sub>O] B)Rate = k[(CH<sub>3</sub>)<sub>2</sub>CHCl] C)Rate = k[(CH<sub>3</sub>)<sub>2</sub>CH]<sup>+</sup>[H<sub>2</sub>O] D)Rate = k[(CH<sub>3</sub>)<sub>2</sub>CH]<sup>+</sup>](https://storage.examlex.com/TB7814/11eac680_9c17_7d7c_a448_a35f1e83c0c2_TB7814_00.jpg)
A)Rate = k[(CH3)2CHCl][H2O]
B)Rate = k[(CH3)2CHCl]
C)Rate = k[(CH3)2CH]+[H2O]
D)Rate = k[(CH3)2CH]+

Unlock Deck
Unlock for access to all 53 flashcards in this deck.
Unlock Deck
k this deck
35
Which of the following statements is not true?
A)Two reactions can have identical values for DH° but very different Ea values.
B)The larger the activation energy,the slower the reaction.
C)DH° determines the height of the energy barrier.
D)The lower the activation energy,the faster the reaction.
A)Two reactions can have identical values for DH° but very different Ea values.
B)The larger the activation energy,the slower the reaction.
C)DH° determines the height of the energy barrier.
D)The lower the activation energy,the faster the reaction.

Unlock Deck
Unlock for access to all 53 flashcards in this deck.
Unlock Deck
k this deck
36
The DG° (free energy change)for the conversion of A to B is predicted to be which of the following? 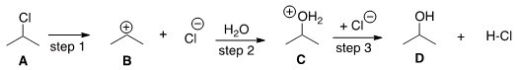
A)DG° = 0
B)DG° < 0
C)DG° > 0
D)Cannot be determined from the information provided

A)DG° = 0
B)DG° < 0
C)DG° > 0
D)Cannot be determined from the information provided

Unlock Deck
Unlock for access to all 53 flashcards in this deck.
Unlock Deck
k this deck
37
Which of the following letters represents DH° for the forward reaction in the following energy diagram? 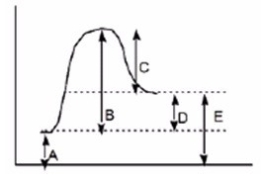
A)A
B)B
C)C
D)D

A)A
B)B
C)C
D)D

Unlock Deck
Unlock for access to all 53 flashcards in this deck.
Unlock Deck
k this deck
38
Which of the following statements is true?
A)Fast reactions have small rate constants.
B)Slow reactions have large rate constants.
C)A rate equation contains concentration terms for all reactants involved in a one-step mechanism.
D)A rate equation contains concentration terms for all the reactants involved in a multi-step reaction.
A)Fast reactions have small rate constants.
B)Slow reactions have large rate constants.
C)A rate equation contains concentration terms for all reactants involved in a one-step mechanism.
D)A rate equation contains concentration terms for all the reactants involved in a multi-step reaction.

Unlock Deck
Unlock for access to all 53 flashcards in this deck.
Unlock Deck
k this deck
39
How many transition states are present in the reaction in the energy diagram? 
A)0
B)1
C)2
D)3

A)0
B)1
C)2
D)3

Unlock Deck
Unlock for access to all 53 flashcards in this deck.
Unlock Deck
k this deck
40
What is the name given to the reaction species that lies at an energy minimum between steps on a reaction energy diagram?
A)Transition state
B)Activation energy
C)Reactive intermediate
D)Equilibrium product
A)Transition state
B)Activation energy
C)Reactive intermediate
D)Equilibrium product

Unlock Deck
Unlock for access to all 53 flashcards in this deck.
Unlock Deck
k this deck
41
The conversion of acetyl chloride to methyl acetate occurs via the following two-step mechanism: 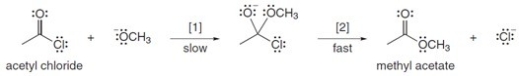 The conversion of acetyl chloride to methyl acetate would be classified as which of the following?
The conversion of acetyl chloride to methyl acetate would be classified as which of the following?
A)Addition
B)Elimination
C)Substitution
D)None of these
 The conversion of acetyl chloride to methyl acetate would be classified as which of the following?
The conversion of acetyl chloride to methyl acetate would be classified as which of the following?A)Addition
B)Elimination
C)Substitution
D)None of these

Unlock Deck
Unlock for access to all 53 flashcards in this deck.
Unlock Deck
k this deck
42
Which of the following statements about a catalyst is true?
A)A catalyst accelerates a reaction by changing the amount of reactant and product at equilibrium.
B)A catalyst accelerates a reaction by lowering the energy of activation.
C)A catalyst accelerates a reaction by raising the energy of activation.
D)A catalyst accelerates a reaction by lowering the equilibrium constant.
A)A catalyst accelerates a reaction by changing the amount of reactant and product at equilibrium.
B)A catalyst accelerates a reaction by lowering the energy of activation.
C)A catalyst accelerates a reaction by raising the energy of activation.
D)A catalyst accelerates a reaction by lowering the equilibrium constant.

Unlock Deck
Unlock for access to all 53 flashcards in this deck.
Unlock Deck
k this deck
43
The symbol Δ stands for ________ in a chemical reaction.
A)light
B)heat
C)reactant
D)product
A)light
B)heat
C)reactant
D)product

Unlock Deck
Unlock for access to all 53 flashcards in this deck.
Unlock Deck
k this deck
44
Which compound would you predict to be highest in energy? 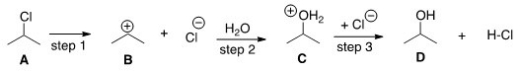
A)A
B)B
C)C
D)D

A)A
B)B
C)C
D)D

Unlock Deck
Unlock for access to all 53 flashcards in this deck.
Unlock Deck
k this deck
45
The conversion of acetyl chloride to methyl acetate occurs via the following two-step mechanism: ![<strong>The conversion of acetyl chloride to methyl acetate occurs via the following two-step mechanism: What is the rate equation for this reaction if the first step is rate determining?</strong> A)Rate = k [acetyl chloride] [<sup>-</sup>OCH<sub>3</sub>] B)Rate = k [acetyl chloride] C)Rate = k [<sup>-</sup>OCH<sub>3</sub>] D)Rate = k [acetyl chloride] [<sup>-</sup>OCH<sub>3</sub>]<sup>2</sup>](https://storage.examlex.com/TB7814/11eac680_9c17_cb9f_a448_7b5063864f4a_TB7814_00.jpg) What is the rate equation for this reaction if the first step is rate determining?
What is the rate equation for this reaction if the first step is rate determining?
A)Rate = k [acetyl chloride] [-OCH3]
B)Rate = k [acetyl chloride]
C)Rate = k [-OCH3]
D)Rate = k [acetyl chloride] [-OCH3]2
![<strong>The conversion of acetyl chloride to methyl acetate occurs via the following two-step mechanism: What is the rate equation for this reaction if the first step is rate determining?</strong> A)Rate = k [acetyl chloride] [<sup>-</sup>OCH<sub>3</sub>] B)Rate = k [acetyl chloride] C)Rate = k [<sup>-</sup>OCH<sub>3</sub>] D)Rate = k [acetyl chloride] [<sup>-</sup>OCH<sub>3</sub>]<sup>2</sup>](https://storage.examlex.com/TB7814/11eac680_9c17_cb9f_a448_7b5063864f4a_TB7814_00.jpg) What is the rate equation for this reaction if the first step is rate determining?
What is the rate equation for this reaction if the first step is rate determining?A)Rate = k [acetyl chloride] [-OCH3]
B)Rate = k [acetyl chloride]
C)Rate = k [-OCH3]
D)Rate = k [acetyl chloride] [-OCH3]2

Unlock Deck
Unlock for access to all 53 flashcards in this deck.
Unlock Deck
k this deck
46
For which of the following reactions is ΔS° a positive value? 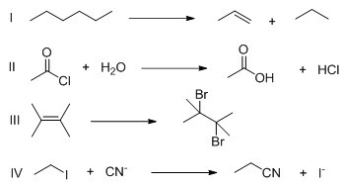
A)I
B)II
C)III
D)IV

A)I
B)II
C)III
D)IV

Unlock Deck
Unlock for access to all 53 flashcards in this deck.
Unlock Deck
k this deck
47
What type of reaction does the following conversion represent? 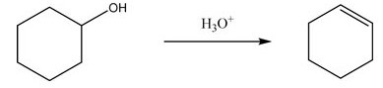
A)Addition reaction
B)Elimination reaction
C)Substitution reaction
D)Oxidation-reduction reaction

A)Addition reaction
B)Elimination reaction
C)Substitution reaction
D)Oxidation-reduction reaction

Unlock Deck
Unlock for access to all 53 flashcards in this deck.
Unlock Deck
k this deck
48
What type of bond cleavage takes place in/what type of intermediate is produced in the following reaction? 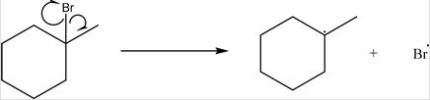
A)Homolysis/Radical
B)Homolysis/Carbocation
C)Heterolysis/Carbocation
D)Heterolysis/Carbanion

A)Homolysis/Radical
B)Homolysis/Carbocation
C)Heterolysis/Carbocation
D)Heterolysis/Carbanion

Unlock Deck
Unlock for access to all 53 flashcards in this deck.
Unlock Deck
k this deck
49
The conversion of acetyl chloride to methyl acetate occurs via the following two-step mechanism: 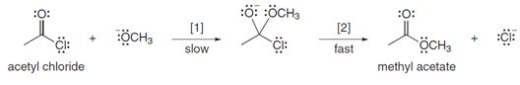 If the concentration of -OCH3 were increased 5 times,what would happen to the rate of the reaction?
If the concentration of -OCH3 were increased 5 times,what would happen to the rate of the reaction?
A)Rate would become one fifth
B)Rate would increase 25 times
C)Rate would increase 5 times
D)Rate would remain unchanged
 If the concentration of -OCH3 were increased 5 times,what would happen to the rate of the reaction?
If the concentration of -OCH3 were increased 5 times,what would happen to the rate of the reaction?A)Rate would become one fifth
B)Rate would increase 25 times
C)Rate would increase 5 times
D)Rate would remain unchanged

Unlock Deck
Unlock for access to all 53 flashcards in this deck.
Unlock Deck
k this deck
50
The conversion of acetyl chloride to methyl acetate occurs via the following two-step mechanism: 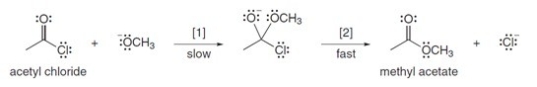 If the concentrations of both -OCH3 and acetyl chloride were increased 2 times,what would happen to the rate of the reaction?
If the concentrations of both -OCH3 and acetyl chloride were increased 2 times,what would happen to the rate of the reaction?
A)Rate would become one-fourth
B)Rate would increase 4 times
C)Rate would increase 16 times
D)Rate would increase 2 times
 If the concentrations of both -OCH3 and acetyl chloride were increased 2 times,what would happen to the rate of the reaction?
If the concentrations of both -OCH3 and acetyl chloride were increased 2 times,what would happen to the rate of the reaction?A)Rate would become one-fourth
B)Rate would increase 4 times
C)Rate would increase 16 times
D)Rate would increase 2 times

Unlock Deck
Unlock for access to all 53 flashcards in this deck.
Unlock Deck
k this deck
51
Which of the following statements about enzymes is true?
A)Enzymes increase the activation energy for a reaction.
B)Enzymes decrease the equilibrium constant.
C)Enzymes shift the equilibrium to favor the product.
D)Enzymes lower the transition state for the rate-determining step.
A)Enzymes increase the activation energy for a reaction.
B)Enzymes decrease the equilibrium constant.
C)Enzymes shift the equilibrium to favor the product.
D)Enzymes lower the transition state for the rate-determining step.

Unlock Deck
Unlock for access to all 53 flashcards in this deck.
Unlock Deck
k this deck
52
What type of bond cleavage takes place in/what type of intermediate is produced in the following reaction? 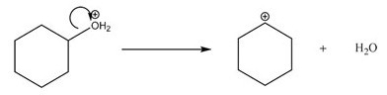
A)Homolysis/Radical
B)Homolysis/Carbocation
C)Heterolysis/Carbocation
D)Heterolysis/Carbanion

A)Homolysis/Radical
B)Homolysis/Carbocation
C)Heterolysis/Carbocation
D)Heterolysis/Carbanion

Unlock Deck
Unlock for access to all 53 flashcards in this deck.
Unlock Deck
k this deck
53
The symbol hν stands for ________ in a chemical reaction.
A)light
B)heat
C)reactant
D)product
A)light
B)heat
C)reactant
D)product

Unlock Deck
Unlock for access to all 53 flashcards in this deck.
Unlock Deck
k this deck


