Deck 3: Introduction to Organic Molecules and Functional Groups
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/56
Play
Full screen (f)
Deck 3: Introduction to Organic Molecules and Functional Groups
1
Why do heteroatoms confer reactivity on a particular molecule?
A)Because they have lone pairs and create electron-rich sites on carbon.
B)Because they have lone pairs and create electron-deficient sites on carbon.
C)Because they are electronegative and act as electrophiles.
D)Because they are electropositive and act as nucleophiles.
A)Because they have lone pairs and create electron-rich sites on carbon.
B)Because they have lone pairs and create electron-deficient sites on carbon.
C)Because they are electronegative and act as electrophiles.
D)Because they are electropositive and act as nucleophiles.
Because they have lone pairs and create electron-deficient sites on carbon.
2
Which of the following correctly matches the molecules to the names of the functional group? I. CH3OCH3 Ether
II) CH3CONH2 Amine
III)CH3SH Thiol
IV)CH3CHO Alcohol
A)I and II
B)II and III
C)III and IV
D)I and III
II) CH3CONH2 Amine
III)CH3SH Thiol
IV)CH3CHO Alcohol
A)I and II
B)II and III
C)III and IV
D)I and III
I and III
3
Which of the following molecules are aliphatic hydrocarbons? 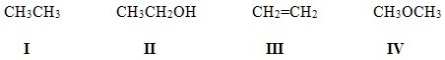
A)I,II,III
B)I and III
C)II,III,IV
D)II and IV
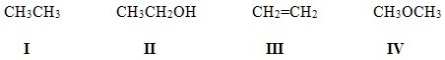
A)I,II,III
B)I and III
C)II,III,IV
D)II and IV
I and III
4
Which of the following structures contains a primary amine? 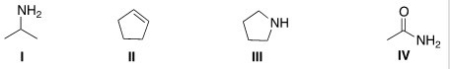
A)I
B)II
C)III
D)IV

A)I
B)II
C)III
D)IV

Unlock Deck
Unlock for access to all 56 flashcards in this deck.
Unlock Deck
k this deck
5
Which of the following molecules contain the same functional groups? 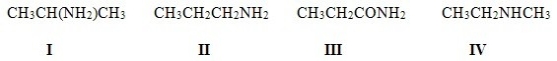
A)I,II,IV
B)I,II,III
C)II,III,IV
D)I,III,IV
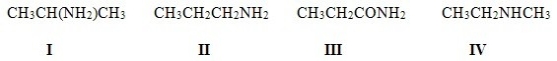
A)I,II,IV
B)I,II,III
C)II,III,IV
D)I,III,IV

Unlock Deck
Unlock for access to all 56 flashcards in this deck.
Unlock Deck
k this deck
6
Why do π bonds confer reactivity on a particular molecule?
A)Because π bonds are difficult to break in chemical reactions.
B)Because π bonds make a molecule an acid.
C)Because π bonds are easily broken in chemical reactions.
D)Because π bonds make a molecule an electrophile.
A)Because π bonds are difficult to break in chemical reactions.
B)Because π bonds make a molecule an acid.
C)Because π bonds are easily broken in chemical reactions.
D)Because π bonds make a molecule an electrophile.

Unlock Deck
Unlock for access to all 56 flashcards in this deck.
Unlock Deck
k this deck
7
Which of the following structures contains an amide? 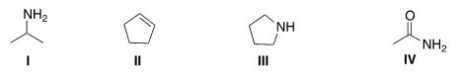
A)I
B)II
C)III
D)IV

A)I
B)II
C)III
D)IV

Unlock Deck
Unlock for access to all 56 flashcards in this deck.
Unlock Deck
k this deck
8
Which of the following correctly matches the molecules to the names of the functional group? I. CH3NH2 Amide
II) CH3SCH3 Sulfide
III)CH3CONH2 Amine
IV)CH3CO2CH3 Ester
A)I and II
B)II and IV
C)III and IV
D)II and III
II) CH3SCH3 Sulfide
III)CH3CONH2 Amine
IV)CH3CO2CH3 Ester
A)I and II
B)II and IV
C)III and IV
D)II and III

Unlock Deck
Unlock for access to all 56 flashcards in this deck.
Unlock Deck
k this deck
9
Which of the following structures contains an alkene? 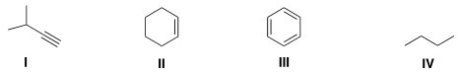
A)I
B)II
C)III
D)IV
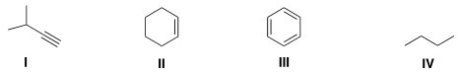
A)I
B)II
C)III
D)IV

Unlock Deck
Unlock for access to all 56 flashcards in this deck.
Unlock Deck
k this deck
10
Consider the molecule donepezil (used to treat Alzheimer's disease).Which of the following lists the correct functional groups present in donepezil? 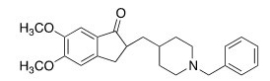
A)Amide,aromatic,ether,ketone
B)Amide,aromatic,ester,ketone
C)Amine,aromatic,ester,ketone
D)Amine,aromatic,ether,ketone

A)Amide,aromatic,ether,ketone
B)Amide,aromatic,ester,ketone
C)Amine,aromatic,ester,ketone
D)Amine,aromatic,ether,ketone

Unlock Deck
Unlock for access to all 56 flashcards in this deck.
Unlock Deck
k this deck
11
Which of the following lists contains common heteroatoms found in organic molecules?
A)N,O,S,P,Cl
B)Na,O,S,P,Cl
C)Na,Mg,S,N,Cl
D)Na,Mg,O,N,Cl
A)N,O,S,P,Cl
B)Na,O,S,P,Cl
C)Na,Mg,S,N,Cl
D)Na,Mg,O,N,Cl

Unlock Deck
Unlock for access to all 56 flashcards in this deck.
Unlock Deck
k this deck
12
Which of the following molecules are aromatic hydrocarbons? 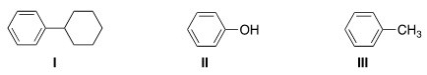
A)I
B)II
C)III
D)I and III

A)I
B)II
C)III
D)I and III

Unlock Deck
Unlock for access to all 56 flashcards in this deck.
Unlock Deck
k this deck
13
Which of the following statements best describes the relationship between the surface area of a molecule and the strength of the intermolecular forces?
A)The larger the surface area,the weaker the intermolecular forces.
B)The larger the surface area,the stronger the intermolecular forces.
C)The smaller the surface area,the stronger the intermolecular forces.
D)There is no relationship between surface area and intermolecular forces.
A)The larger the surface area,the weaker the intermolecular forces.
B)The larger the surface area,the stronger the intermolecular forces.
C)The smaller the surface area,the stronger the intermolecular forces.
D)There is no relationship between surface area and intermolecular forces.

Unlock Deck
Unlock for access to all 56 flashcards in this deck.
Unlock Deck
k this deck
14
Rank the following compounds in order of increasing strength of intermolecular forces,putting the molecule with the weakest intermolecular force first. 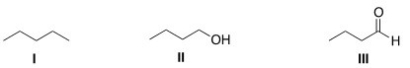
A)I < II < III
B)II < I < III
C)I < III < II
D)II < III < I

A)I < II < III
B)II < I < III
C)I < III < II
D)II < III < I

Unlock Deck
Unlock for access to all 56 flashcards in this deck.
Unlock Deck
k this deck
15
Which of the following is a tertiary amine? 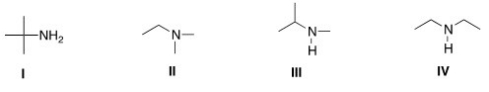
A)I
B)II
C)III
D)IV
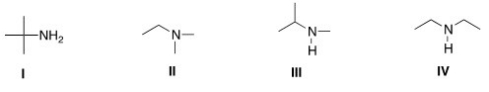
A)I
B)II
C)III
D)IV

Unlock Deck
Unlock for access to all 56 flashcards in this deck.
Unlock Deck
k this deck
16
Consider the molecule atenolol (a β blocker used to treat hypertension).Which of the following lists the correct functional groups present in atenolol? 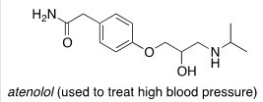
A)Primary alcohol,amide,primary amine,aromatic,ether
B)Secondary alcohol,amide,secondary amine,aromatic,ether
C)Secondary alcohol,amide,primary amine,aromatic,ether
D)Secondary alcohol,amide,secondary amine,aromatic,ester

A)Primary alcohol,amide,primary amine,aromatic,ether
B)Secondary alcohol,amide,secondary amine,aromatic,ether
C)Secondary alcohol,amide,primary amine,aromatic,ether
D)Secondary alcohol,amide,secondary amine,aromatic,ester

Unlock Deck
Unlock for access to all 56 flashcards in this deck.
Unlock Deck
k this deck
17
Which of the following structures contains a secondary amine? 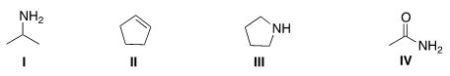
A)I
B)II
C)III
D)IV

A)I
B)II
C)III
D)IV

Unlock Deck
Unlock for access to all 56 flashcards in this deck.
Unlock Deck
k this deck
18
Which of the following molecules contain the same functional groups? 
A)I,II,III
B)I,II,IV
C)II,III,IV
D)I,III,IV

A)I,II,III
B)I,II,IV
C)II,III,IV
D)I,III,IV

Unlock Deck
Unlock for access to all 56 flashcards in this deck.
Unlock Deck
k this deck
19
Which of the following correctly matches the molecules to the names of the functional group? I. CH3OH Carboxylic acid
II) CH3CO2CH3 Ester
III) CH3COCH3 Ketone
IV) H2CO Alcohol
A)I and II
B)III and IV
C)II and III
D)II and IV
II) CH3CO2CH3 Ester
III) CH3COCH3 Ketone
IV) H2CO Alcohol
A)I and II
B)III and IV
C)II and III
D)II and IV

Unlock Deck
Unlock for access to all 56 flashcards in this deck.
Unlock Deck
k this deck
20
Which of the following is a secondary alcohol? 
A)I
B)II
C)III
D)IV

A)I
B)II
C)III
D)IV

Unlock Deck
Unlock for access to all 56 flashcards in this deck.
Unlock Deck
k this deck
21
Which of the following compounds can form intermolecular hydrogen bonds with a molecule similar to itself? 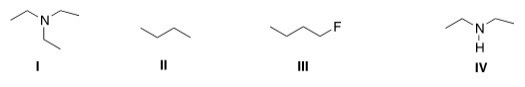
A)I
B)II
C)III
D)IV

A)I
B)II
C)III
D)IV

Unlock Deck
Unlock for access to all 56 flashcards in this deck.
Unlock Deck
k this deck
22
Which of the following compounds is expected to be H2O soluble? 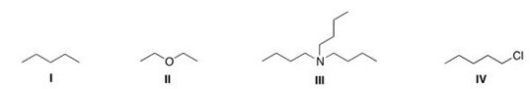
A)I
B)II
C)III
D)IV

A)I
B)II
C)III
D)IV

Unlock Deck
Unlock for access to all 56 flashcards in this deck.
Unlock Deck
k this deck
23
Rank the following compounds in order of increasing melting point,putting the compound with the least melting point first. 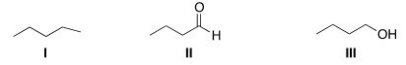
A)II < I < III
B)I < III < II
C)I < II < III
D)III < II < I

A)II < I < III
B)I < III < II
C)I < II < III
D)III < II < I

Unlock Deck
Unlock for access to all 56 flashcards in this deck.
Unlock Deck
k this deck
24
Which of the following compounds has the highest boiling point? 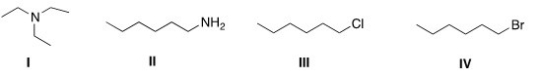
A)I
B)II
C)III
D)IV

A)I
B)II
C)III
D)IV

Unlock Deck
Unlock for access to all 56 flashcards in this deck.
Unlock Deck
k this deck
25
Rank the following compounds in order of decreasing boiling point,putting the compound with the highest boiling point first. 
A)I > II > III > IV
B)III > IV > II > I
C)III > II > IV > I
D)I > IV > II > III

A)I > II > III > IV
B)III > IV > II > I
C)III > II > IV > I
D)I > IV > II > III

Unlock Deck
Unlock for access to all 56 flashcards in this deck.
Unlock Deck
k this deck
26
Which of the following compounds has the highest boiling point? 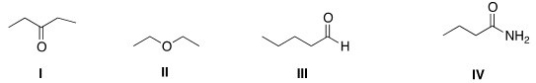
A)I
B)II
C)III
D)IV

A)I
B)II
C)III
D)IV

Unlock Deck
Unlock for access to all 56 flashcards in this deck.
Unlock Deck
k this deck
27
What molecular features are required for soap to properly dissolve grease and oil?
A)The molecule must be large.
B)The molecule must contain a polar head.
C)The molecule must contain a non-polar tail.
D)The molecule must contain both a polar head and a non-polar tail.
A)The molecule must be large.
B)The molecule must contain a polar head.
C)The molecule must contain a non-polar tail.
D)The molecule must contain both a polar head and a non-polar tail.

Unlock Deck
Unlock for access to all 56 flashcards in this deck.
Unlock Deck
k this deck
28
Which of the following alkanes is expected to have the highest melting point? 
A)I
B)II
C)III
D)IV

A)I
B)II
C)III
D)IV

Unlock Deck
Unlock for access to all 56 flashcards in this deck.
Unlock Deck
k this deck
29
Which of the following compounds has the lowest boiling point? 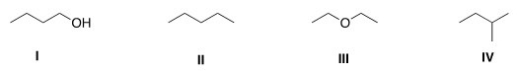
A)I
B)II
C)III
D)IV

A)I
B)II
C)III
D)IV

Unlock Deck
Unlock for access to all 56 flashcards in this deck.
Unlock Deck
k this deck
30
What is the strongest intermolecular force present in 1-propanol? 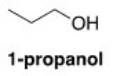
A)Ion-ion
B)Hydrogen bonding
C)Dipole-dipole
D)Induced dipole-induced dipole

A)Ion-ion
B)Hydrogen bonding
C)Dipole-dipole
D)Induced dipole-induced dipole

Unlock Deck
Unlock for access to all 56 flashcards in this deck.
Unlock Deck
k this deck
31
What intermolecular force is generally considered the strongest?
A)Hydrogen bonding
B)London dispersion forces
C)Covalent bonds
D)Dipole-dipole
A)Hydrogen bonding
B)London dispersion forces
C)Covalent bonds
D)Dipole-dipole

Unlock Deck
Unlock for access to all 56 flashcards in this deck.
Unlock Deck
k this deck
32
Which of the following statements about the solubility of organic compounds in H2O is true?
A)The non-polar part of a molecule that is not attracted to water is said to be hydrophilic.
B)The non-polar part of a molecule that is not attracted to water is said to be hydrophobic.
C)The polar part of a molecule that can that can bond hydrogen to water is said to be hydrophobic.
D)For an organic compound with one functional group that contains an O or N atom,the compound is water soluble only if it has 35 carbons.
A)The non-polar part of a molecule that is not attracted to water is said to be hydrophilic.
B)The non-polar part of a molecule that is not attracted to water is said to be hydrophobic.
C)The polar part of a molecule that can that can bond hydrogen to water is said to be hydrophobic.
D)For an organic compound with one functional group that contains an O or N atom,the compound is water soluble only if it has 35 carbons.

Unlock Deck
Unlock for access to all 56 flashcards in this deck.
Unlock Deck
k this deck
33
Which of the following compounds is expected to be the most soluble in H2O? 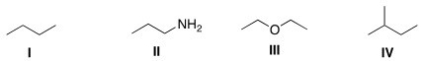
A)I
B)II
C)III
D)IV

A)I
B)II
C)III
D)IV

Unlock Deck
Unlock for access to all 56 flashcards in this deck.
Unlock Deck
k this deck
34
Which of the following compounds has the highest boiling point? 
A)I
B)II
C)III
D)IV

A)I
B)II
C)III
D)IV

Unlock Deck
Unlock for access to all 56 flashcards in this deck.
Unlock Deck
k this deck
35
Which of the following intermolecular forces would not form between similar molecules of the structure below? 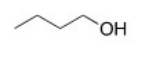
A)London dispersion forces
B)Ion-ion
C)Hydrogen bonding
D)Dipole-dipole

A)London dispersion forces
B)Ion-ion
C)Hydrogen bonding
D)Dipole-dipole

Unlock Deck
Unlock for access to all 56 flashcards in this deck.
Unlock Deck
k this deck
36
Which of the following compounds is expected to be the least soluble in H2O? 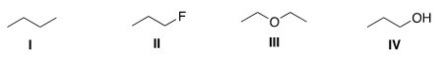
A)I
B)II
C)III
D)IV
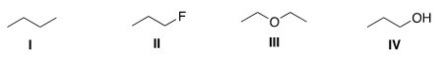
A)I
B)II
C)III
D)IV

Unlock Deck
Unlock for access to all 56 flashcards in this deck.
Unlock Deck
k this deck
37
Rank the following compounds in order of decreasing melting point,putting the compound with the highest melting point first. 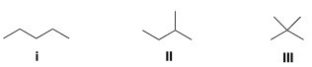
A)I > II > III
B)II > III > I
C)III > II > I
D)III > I > II

A)I > II > III
B)II > III > I
C)III > II > I
D)III > I > II

Unlock Deck
Unlock for access to all 56 flashcards in this deck.
Unlock Deck
k this deck
38
Which of the following compounds has the highest boiling point? 
A)I
B)II
C)III
D)IV

A)I
B)II
C)III
D)IV

Unlock Deck
Unlock for access to all 56 flashcards in this deck.
Unlock Deck
k this deck
39
What intermolecular force is generally considered the weakest?
A)Hydrogen bonding
B)London dispersion forces
C)Dipole-dipole
D)Ion-ion
A)Hydrogen bonding
B)London dispersion forces
C)Dipole-dipole
D)Ion-ion

Unlock Deck
Unlock for access to all 56 flashcards in this deck.
Unlock Deck
k this deck
40
Which of the following compounds would be expected to be more soluble in hexane (C6H14)? 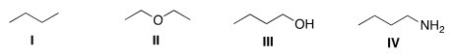
A)I
B)II
C)III
D)IV

A)I
B)II
C)III
D)IV

Unlock Deck
Unlock for access to all 56 flashcards in this deck.
Unlock Deck
k this deck
41
List the intermolecular forces present in the following molecule: 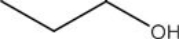
A)Van der Waals
B)Dipole-dipole interactions
C)Hydrogen bonding
D)More than one of these answer choices is correct.

A)Van der Waals
B)Dipole-dipole interactions
C)Hydrogen bonding
D)More than one of these answer choices is correct.

Unlock Deck
Unlock for access to all 56 flashcards in this deck.
Unlock Deck
k this deck
42
Rank the following compounds in order of increasing strength of intermolecular forces. 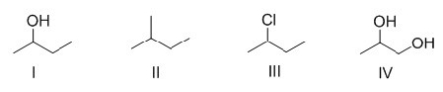
A)I > III > IV > II
B)IV > II > I > III
C)IV > I > III > II
D)I > IV > II > III

A)I > III > IV > II
B)IV > II > I > III
C)IV > I > III > II
D)I > IV > II > III

Unlock Deck
Unlock for access to all 56 flashcards in this deck.
Unlock Deck
k this deck
43
Which of the following molecules can bond hydrogen to another molecule of itself? 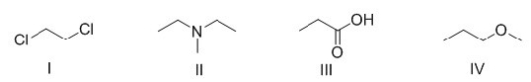
A)I
B)II
C)III
D)IV

A)I
B)II
C)III
D)IV

Unlock Deck
Unlock for access to all 56 flashcards in this deck.
Unlock Deck
k this deck
44
List the intermolecular forces present in the following molecule: 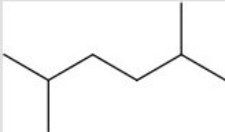
A)Van der Waals
B)Dipole-dipole interactions
C)Hydrogen bonding
D)More than one of these answer choices is correct.

A)Van der Waals
B)Dipole-dipole interactions
C)Hydrogen bonding
D)More than one of these answer choices is correct.

Unlock Deck
Unlock for access to all 56 flashcards in this deck.
Unlock Deck
k this deck
45
The indicated bond is: 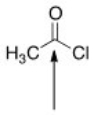
A)Nucleophilic because it is electron-deficient.
B)Electrophilic because it is electron-rich.
C)Nucleophilic because it is electron-rich.
D)Electrophilic because it is electron-deficient.

A)Nucleophilic because it is electron-deficient.
B)Electrophilic because it is electron-rich.
C)Nucleophilic because it is electron-rich.
D)Electrophilic because it is electron-deficient.

Unlock Deck
Unlock for access to all 56 flashcards in this deck.
Unlock Deck
k this deck
46
Which of the following could most likely serve as an ionophore? 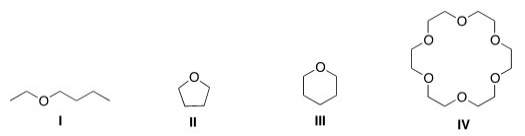
A)I
B)II
C)III
D)IV

A)I
B)II
C)III
D)IV

Unlock Deck
Unlock for access to all 56 flashcards in this deck.
Unlock Deck
k this deck
47
List the intermolecular forces present in the following molecule: 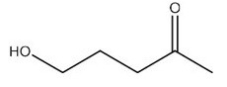
A)Van der Waals
B)Dipole-dipole interactions
C)Hydrogen bonding
D)More than one of these answer choices is correct.

A)Van der Waals
B)Dipole-dipole interactions
C)Hydrogen bonding
D)More than one of these answer choices is correct.

Unlock Deck
Unlock for access to all 56 flashcards in this deck.
Unlock Deck
k this deck
48
Which C=O functional group is present in the following molecule? 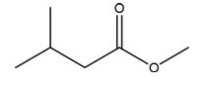
A)Carboxylic acid
B)Ester
C)Ketone
D)Aldehyde

A)Carboxylic acid
B)Ester
C)Ketone
D)Aldehyde

Unlock Deck
Unlock for access to all 56 flashcards in this deck.
Unlock Deck
k this deck
49
Which C=O functional group is present in the following molecule? 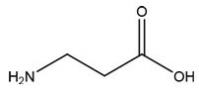
A)Carboxylic Acid
B)Ester
C)Ketone
D)Aldehyde

A)Carboxylic Acid
B)Ester
C)Ketone
D)Aldehyde

Unlock Deck
Unlock for access to all 56 flashcards in this deck.
Unlock Deck
k this deck
50
Rank the following compounds in order of decreasing boiling point,putting the compound with the highest boiling point first. 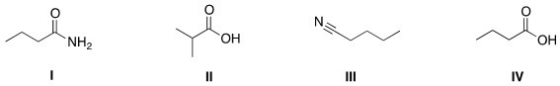
A)I > III > IV > II
B)IV > II > I > III
C)IV > I > II > III
D)I > IV > II > III

A)I > III > IV > II
B)IV > II > I > III
C)IV > I > II > III
D)I > IV > II > III

Unlock Deck
Unlock for access to all 56 flashcards in this deck.
Unlock Deck
k this deck
51
Which of the following statements about vitamin C,drawn below,is true? 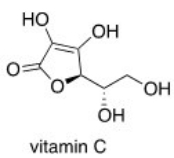
A)Vitamin C is insoluble in H2O.
B)Vitamin C is soluble in H2O.
C)Vitamin C is an aliphatic hydrocarbon.
D)Vitamin C contains a ketone functional group.

A)Vitamin C is insoluble in H2O.
B)Vitamin C is soluble in H2O.
C)Vitamin C is an aliphatic hydrocarbon.
D)Vitamin C contains a ketone functional group.

Unlock Deck
Unlock for access to all 56 flashcards in this deck.
Unlock Deck
k this deck
52
The indicated bond is: 
A)Nucleophilic because it is electron-deficient.
B)Electrophilic because it is electron-deficient.
C)Nucleophilic because it electron-rich.
D)Electrophilic because it is electron-rich.

A)Nucleophilic because it is electron-deficient.
B)Electrophilic because it is electron-deficient.
C)Nucleophilic because it electron-rich.
D)Electrophilic because it is electron-rich.

Unlock Deck
Unlock for access to all 56 flashcards in this deck.
Unlock Deck
k this deck
53
Which of the following statements about vitamin A,drawn below,is true? 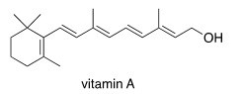
A)Vitamin A is soluble in H2O.
B)Vitamin A is insoluble in organic solvents.
C)Vitamin A contains an aromatic ring.
D)Vitamin A is insoluble in H2O.

A)Vitamin A is soluble in H2O.
B)Vitamin A is insoluble in organic solvents.
C)Vitamin A contains an aromatic ring.
D)Vitamin A is insoluble in H2O.

Unlock Deck
Unlock for access to all 56 flashcards in this deck.
Unlock Deck
k this deck
54
Which C=O functional group is present in the following molecule? 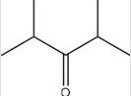
A)Carboxylic acid
B)Ester
C)Ketone
D)Aldehyde

A)Carboxylic acid
B)Ester
C)Ketone
D)Aldehyde

Unlock Deck
Unlock for access to all 56 flashcards in this deck.
Unlock Deck
k this deck
55
The indicated carbon atom is: 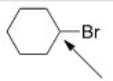
A)Electrophilic because it is electron-deficient.
B)Nucleophilic because it is electron-deficient.
C)Electrophilic because it is electron-rich.
D)Nucleophilic because it is electron-rich.

A)Electrophilic because it is electron-deficient.
B)Nucleophilic because it is electron-deficient.
C)Electrophilic because it is electron-rich.
D)Nucleophilic because it is electron-rich.

Unlock Deck
Unlock for access to all 56 flashcards in this deck.
Unlock Deck
k this deck
56
Which of the following lists the correct functional groups found in aspartame,the artificial sweetener? 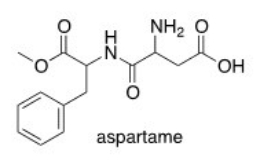
A)Amine,aromatic,carboxylic acid,ether,ketone
B)Amine,amide,aromatic,carboxylic acid,ester
C)Amide,alcohol,aromatic,carboxylic acid,ether
D)Amine,aromatic,carboxylic acid,ester,nitrile

A)Amine,aromatic,carboxylic acid,ether,ketone
B)Amine,amide,aromatic,carboxylic acid,ester
C)Amide,alcohol,aromatic,carboxylic acid,ether
D)Amine,aromatic,carboxylic acid,ester,nitrile

Unlock Deck
Unlock for access to all 56 flashcards in this deck.
Unlock Deck
k this deck


