Deck 11: Theories of Covalent Bonding
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/48
Play
Full screen (f)
Deck 11: Theories of Covalent Bonding
1
Valence bond theory predicts that sulfur will use _____ hybrid orbitals in sulfur dioxide, SO2.
A) sp
B) sp2
C) sp3
D) sp3d
E) sp3d2
A) sp
B) sp2
C) sp3
D) sp3d
E) sp3d2
sp2
2
A molecule with the formula AX4 uses _________ to form its bonds.
A) sp hybrid orbitals
B) sp2 hybrid orbitals
C) sp3 hybrid orbitals
D) sp3d hybrid orbitals
E) sp3d2 hybrid orbitals
A) sp hybrid orbitals
B) sp2 hybrid orbitals
C) sp3 hybrid orbitals
D) sp3d hybrid orbitals
E) sp3d2 hybrid orbitals
sp3 hybrid orbitals
3
According to valence bond theory, which orbitals on N and H overlap in the NH3 molecule?
A) 2p on N overlaps with 2s on H
B) 2p on N overlaps with 1s on H
C) 2s on N overlaps with 1s on H
D) sp3 on N overlaps with sp on H
E) sp3 on N overlaps with 1s on H
A) 2p on N overlaps with 2s on H
B) 2p on N overlaps with 1s on H
C) 2s on N overlaps with 1s on H
D) sp3 on N overlaps with sp on H
E) sp3 on N overlaps with 1s on H
sp3 on N overlaps with 1s on H
4
A molecule with the formula AX3 uses __________ to form its bonds.
A) sp hybrid orbitals
B) sp2 hybrid orbitals
C) sp3 hybrid orbitals
D) sp3d hybrid orbitals
E) sp3d2 hybrid orbitals
A) sp hybrid orbitals
B) sp2 hybrid orbitals
C) sp3 hybrid orbitals
D) sp3d hybrid orbitals
E) sp3d2 hybrid orbitals

Unlock Deck
Unlock for access to all 48 flashcards in this deck.
Unlock Deck
k this deck
5
Carbon uses ______ hybrid orbitals in ClCN.
A) sp
B) sp2
C) sp3
D) sp3d
E) sp3d2
A) sp
B) sp2
C) sp3
D) sp3d
E) sp3d2

Unlock Deck
Unlock for access to all 48 flashcards in this deck.
Unlock Deck
k this deck
6
Valence bond theory predicts that carbon will use _____ hybrid orbitals in the carbonate anion, CO3-.
A) sp
B) sp2
C) sp3
D) sp3d
E) sp3d2
A) sp
B) sp2
C) sp3
D) sp3d
E) sp3d2

Unlock Deck
Unlock for access to all 48 flashcards in this deck.
Unlock Deck
k this deck
7
According to valence bond theory, which of the following molecules involves sp2 hybridization of orbitals on the central atom? (central atom is bold)
A) C2H2
B) C2H4
C) C2H6
D) CO2
E) H2O
A) C2H2
B) C2H4
C) C2H6
D) CO2
E) H2O

Unlock Deck
Unlock for access to all 48 flashcards in this deck.
Unlock Deck
k this deck
8
For which one of the following molecules is the indicated type of hybridization not appropriate for the central atom?
A) BeCl2 sp2
B) SiH4 sp3
C) BF3 sp2
D) C2H2 sp
E) H2O sp3
A) BeCl2 sp2
B) SiH4 sp3
C) BF3 sp2
D) C2H2 sp
E) H2O sp3

Unlock Deck
Unlock for access to all 48 flashcards in this deck.
Unlock Deck
k this deck
9
A molecule with the formula AX4E2 uses _________ to form its bonds.
A) sp hybrid orbitals
B) sp2 hybrid orbitals
C) sp3 hybrid orbitals
D) sp3d hybrid orbitals
E) sp3d2 hybrid orbitals
A) sp hybrid orbitals
B) sp2 hybrid orbitals
C) sp3 hybrid orbitals
D) sp3d hybrid orbitals
E) sp3d2 hybrid orbitals

Unlock Deck
Unlock for access to all 48 flashcards in this deck.
Unlock Deck
k this deck
10
A molecule with the formula AX2 uses _________ to form its bonds.
A) sp hybrid orbitals
B) sp2 hybrid orbitals
C) sp3 hybrid orbitals
D) sp3d hybrid orbitals
E) sp3d2 hybrid orbitals
A) sp hybrid orbitals
B) sp2 hybrid orbitals
C) sp3 hybrid orbitals
D) sp3d hybrid orbitals
E) sp3d2 hybrid orbitals

Unlock Deck
Unlock for access to all 48 flashcards in this deck.
Unlock Deck
k this deck
11
Valence bond theory predicts that bromine will use _____ hybrid orbitals in BrF3.
A) sp2
B) sp3
C) sp3d
D) sp4
E) sp3d2
A) sp2
B) sp3
C) sp3d
D) sp4
E) sp3d2

Unlock Deck
Unlock for access to all 48 flashcards in this deck.
Unlock Deck
k this deck
12
Valence bond theory predicts that tin will use _____ hybrid orbitals in SnCl3-.
A) sp
B) sp2
C) sp3
D) sp3d
E) sp3d2
A) sp
B) sp2
C) sp3
D) sp3d
E) sp3d2

Unlock Deck
Unlock for access to all 48 flashcards in this deck.
Unlock Deck
k this deck
13
Valence bond theory predicts that tin will use _____ hybrid orbitals in SnF5-.
A) sp2
B) sp3
C) sp3d
D) sp3d2
E) d2sp3
A) sp2
B) sp3
C) sp3d
D) sp3d2
E) d2sp3

Unlock Deck
Unlock for access to all 48 flashcards in this deck.
Unlock Deck
k this deck
14
A molecule with the formula AX3E uses _________ to form its bonds.
A) s and p atomic orbitals
B) sp3 hybrid orbitals
C) sp2 hybrid orbitals
D) sp hybrid orbitals
E) sp3d2 hybrid orbitals
A) s and p atomic orbitals
B) sp3 hybrid orbitals
C) sp2 hybrid orbitals
D) sp hybrid orbitals
E) sp3d2 hybrid orbitals

Unlock Deck
Unlock for access to all 48 flashcards in this deck.
Unlock Deck
k this deck
15
Valence bond theory predicts that xenon will use _____ hybrid orbitals in XeOF4.
A) sp
B) sp2
C) sp3
D) sp3d
E) sp3d2
A) sp
B) sp2
C) sp3
D) sp3d
E) sp3d2

Unlock Deck
Unlock for access to all 48 flashcards in this deck.
Unlock Deck
k this deck
16
Which one of the following statements about orbital hybridization is incorrect?
A) The carbon atom in CH4 is sp3 hybridized.
B) The carbon atom in CO2 is sp hybridized.
C) The nitrogen atom in NH3 is sp2 hybridized.
D) sp2 hybrid orbitals are coplanar, and at 120° to each other.
E) sp hybrid orbitals lie at 180° to each other.
A) The carbon atom in CH4 is sp3 hybridized.
B) The carbon atom in CO2 is sp hybridized.
C) The nitrogen atom in NH3 is sp2 hybridized.
D) sp2 hybrid orbitals are coplanar, and at 120° to each other.
E) sp hybrid orbitals lie at 180° to each other.

Unlock Deck
Unlock for access to all 48 flashcards in this deck.
Unlock Deck
k this deck
17
A molecule with the formula AX4E uses _________ to form its bonds.
A) sp2 hybrid orbitals
B) sp3 hybrid orbitals
C) sp3d hybrid orbitals
D) sp3d2 hybrid orbitals
E) None of these choices are correct.
A) sp2 hybrid orbitals
B) sp3 hybrid orbitals
C) sp3d hybrid orbitals
D) sp3d2 hybrid orbitals
E) None of these choices are correct.

Unlock Deck
Unlock for access to all 48 flashcards in this deck.
Unlock Deck
k this deck
18
Valence bond theory predicts that bromine will use _____ hybrid orbitals in BrF5.
A) sp2
B) sp3
C) sp3d
D) sp3d2
E) None of these choices are correct.
A) sp2
B) sp3
C) sp3d
D) sp3d2
E) None of these choices are correct.

Unlock Deck
Unlock for access to all 48 flashcards in this deck.
Unlock Deck
k this deck
19
Valence bond theory predicts that iodine will use _____ hybrid orbitals in ICl2-.
A) sp2
B) sp3
C) sp3d
D) sp3d2
E) None of these choices are correct.
A) sp2
B) sp3
C) sp3d
D) sp3d2
E) None of these choices are correct.

Unlock Deck
Unlock for access to all 48 flashcards in this deck.
Unlock Deck
k this deck
20
When PCl5 solidifies it forms PCl4+ cations and PCl6-anions. According to valence bond theory, what hybrid orbitals are used by phosphorus in the PCl4+ cations?
A) sp
B) sp2
C) sp3
D) sp3d
E) sp3d2
A) sp
B) sp2
C) sp3
D) sp3d
E) sp3d2

Unlock Deck
Unlock for access to all 48 flashcards in this deck.
Unlock Deck
k this deck
21
According to valence bond theory, the triple bond in ethyne (acetylene, C2H2) consists of
A) three σ bonds and no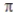 bonds.
bonds.
B) two σ bonds and one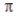 bond.
bond.
C) one σ bond and two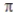 bonds.
bonds.
D) no σ bonds and three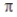 bonds.
bonds.
E) None of these choices are correct.
A) three σ bonds and no
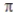 bonds.
bonds.B) two σ bonds and one
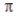 bond.
bond.C) one σ bond and two
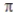 bonds.
bonds.D) no σ bonds and three
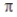 bonds.
bonds.E) None of these choices are correct.

Unlock Deck
Unlock for access to all 48 flashcards in this deck.
Unlock Deck
k this deck
22
According to molecular orbital theory, what is the bond order in the O2+ ion?
A) 5.5
B) 5
C) 4
D) 2.5
E) 1.5
A) 5.5
B) 5
C) 4
D) 2.5
E) 1.5

Unlock Deck
Unlock for access to all 48 flashcards in this deck.
Unlock Deck
k this deck
23
Determine the shape (geometry) of PCl3 and then decide on the appropriate hybridization of phosphorus in this molecule. (Phosphorus is the central atom.)
A) sp3
B) sp2
C) sp
D) sp3d
E) sp3d2
A) sp3
B) sp2
C) sp
D) sp3d
E) sp3d2

Unlock Deck
Unlock for access to all 48 flashcards in this deck.
Unlock Deck
k this deck
24
Overlap of two sp2 hybrid orbitals produces a 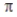 bond.
bond.
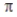 bond.
bond.
Unlock Deck
Unlock for access to all 48 flashcards in this deck.
Unlock Deck
k this deck
25
The angles between sp2 hybrid orbitals are 109.5°.

Unlock Deck
Unlock for access to all 48 flashcards in this deck.
Unlock Deck
k this deck
26
Which of the following statements relating to molecular orbital (MO) theory is incorrect?
A) Combination of two atomic orbitals produces one bonding and one antibonding MO.
B) A bonding MO is lower in energy than the two atomic orbitals from which it is formed.
C) Combination of two 2p orbitals may result in either σ or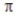 MOs.
MOs.
D) A species with a bond order of zero will not be stable.
E) In a stable molecule having an even number of electrons, all electrons must be paired.
A) Combination of two atomic orbitals produces one bonding and one antibonding MO.
B) A bonding MO is lower in energy than the two atomic orbitals from which it is formed.
C) Combination of two 2p orbitals may result in either σ or
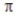 MOs.
MOs.D) A species with a bond order of zero will not be stable.
E) In a stable molecule having an even number of electrons, all electrons must be paired.

Unlock Deck
Unlock for access to all 48 flashcards in this deck.
Unlock Deck
k this deck
27
The nitrosonium ion, NO+, forms a number of interesting complexes with nickel, cobalt, and iron. According to molecular orbital theory, which of the following statements about NO+ is correct?
A) NO+ has a bond order of 2 and is paramagnetic.
B) NO+ has a bond order of 2 and is diamagnetic.
C) NO+ has a bond order of 3 and is paramagnetic.
D) NO+ has a bond order of 3 and is diamagnetic.
E) None of these choices are correct.
A) NO+ has a bond order of 2 and is paramagnetic.
B) NO+ has a bond order of 2 and is diamagnetic.
C) NO+ has a bond order of 3 and is paramagnetic.
D) NO+ has a bond order of 3 and is diamagnetic.
E) None of these choices are correct.

Unlock Deck
Unlock for access to all 48 flashcards in this deck.
Unlock Deck
k this deck
28
Hybrid orbitals of the sp3d type occur in sets of four.

Unlock Deck
Unlock for access to all 48 flashcards in this deck.
Unlock Deck
k this deck
29
According to valence bond theory, overlap of bonding orbitals of atoms will weaken a bond, due to electron-electron repulsion.

Unlock Deck
Unlock for access to all 48 flashcards in this deck.
Unlock Deck
k this deck
30
In the valence bond treatment, overlap of an s orbital on one atom with an sp3 orbital on another atom can give rise to a σ bond.

Unlock Deck
Unlock for access to all 48 flashcards in this deck.
Unlock Deck
k this deck
31
Which one, if any, of the following statements about the MO treatment of the bonding in benzene is correct?
A) MO theory uses resonance to describe the delocalized nature of the bonding in benzene.
B) The lowest energy pi-bonding orbital in benzene can hold up to 6 electrons.
C) MO theory predicts that benzene should be paramagnetic.
D) The lowest energy pi-bonding MO in benzene has two hexagonal lobes, above and below the plane of the carbon atoms.
E) None of these choices are correct.
A) MO theory uses resonance to describe the delocalized nature of the bonding in benzene.
B) The lowest energy pi-bonding orbital in benzene can hold up to 6 electrons.
C) MO theory predicts that benzene should be paramagnetic.
D) The lowest energy pi-bonding MO in benzene has two hexagonal lobes, above and below the plane of the carbon atoms.
E) None of these choices are correct.

Unlock Deck
Unlock for access to all 48 flashcards in this deck.
Unlock Deck
k this deck
32
Atoms of period 3 and beyond can undergo sp3d2 hybridization, but atoms of period 2 cannot.

Unlock Deck
Unlock for access to all 48 flashcards in this deck.
Unlock Deck
k this deck
33
A carbon-carbon double bond in a molecule may give rise to the existence of cis and trans isomers.

Unlock Deck
Unlock for access to all 48 flashcards in this deck.
Unlock Deck
k this deck
34
Select the correct statement about 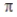 -bonds in valence bond theory.
-bonds in valence bond theory.
A) A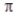 bond is stronger than a sigma bond.
bond is stronger than a sigma bond.
B) A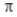 bond can hold 4 electrons, two above and two below the σ-bond axis.
bond can hold 4 electrons, two above and two below the σ-bond axis.
C) A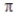 carbon-carbon double bond consists of two bonds.
carbon-carbon double bond consists of two bonds.
D) A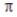 bond is the same strength as a σ bond.
bond is the same strength as a σ bond.
E) A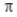 bond between two carbon atoms restricts rotation about the C-C axis.
bond between two carbon atoms restricts rotation about the C-C axis.
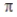 -bonds in valence bond theory.
-bonds in valence bond theory.A) A
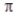 bond is stronger than a sigma bond.
bond is stronger than a sigma bond.B) A
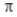 bond can hold 4 electrons, two above and two below the σ-bond axis.
bond can hold 4 electrons, two above and two below the σ-bond axis.C) A
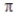 carbon-carbon double bond consists of two bonds.
carbon-carbon double bond consists of two bonds.D) A
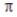 bond is the same strength as a σ bond.
bond is the same strength as a σ bond.E) A
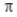 bond between two carbon atoms restricts rotation about the C-C axis.
bond between two carbon atoms restricts rotation about the C-C axis.
Unlock Deck
Unlock for access to all 48 flashcards in this deck.
Unlock Deck
k this deck
35
According to the molecular orbital (MO) treatment of the NO molecule, what are the bond order and the number of unpaired electrons, respectively?
A) 2, 2
B) 3, 3
C) 1, 1
D) 1.5, 2
E) 2.5, 1
A) 2, 2
B) 3, 3
C) 1, 1
D) 1.5, 2
E) 2.5, 1

Unlock Deck
Unlock for access to all 48 flashcards in this deck.
Unlock Deck
k this deck
36
One can safely assume that the 3s- and 3p-orbitals will form molecular orbitals similar to those formed when 2s- and 2p-orbitals interact. According to molecular orbital theory, what will be the bond order for the Cl2+ ion?
A) 0.5
B) 1
C) 1.5
D) 2
E) None of these choices are correct.
A) 0.5
B) 1
C) 1.5
D) 2
E) None of these choices are correct.

Unlock Deck
Unlock for access to all 48 flashcards in this deck.
Unlock Deck
k this deck
37
How many sigma and pi bonds, respectively, are in the molecule below? CH3CH2CHCHCH3?
A) 16, 1
B) 16, 0
C) 15, 1
D) 15, 0
E) 14, 1
A) 16, 1
B) 16, 0
C) 15, 1
D) 15, 0
E) 14, 1

Unlock Deck
Unlock for access to all 48 flashcards in this deck.
Unlock Deck
k this deck
38
Valence bond theory explains the bonding in diatomic molecules such as HCl without resorting to the use of hybrid orbitals.

Unlock Deck
Unlock for access to all 48 flashcards in this deck.
Unlock Deck
k this deck
39
According to molecular orbital (MO) theory, the twelve outermost electrons in the O2 molecule are distributed as follows:
A) 12 in bonding MOs, 0 in antibonding MOs.
B) 10 in bonding MOs, 2 in antibonding MOs.
C) 9 in bonding MOs, 3 in antibonding MOs.
D) 8 in bonding MOs, 4 in antibonding MOs.
E) 7 in bonding MOs, 5 in antibonding MOs.
A) 12 in bonding MOs, 0 in antibonding MOs.
B) 10 in bonding MOs, 2 in antibonding MOs.
C) 9 in bonding MOs, 3 in antibonding MOs.
D) 8 in bonding MOs, 4 in antibonding MOs.
E) 7 in bonding MOs, 5 in antibonding MOs.

Unlock Deck
Unlock for access to all 48 flashcards in this deck.
Unlock Deck
k this deck
40
In the valence bond treatment, a 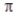 bond is formed when two 2p orbitals overlap side to side.
bond is formed when two 2p orbitals overlap side to side.
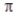 bond is formed when two 2p orbitals overlap side to side.
bond is formed when two 2p orbitals overlap side to side.
Unlock Deck
Unlock for access to all 48 flashcards in this deck.
Unlock Deck
k this deck
41
Valence bond theory is able to explain why molecular oxygen is paramagnetic.

Unlock Deck
Unlock for access to all 48 flashcards in this deck.
Unlock Deck
k this deck
42
In molecular orbital theory, combination of two 2p atomic orbitals may give rise to either σ or π type molecular orbitals.

Unlock Deck
Unlock for access to all 48 flashcards in this deck.
Unlock Deck
k this deck
43
The lone pair of electrons on sulfur in the sulfite ion (SO32-) is in
A) a p orbital.
B) an s orbital.
C) an sp2 hybrid orbital.
D) an sp3 hybrid orbital.
E) none of these orbital types.
A) a p orbital.
B) an s orbital.
C) an sp2 hybrid orbital.
D) an sp3 hybrid orbital.
E) none of these orbital types.

Unlock Deck
Unlock for access to all 48 flashcards in this deck.
Unlock Deck
k this deck
44
In applying molecular orbital theory, the bond order is calculated in the same way as with Lewis structures.

Unlock Deck
Unlock for access to all 48 flashcards in this deck.
Unlock Deck
k this deck
45
In molecular orbital theory, combination of two atomic orbitals produces two molecular orbitals.

Unlock Deck
Unlock for access to all 48 flashcards in this deck.
Unlock Deck
k this deck
46
According to molecular orbital theory, all diatomic molecules with an even number of electrons will be diamagnetic.

Unlock Deck
Unlock for access to all 48 flashcards in this deck.
Unlock Deck
k this deck
47
A carbon-carbon triple bond in a molecule may give rise to the existence of cis and trans isomers.

Unlock Deck
Unlock for access to all 48 flashcards in this deck.
Unlock Deck
k this deck
48
In molecular orbital theory, molecules with an even number of electrons will have bond orders which are whole numbers.

Unlock Deck
Unlock for access to all 48 flashcards in this deck.
Unlock Deck
k this deck


