Deck 3: Matter and Minerals
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/74
Play
Full screen (f)
Deck 3: Matter and Minerals
1
Which is the strongest of the atomic bonds?
A)Ionic
B)Covalent
C)Metallic
D)Hybrid
A)Ionic
B)Covalent
C)Metallic
D)Hybrid
B
2
An isotope of oxygen has 8 protons, 10 neutrons, and 8 electrons.What is the atomic mass of this isotope?
A)16
B)18
C)21
D)26
A)16
B)18
C)21
D)26
B
3
________ refers to a mineral's intensity and quality of reflected light.
A)Cleavage
B)Luster
C)Tenacity
D)Streak
A)Cleavage
B)Luster
C)Tenacity
D)Streak
B
4
Which of the following minerals has good cleavage in two directions, which creates a rectangular-shaped crystal?
A)Halite
B)Quartz
C)Plagioclase
D)Biotite
A)Halite
B)Quartz
C)Plagioclase
D)Biotite

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
5
Most marine organisms produce the mineral substance ________, which will later become limestone.
A)SiO2
B)SO2
C)H2CO3
D)CaCO3
A)SiO2
B)SO2
C)H2CO3
D)CaCO3

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
6
________ is defined as a mineral's resistance to scratching.
A)Hardness
B)Cleavage
C)Fracture
D)Streak
A)Hardness
B)Cleavage
C)Fracture
D)Streak

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
7
What is the charge of a single proton?
A)+1
B)-1
C)+2
D)-3
A)+1
B)-1
C)+2
D)-3

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
8
Which of the following mineral identification techniques would most easily identify calcite?
A)Taste
B)Streak
C)Magnetism
D)Effervescence
A)Taste
B)Streak
C)Magnetism
D)Effervescence

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
9
In an aqueous solution that has not reached the saturation point, why won't the ions bond to create mineral crystals?
A)The opposite charges of the ions repel each other.
B)The ions are too close together to form a regular internal crystalline structure.
C)The motion of the dissolved ions keeps them from joining.
D)There are too few ions to make any minerals.
A)The opposite charges of the ions repel each other.
B)The ions are too close together to form a regular internal crystalline structure.
C)The motion of the dissolved ions keeps them from joining.
D)There are too few ions to make any minerals.

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
10
A ________ is a solid, naturally occurring, cohesive substance composed of minerals or mineral-like materials.
A)tetrahedron
B)mineral
C)rock
D)mixture
A)tetrahedron
B)mineral
C)rock
D)mixture

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
11
________ is the study of mineral materials.
A)Mineralogy
B)Lithology
C)Mineralosophy
D)Historical Geology
A)Mineralogy
B)Lithology
C)Mineralosophy
D)Historical Geology

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
12
Crystallization of molten rock will produce ________ rocks.
A)metamorphic
B)igneous
C)sedimentary
A)metamorphic
B)igneous
C)sedimentary

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
13
What is the name of the chart that organizes elements in order of chemical properties?
A)Moh's hardness scale
B)Atomic symbols
C)Elemental flowchart
D)Periodic table of elements
A)Moh's hardness scale
B)Atomic symbols
C)Elemental flowchart
D)Periodic table of elements

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
14
________ are groups of the same kinds of atoms that cannot be broken down into other substances by ordinary chemical means.
A)Molecules
B)Electrons
C)Elements
D)Minerals
A)Molecules
B)Electrons
C)Elements
D)Minerals

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
15
Which of the following is not part of the definition of a mineral?
A)Naturally occurring
B)Organic
C)Orderly crystalline structure
D)Definite chemical composition
A)Naturally occurring
B)Organic
C)Orderly crystalline structure
D)Definite chemical composition

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
16
Which of the following is the hardest known natural substance?
A)Talc
B)Diamond
C)Quartz
D)Sapphire
A)Talc
B)Diamond
C)Quartz
D)Sapphire

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
17
Which of the following is not one of the three primary ways minerals can form?
A)Hybridization
B)Precipitation
C)Crystallization of molten material
D)Biological Processes
A)Hybridization
B)Precipitation
C)Crystallization of molten material
D)Biological Processes

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
18
Which of the following is a rock composed of nonmineral matter?
A)Granite
B)Coal
C)Limestone
D)Basalt
A)Granite
B)Coal
C)Limestone
D)Basalt

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
19
According to the octet rule, atoms tend to gain, lose, or share electrons until they are surrounded by ________ valence electrons.
A)2
B)6
C)8
D)10
A)2
B)6
C)8
D)10

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
20
What is an ion?
A)An atom that has more or fewer protons than it should
B)An atom that has more or fewer neutrons than it should
C)An atom that has more or fewer electrons than it should
A)An atom that has more or fewer protons than it should
B)An atom that has more or fewer neutrons than it should
C)An atom that has more or fewer electrons than it should

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
21
Why will an iron-rich olivine have a higher density than a magnesium-rich olivine?
A)More magnesium is needed to equal the same atomic charge as iron.
B)Iron-rich olivine has smaller crystals, so more of them will fit into the same area.
C)Magnesium-rich olivine has more silicon, which is less dense.
D)Iron has a greater atomic mass than magnesium and therefore is heavier.
A)More magnesium is needed to equal the same atomic charge as iron.
B)Iron-rich olivine has smaller crystals, so more of them will fit into the same area.
C)Magnesium-rich olivine has more silicon, which is less dense.
D)Iron has a greater atomic mass than magnesium and therefore is heavier.

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
22
The addition of which of the following will control a mineral's color?
A)Water
B)Biologic secretions
C)Pigment
D)Trace elements
A)Water
B)Biologic secretions
C)Pigment
D)Trace elements

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
23
Define the tenacity of a mineral.
A)Resistance to scratching
B)Parting along a plane of weakness
C)A specific pattern of fracturing
D)Resistance to breaking or deforming
A)Resistance to scratching
B)Parting along a plane of weakness
C)A specific pattern of fracturing
D)Resistance to breaking or deforming

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
24
Which ferromagnesian mineral is believed to constitute up to 50 percent of the mantle?
A)Biotite
B)Amphibole
C)Olivine
D)Garnet
A)Biotite
B)Amphibole
C)Olivine
D)Garnet

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
25
The ________ are the mineral class that accounts for more than 90 percent of the Earth's crust.
A)silicates
B)sulfates
C)carbonates
D)sulfides
A)silicates
B)sulfates
C)carbonates
D)sulfides

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
26
Only eight elements make up the vast majority of rock-forming minerals.Which of the following elements is not one of the eight?
A)Oxygen
B)Hydrogen
C)Iron
D)Potassium
A)Oxygen
B)Hydrogen
C)Iron
D)Potassium

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
27
What are the two groups of feldspar minerals called?
A)Microcline and orthoclase
B)Potassium feldspar and plagioclase
C)Sodium and calcium
D)Albite and anorthite
A)Microcline and orthoclase
B)Potassium feldspar and plagioclase
C)Sodium and calcium
D)Albite and anorthite

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
28
A(n)________ is a silicate structure where no silica tetrahedra share any oxygen ions.
A)single chain
B)double chain
C)framework
D)independent
A)single chain
B)double chain
C)framework
D)independent

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
29
What is the mineral name for table salt?
A)Halite
B)Calcite
C)Dolomite
D)Gypsum
A)Halite
B)Calcite
C)Dolomite
D)Gypsum

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
30
________ states that the angles between equivalent faces of crystals of the same mineral are always the same.
A)The law of conservation of mass
B)The law of constancy of interfacial angles
C)The law of superposition
D)The law of opposite interior angles
A)The law of conservation of mass
B)The law of constancy of interfacial angles
C)The law of superposition
D)The law of opposite interior angles

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
31
Which negative ion forms the basis of the oxide minerals?
A)F-
B)Cl-
C)O22-
D)S-
A)F-
B)Cl-
C)O22-
D)S-

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
32
Which group of silicate minerals has two planes of cleavage that are oriented at approximately 60° and 120°?
A)Pyroxenes
B)Potassium Feldspars
C)Amphiboles
D)Galena
A)Pyroxenes
B)Potassium Feldspars
C)Amphiboles
D)Galena

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
33
Which intermediary helps to break down minerals into a form digestible by humans?
A)Meteorites
B)Rocks
C)Plants
D)Gravel
A)Meteorites
B)Rocks
C)Plants
D)Gravel

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
34
Ferromagnesian minerals are those that contain a great deal of ________.
A)manganese and iron
B)potassium and aluminum
C)magnesium and iron
D)calcium and silicon
A)manganese and iron
B)potassium and aluminum
C)magnesium and iron
D)calcium and silicon

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
35
Which ions-positive ions or negative ions-tend to be larger and why?
A)Positive ions because they have more protons
B)Positive ions because they have more electrons
C)Negative ions because they have more neutrons
D)Negative ions because they have more electrons
A)Positive ions because they have more protons
B)Positive ions because they have more electrons
C)Negative ions because they have more neutrons
D)Negative ions because they have more electrons

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
36
Which kind of mineral tenacity refers to a mineral being deformed by being hammered without breaking?
A)Malleable
B)Sectile
C)Fibrous
D)Elastic
A)Malleable
B)Sectile
C)Fibrous
D)Elastic

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
37
What is the best method to distinguish between muscovite mica and biotite mica?
A)Cleavage
B)Color
C)Hardness
D)Streak
A)Cleavage
B)Color
C)Hardness
D)Streak

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
38
Conchoidal fractures are indicative of the mineral ________.
A)muscovite
B)quartz
C)fluorite
D)pyroxene
A)muscovite
B)quartz
C)fluorite
D)pyroxene

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
39
One defining characteristic of minerals is that they are found in a solid state in nature.Which of the following minerals is an exception to this rule, naturally occurring in a liquid state at room temperature?
A)Diamond
B)Ice
C)Carbon
D)Calcite
A)Diamond
B)Ice
C)Carbon
D)Calcite

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
40
Silica tetrahedra are able to link into long chains that share oxygen ions through the process of ________.
A)isolation
B)precipitation
C)fission
D)polymerization
A)isolation
B)precipitation
C)fission
D)polymerization

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
41
A(n)________ is the fundamental particle of matter.
A)electron
B)mineral
C)nucleus
D)atom
A)electron
B)mineral
C)nucleus
D)atom

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
42
Explain how differences in molecular bonds in minerals can result in cleavage versus fracture.

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
43
A rock can be composed of almost entirely one mineral.

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
44
Consider the definition of a mineral.Why would a synthetic gemstone fail this definition and not be considered a true mineral?

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
45
Color is a reliable identification technique for minerals.

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
46
Which of the following rocks is composed of only a single mineral?
A)Limestone
B)Granite
C)Conglomerate
D)Gabbro
A)Limestone
B)Granite
C)Conglomerate
D)Gabbro

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
47
Quartz has only one color.

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
48
A geode contains minerals that form through biological processes.

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
49
Which of the following materials will form through evaporation?
A)Feldspars
B)Geodes
C)Salt
D)Limestone
A)Feldspars
B)Geodes
C)Salt
D)Limestone

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
50
The more oxygen ions that are shared in silicate minerals, the higher the oxygen-to-silicon ratio.

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
51
All minerals have cleavage.

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
52
Graphite and diamonds are polymorphs because both are made up of carbon atoms.

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
53
Ferromagnesian minerals have a higher specific gravity than nonferromagnesian minerals.

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
54
You have a solution of salt dissolved in water.Why might a drop in temperature result in the dissolved minerals precipitating out of solution?

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
55
Mineral species can be further subdivided into ________.
A)mineral classes
B)mineraloids
C)evaporites
D)varieties
A)mineral classes
B)mineraloids
C)evaporites
D)varieties

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
56
Polymorphs are minerals that will have two identical mineral structures, but different chemical compositions.

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
57
There can be no variation of mineral composition in order for the substance to remain the same mineral.

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
58
Limestone is a sedimentary rock that is found over large areas of the United States.Using this information, explain what the environment of deposition was like at the time of limestone formation and how the mineral in the limestone formed?

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
59
If there are over 100 elements on the periodic table of elements, it would stand to reason that there would be millions of ways these elements could combine to form minerals.However, there are only 4000 named minerals.Why might there be so few minerals on Earth when there are so many possible combinations?

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
60
What is the significance of valence electrons in connection to mineral formation?

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
61
 Assuming well-formed crystals, how many cleavage surfaces are present on the sample in this image? (Note: Cleavage surfaces are not cleavage planes.)
Assuming well-formed crystals, how many cleavage surfaces are present on the sample in this image? (Note: Cleavage surfaces are not cleavage planes.)
Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
62
Write a simple chemical formula showing how the component elements of the silicate ion affect its net charge.

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
63
 Using the samples in this image, indicate the number of cleavage directions this crystal displays.(Please note that cleavage surfaces do not equate to cleavage directions.)
Using the samples in this image, indicate the number of cleavage directions this crystal displays.(Please note that cleavage surfaces do not equate to cleavage directions.)
Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
64
Describe how magnesium can be exchanged for iron in olivine without destroying the internal framework of the mineral.

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
65
Certain diamond companies use the advertising slogan "Diamonds are forever." Why, from a geologic perspective, might this not be accurate?

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
66
Restricted ocean basins located in warm climates will experience high rates of evaporation, which will result in the creation of evaporate minerals such as gypsum or halite.When such a solution containing dissolved mineral matter evaporates, why does the mineral matter precipitate instead of evaporating with the water?

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
67
 Assuming well-formed crystals, how many cleavage surfaces are present on the sample in this image? (Note: Cleavage surfaces are not cleavage planes.)
Assuming well-formed crystals, how many cleavage surfaces are present on the sample in this image? (Note: Cleavage surfaces are not cleavage planes.)
Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
68
If concrete is made by mixing cement with gravels and sands, is concrete a rock? Why or why not?

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
69
 Using the samples in this image, indicate the number of cleavage directions this crystal displays.(Please note that cleavage surfaces do not equate to cleavage directions.)
Using the samples in this image, indicate the number of cleavage directions this crystal displays.(Please note that cleavage surfaces do not equate to cleavage directions.)
Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
70
Students new to mineral identification often confuse shiny, dark-colored nonmetallic minerals for those minerals with a metallic luster.Explain how streak can be useful for telling these minerals apart.

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
71
Which of the following substances is not a mineral: gold, water, ice? Why?

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
72
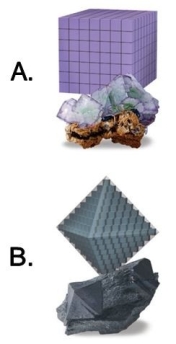 Using the cleavage planes and shapes in this image, list one mineral example for each crystal.
Using the cleavage planes and shapes in this image, list one mineral example for each crystal.
Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
73
Two unknown minerals are placed before you for identification.You decide to test each mineral's hardness.Mineral A will scratch a piece of glass.Mineral B will not.Which mineral is harder? How do they compare to the specific hardness of the glass?

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
74
Why do atoms exchange or share electrons?

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck


