Deck 11: Oxidative Phosphorylation
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/99
Play
Full screen (f)
Deck 11: Oxidative Phosphorylation
1
Which reaction does the concept of oxidative phosphorylation refer to?
A) O2 + ADP + Pi 2 H2O + ATP
B) NADH + H+ + 1/2 O2 NAD+ + H2O
C) ADP + Pi ATP
D) NADH + 1/2 O2 + H+ + ADP + Pi NAD+ + ATP + H2O
A) O2 + ADP + Pi 2 H2O + ATP
B) NADH + H+ + 1/2 O2 NAD+ + H2O
C) ADP + Pi ATP
D) NADH + 1/2 O2 + H+ + ADP + Pi NAD+ + ATP + H2O
NADH + 1/2 O2 + H+ + ADP + Pi NAD+ + ATP + H2O
2
Approximately how many more ATPs are made from one glucose molecule under aerobic conditions with oxidative phosphorylation than under anaerobic conditions?
A) 0
B) 2
C) 30
D) 104
A) 0
B) 2
C) 30
D) 104
30
3
What are the principle physiological electron donors for the mitochondrial electron transport pathway?
A) FADH2 and NADH
B) FADH2, NADH, and NADPH
C) NADH only
D) UQH2, NADH, and FADH2
A) FADH2 and NADH
B) FADH2, NADH, and NADPH
C) NADH only
D) UQH2, NADH, and FADH2
FADH2 and NADH
4
Which process or pathway describes the coupling of the oxidation reaction of NADH with the formation of ATP?
A) electron transport system
B) chemiosmotic theory
C) proton motive force
D) oxidative phosphorylation
A) electron transport system
B) chemiosmotic theory
C) proton motive force
D) oxidative phosphorylation

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
5
Protons are pumped by mitochondria during active electron transport
A) into the thylakoid lumen.
B) outside the outer mitochondrial membrane.
C) into the mitochondrial matrix.
D) outside the inner mitochondrial membrane.
A) into the thylakoid lumen.
B) outside the outer mitochondrial membrane.
C) into the mitochondrial matrix.
D) outside the inner mitochondrial membrane.

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
6
Mitochondria
A) have a porous inner membrane and nonporous outer membrane.
B) have a higher pH inside the matrix than outside during active electron transport.
C) have an outer membrane that is composed of lipids and protein electron transport complexes.
D) generate ATP for the cell under anaerobic and aerobic conditions.
A) have a porous inner membrane and nonporous outer membrane.
B) have a higher pH inside the matrix than outside during active electron transport.
C) have an outer membrane that is composed of lipids and protein electron transport complexes.
D) generate ATP for the cell under anaerobic and aerobic conditions.

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
7
As electrons from NADH pass through the electron transport system,
A) the oxidized form of ADP is reduced to ATP.
B) the reduction potential of the mitochondria becomes more thermodynamically favorable.
C) the resulting electron gradient is used to make ATP.
D) protons are pumped across the mitochondrial membrane to create a pH gradient.
A) the oxidized form of ADP is reduced to ATP.
B) the reduction potential of the mitochondria becomes more thermodynamically favorable.
C) the resulting electron gradient is used to make ATP.
D) protons are pumped across the mitochondrial membrane to create a pH gradient.

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
8
Use the table below to answer the question. 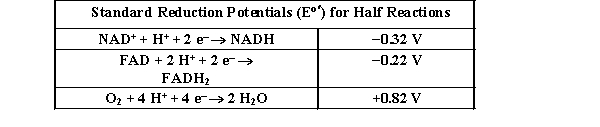 In the direction indicated, which of the following reactions are thermodynamically favored?
In the direction indicated, which of the following reactions are thermodynamically favored?
I. NAD+ + H2O O2 + NADH
II. FADH2 + H2O O2 + FAD
III. NADH + O2 H2O + NAD+
A) I and II
B) I and III
C) I, II, and III
D) III only
 In the direction indicated, which of the following reactions are thermodynamically favored?
In the direction indicated, which of the following reactions are thermodynamically favored? I. NAD+ + H2O O2 + NADH
II. FADH2 + H2O O2 + FAD
III. NADH + O2 H2O + NAD+
A) I and II
B) I and III
C) I, II, and III
D) III only

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
9
The major purpose of the electron transport system is to
A) reduce oxygen to water.
B) reoxidize NADH and use that energy to pump protons across a membrane.
C) produce ATP.
D) produce NADH for cellular respiration.
A) reduce oxygen to water.
B) reoxidize NADH and use that energy to pump protons across a membrane.
C) produce ATP.
D) produce NADH for cellular respiration.

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
10
Studies of the inner mitochondrial membrane reveal its composition to be approximately 20% lipid bilayer and 80% protein. What is true of these proteins?
A) Abundant collagen proteins form connective tissues to strengthen the membrane against the pH gradient.
B) High levels of proteins are required to metabolize glucose in the glycolysis pathway for rapid energy production.
C) The proteins form highly folded cristae structures.
D) The proteins are largely electron transport complexes and ATP synthase enzymes.
A) Abundant collagen proteins form connective tissues to strengthen the membrane against the pH gradient.
B) High levels of proteins are required to metabolize glucose in the glycolysis pathway for rapid energy production.
C) The proteins form highly folded cristae structures.
D) The proteins are largely electron transport complexes and ATP synthase enzymes.

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
11
Which statement comparing chloroplasts and mitochondria is true?
A) Chloroplasts and mitochondria each contain two membranes.
B) Chloroplasts and mitochondria use electrons from NADH to create a proton gradient.
C) Chloroplasts pump protons outside the organelle, while mitochondria pump protons inside the inner part of the organelle.
D) Chloroplasts and mitochondria use energy from a proton gradient to make ATP.
A) Chloroplasts and mitochondria each contain two membranes.
B) Chloroplasts and mitochondria use electrons from NADH to create a proton gradient.
C) Chloroplasts pump protons outside the organelle, while mitochondria pump protons inside the inner part of the organelle.
D) Chloroplasts and mitochondria use energy from a proton gradient to make ATP.

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
12
What is the fate of NADH after it donates its electrons to the electron transport system?
A) NAD+ is excreted from the cell and sent to the liver for final degradation.
B) NADH is used for cellular biosynthesis.
C) NAD+ is re-reduced by the TCA cycle or glycolysis and returns to electron transport system, where the process is repeated.
D) NAD+ is fed into the TCA cycle for oxidation of CO2.
A) NAD+ is excreted from the cell and sent to the liver for final degradation.
B) NADH is used for cellular biosynthesis.
C) NAD+ is re-reduced by the TCA cycle or glycolysis and returns to electron transport system, where the process is repeated.
D) NAD+ is fed into the TCA cycle for oxidation of CO2.

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
13
The TCA cycle is dependent on O2 to
A) enable the regeneration of the NAD+ by the electron transport system.
B) oxidize the sugar carbons to CO2.
C) support cellular combustion reactions.
D) serve as a substrate for the oxidoreductase enzymes.
A) enable the regeneration of the NAD+ by the electron transport system.
B) oxidize the sugar carbons to CO2.
C) support cellular combustion reactions.
D) serve as a substrate for the oxidoreductase enzymes.

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
14
The energy released during mitochondrial electron transport processes is used to
A) make ATP.
B) pump protons across the membrane.
C) heat the mitochondria.
D) synthesize carbohydrates.
A) make ATP.
B) pump protons across the membrane.
C) heat the mitochondria.
D) synthesize carbohydrates.

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
15
A decrease in would be LEAST likely to affect the processes of the electron transport system.
A) oxygen concentrations in the cell
B) the TCA cycle activity
C) cellular CO2 concentrations
D) the concentration of cellular NADH
A) oxygen concentrations in the cell
B) the TCA cycle activity
C) cellular CO2 concentrations
D) the concentration of cellular NADH

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
16
What would happen to the ETC and oxidative phosphorylation pathway in the presence of excess NADH if the mitochondrial matrix were not closed, but opened up to the cytoplasm?
A) The ETC complexes would function as normal, but no ATP would be made.
B) The ETC and synthesis of ATP would continue as normal.
C) The ETC complexes would transfer electrons from NADH to O2, but no protons would be pumped.
D) The NADH would react directly with O2, generating excess heat.
A) The ETC complexes would function as normal, but no ATP would be made.
B) The ETC and synthesis of ATP would continue as normal.
C) The ETC complexes would transfer electrons from NADH to O2, but no protons would be pumped.
D) The NADH would react directly with O2, generating excess heat.

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
17
The ultimate electron acceptor of the mitochondrial electron transport system is
A) O2.
B) NADH.
C) H2O.
D) cytochrome c.
A) O2.
B) NADH.
C) H2O.
D) cytochrome c.

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
18
Mitochondria
A) selectively transport molecules from the cytoplasm to the intermembrane space.
B) maintain a pH gradient across the inner mitochondrial membrane.
C) are found one per cell.
D) have a matrix that is continuous with the cytoplasm.
A) selectively transport molecules from the cytoplasm to the intermembrane space.
B) maintain a pH gradient across the inner mitochondrial membrane.
C) are found one per cell.
D) have a matrix that is continuous with the cytoplasm.

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
19
In the mitochondrial electron transport system, the electron from NADH moves from __________ the mitochondria through the protein complexes to the
Mitochondria.
A) outside; outside
B) outside; inside
C) inside; inside
D) inside; outside
Mitochondria.
A) outside; outside
B) outside; inside
C) inside; inside
D) inside; outside

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
20
Which of the following statements about the chemiosmotic theory is true?
A) It requires an enclosed mitochondrial membrane.
B) The membrane ATPase (or ATP synthase) has no significant role in the theory.
C) Energy is coupled through a transmembrane electron gradient.
D) It explains how ATP energy is used to create a pH gradient.
A) It requires an enclosed mitochondrial membrane.
B) The membrane ATPase (or ATP synthase) has no significant role in the theory.
C) Energy is coupled through a transmembrane electron gradient.
D) It explains how ATP energy is used to create a pH gradient.

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
21
The electron transport complexes found in the electron transport system
A) contain multiple electron transfer cofactors that facilitate electron transfer through the complexes.
B) are bound to the outer mitochondrial membrane.
C) pump protons from outside the mitochondria to the mitochondrial matrix on electron transfer.
D) absorb light energy that results in electron transfer.
A) contain multiple electron transfer cofactors that facilitate electron transfer through the complexes.
B) are bound to the outer mitochondrial membrane.
C) pump protons from outside the mitochondria to the mitochondrial matrix on electron transfer.
D) absorb light energy that results in electron transfer.

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
22
What is the reaction catalyzed by complex III in the electron transport system?
A) UQH2 + O2 UQ + H2O
B) UQH2 + complex IV+ UQ + complex IV
C) FADH2 + UQ FAD + UQH2
D) UQH2 + cytochrome c+ UQ + cytochrome c
A) UQH2 + O2 UQ + H2O
B) UQH2 + complex IV+ UQ + complex IV
C) FADH2 + UQ FAD + UQH2
D) UQH2 + cytochrome c+ UQ + cytochrome c

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
23
Which of the following is NOT a part of oxidative phosphorylation?
A) NADH is oxidized to NAD+.
B) O2 is reduced to H2O.
C) Electrons from O2 are transferred to ATP.
D) The pumping of protons is coupled with the formation of ATP.
A) NADH is oxidized to NAD+.
B) O2 is reduced to H2O.
C) Electrons from O2 are transferred to ATP.
D) The pumping of protons is coupled with the formation of ATP.

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
24
What active site cofactor is found in the electron transport system protein called cytochrome c?
A) heme
B) FeS cluster
C) Flavin
D) quinone
A) heme
B) FeS cluster
C) Flavin
D) quinone

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
25
A characteristic of complex III is that it
A) transports electrons from cytochrome c to complex IV.
B) is reduced by FADH2.
C) uses the Q cycle mechanism to oxidize ubiquinone.
D) participates in electron transfer when the donor is NADH but not when the donor is succinate (or FADH2).
A) transports electrons from cytochrome c to complex IV.
B) is reduced by FADH2.
C) uses the Q cycle mechanism to oxidize ubiquinone.
D) participates in electron transfer when the donor is NADH but not when the donor is succinate (or FADH2).

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
26
The electron transport chain component that transfers electrons directly to oxygen is
A) complex I.
B) cytochrome c.
C) complex IV.
D) NADH.
A) complex I.
B) cytochrome c.
C) complex IV.
D) NADH.

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
27
The last enzyme in the electron transport system, where O2 is reduced to water, is called
A) NADH-ubiquinone oxidoreductase.
B) ATP synthase.
C) ubiquinone cytochrome c oxidoreductase.
D) cytochrome c oxidase.
A) NADH-ubiquinone oxidoreductase.
B) ATP synthase.
C) ubiquinone cytochrome c oxidoreductase.
D) cytochrome c oxidase.

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
28
Identify the correct order of electron transfers in the electron transport chain starting from FADH2.
A) complex I complex II complex III complex IV
B) complex II complex III cytochrome c complex IV
C) complex II coenzyme Q complex IV ATP synthase
D) complex I coenzyme Q complex III complex IV
A) complex I complex II complex III complex IV
B) complex II complex III cytochrome c complex IV
C) complex II coenzyme Q complex IV ATP synthase
D) complex I coenzyme Q complex III complex IV

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
29
What best describes the driving force for ATP synthesis in the mitochondria?
A) Electron transport from electron transport system complexes.
B) The higher pH inside the mitochondria that results from electron transfer.
C) The large drops in G resulting from electron transfer in the ETC.
D) The substrate level phosphorylations of the TCA cycle and glycolysis.
A) Electron transport from electron transport system complexes.
B) The higher pH inside the mitochondria that results from electron transfer.
C) The large drops in G resulting from electron transfer in the ETC.
D) The substrate level phosphorylations of the TCA cycle and glycolysis.

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
30
What enzyme uses the proton motive force for its driving force?
A) complex IV
B) ATP synthase
C) complex III
D) complex I
A) complex IV
B) ATP synthase
C) complex III
D) complex I

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
31
Which statement is true of the mitochondrial electron transport system?
A) All the electron carriers are located in enzyme complexes.
B) All the electrons in the chain end up on O2 to produce water.
C) The pH drops in the mitochondria as electrons pass through the system.
D) Protons are pumped from the inner membrane space to the mitochondrial matrix during the electron transfer.
A) All the electron carriers are located in enzyme complexes.
B) All the electrons in the chain end up on O2 to produce water.
C) The pH drops in the mitochondria as electrons pass through the system.
D) Protons are pumped from the inner membrane space to the mitochondrial matrix during the electron transfer.

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
32
Which one of the following is involved in the flow of electrons from NADH through the electron transport system to molecular oxygen (O2)?
A) ATP synthase
B) complex II
C) complex III
D) ATP
A) ATP synthase
B) complex II
C) complex III
D) ATP

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
33
What is the location of the electron transport system protein called cytochrome c?
A) cytoplasm
B) mitochondrial matrix
C) inner mitochondrial membrane
D) intermembrane space
A) cytoplasm
B) mitochondrial matrix
C) inner mitochondrial membrane
D) intermembrane space

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
34
Which is NOT involved in the transfer of reducing equivalents from succinate to molecular oxygen (O2)?
A) cytochrome c
B) coenzyme Q
C) complex I
D) complex II
A) cytochrome c
B) coenzyme Q
C) complex I
D) complex II

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
35
The first enzyme in the electron transport system is referred to as complex I, otherwise known as
A) ATP synthase.
B) NADH-ubiquinone oxidoreductase.
C) succinate dehydrogenase.
D) cytochrome c oxidase.
A) ATP synthase.
B) NADH-ubiquinone oxidoreductase.
C) succinate dehydrogenase.
D) cytochrome c oxidase.

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
36
The structure of the electron transport system protein called cytochrome c is highly conserved in nature because the
A) enzyme has the rare ability to bind O2 and reduce it.
B) protein active site contains a cofactor unique to the electron transport system.
C) protein is located inside the mitochondria of eukaryotic cells.
D) protein plays an important role in the electron transport system and in other critical cellular pathways such as apoptosis.
A) enzyme has the rare ability to bind O2 and reduce it.
B) protein active site contains a cofactor unique to the electron transport system.
C) protein is located inside the mitochondria of eukaryotic cells.
D) protein plays an important role in the electron transport system and in other critical cellular pathways such as apoptosis.

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
37
Which of the following is an electron carrier in the mitochondrial electron transport system?
A) proton
B) water
C) quinone
D) ATP
A) proton
B) water
C) quinone
D) ATP

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
38
Complex IV in the mitochondrial electron transport chain belongs to which enzyme class?
A) lyase
B) hydrolase
C) transferase
D) oxidoreductase
A) lyase
B) hydrolase
C) transferase
D) oxidoreductase

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
39
For one electron entering the electron transport chain at complex I, how many times is it handed off between redox active cofactors on its way through the enzyme complexes to end up on O2?
A) ~2000
B) ~200
C) ~20
D) ~2
A) ~2000
B) ~200
C) ~20
D) ~2

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
40
Which is the only electron carrier in the electron transport system that is not embedded in a membrane?
A) ATP
B) cytochrome c
C) coenzyme Q
D) complex I
A) ATP
B) cytochrome c
C) coenzyme Q
D) complex I

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
41
The __________ causes the catalytic headpiece of ATP synthase to change conformation.
A) rotation of the circle of the 3 3 subunits
B) movement of the circle of ~10 c subunits
C) interaction with the rotating central subunit
D) binding of ADP and Pi
A) rotation of the circle of the 3 3 subunits
B) movement of the circle of ~10 c subunits
C) interaction with the rotating central subunit
D) binding of ADP and Pi

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
42
ATP synthase is located in or on the
A) cytoplasm.
B) intermembrane space of the mitochondria.
C) inner mitochondrial membrane.
D) outer mitochondrial membrane.
A) cytoplasm.
B) intermembrane space of the mitochondria.
C) inner mitochondrial membrane.
D) outer mitochondrial membrane.

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
43
The resting state of the three subunits in the ATP synthase enzyme is best described as
A) one O, one L, and one T conformation.
B) all in O conformations.
C) all in L conformations.
D) one L and two O conformations.
A) one O, one L, and one T conformation.
B) all in O conformations.
C) all in L conformations.
D) one L and two O conformations.

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
44
Which part of the native ATP synthase enzyme is stationary and does NOT rotate during ATP synthesis?
A) the rotor
B) the circle of c subunits
C) the central subunit connecting the rotor to the catalytic headpiece
D) the catalytic headpiece
A) the rotor
B) the circle of c subunits
C) the central subunit connecting the rotor to the catalytic headpiece
D) the catalytic headpiece

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
45
Which one of the following statements about the glycerol phosphate shuttle is true?
A) It involves the transfer of electrons from cytoplasmic NADH to dihydroxyacetone phosphate (DHAP) to yield glycerol phosphate.
B) It is more efficient than the malate aspartate shuttle.
C) NADH produced in the cytoplasm by glycolysis ultimately leads to NADH in mitochondria.
D) Glycerol phosphate diffuses into the mitochondria.
A) It involves the transfer of electrons from cytoplasmic NADH to dihydroxyacetone phosphate (DHAP) to yield glycerol phosphate.
B) It is more efficient than the malate aspartate shuttle.
C) NADH produced in the cytoplasm by glycolysis ultimately leads to NADH in mitochondria.
D) Glycerol phosphate diffuses into the mitochondria.

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
46
How many ATPs are obtained from one acetyl-CoA run once through the TCA cycle, assuming that all resulting NADH and FADH2 is used by the electron transport chain and oxidative phosphorylation to make ATP?
A) 6.5
B) 9
C) 10
D) 11
A) 6.5
B) 9
C) 10
D) 11

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
47
An ATP synthase enzyme with more than 10 c subunits in the F0 stalk would
A) require more protons to complete one 360 rotation.
B) result in more ATP synthesis per 360 turn.
C) require fewer protons to rotate 360 .
D) result in less ATP synthesis per 360 turn.
A) require more protons to complete one 360 rotation.
B) result in more ATP synthesis per 360 turn.
C) require fewer protons to rotate 360 .
D) result in less ATP synthesis per 360 turn.

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
48
Which one of the following correctly designates the number of ATPs generated by the reducing molecules shown below?
A) NADH from the TCA cycle-1.5 ATP
B) NADH from the cytosol (glycerol phosphate shuttle)-2.25 ATP
C) NADH from the cytosol (malate-aspartate shuttle)-1.5 ATP
D) FADH2 from the TCA cycle-1.5 ATP
A) NADH from the TCA cycle-1.5 ATP
B) NADH from the cytosol (glycerol phosphate shuttle)-2.25 ATP
C) NADH from the cytosol (malate-aspartate shuttle)-1.5 ATP
D) FADH2 from the TCA cycle-1.5 ATP

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
49
How many ATPs are produced from the complete metabolism of one glucose molecule, assuming that all resulting NADH and FADH2 is used by the electron transport chain and oxidative phosphorylation to make ATP?
A) 4
B) 16
C) 32
D) 102
A) 4
B) 16
C) 32
D) 102

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
50
Electrons from a succinate molecule can enter into the ETC and result in enough pumped protons to make how many ATPs?
A) 2.0
B) 1.5
C) 1.0
D) 2.5
A) 2.0
B) 1.5
C) 1.0
D) 2.5

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
51
Protons in the mitochondria are
A) the driving force for the electron transfers in the electron transport system.
B) pumped by mitochondrial electron transport system enzymes.
C) pumped inside the mitochondria using ATP energy.
D) the cause of a lower pH inside the mitochondria than outside the mitochondria.
A) the driving force for the electron transfers in the electron transport system.
B) pumped by mitochondrial electron transport system enzymes.
C) pumped inside the mitochondria using ATP energy.
D) the cause of a lower pH inside the mitochondria than outside the mitochondria.

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
52
What would happen to a mutated ATP synthase enzyme where the proton binding aspartate residue on the c subunits was mutated to an alanine?
A) The enzyme would make ATP as normal in the presence of a proton gradient.
B) The enzyme would not make ATP in the presence of a proton gradient.
C) The enzyme would make ATP without the need for a proton gradient.
D) The rotor would rotate in response to a proton gradient, but no ATP would be made.
A) The enzyme would make ATP as normal in the presence of a proton gradient.
B) The enzyme would not make ATP in the presence of a proton gradient.
C) The enzyme would make ATP without the need for a proton gradient.
D) The rotor would rotate in response to a proton gradient, but no ATP would be made.

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
53
What is the driving force for ATP synthesis by the ATP synthase enzyme?
A) The electron transfers through the protein complexes.
B) ADP + Pi ATP
C) The oxidation of NADH to NAD+.
D) The pH gradient across the inner mitochondrial membrane.
A) The electron transfers through the protein complexes.
B) ADP + Pi ATP
C) The oxidation of NADH to NAD+.
D) The pH gradient across the inner mitochondrial membrane.

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
54
In yeast, it is estimated that approximately H+ are required by ATP synthase per ATP synthesized.
A) 1
B) 3
C) 9
D) 10
A) 1
B) 3
C) 9
D) 10

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
55
The ATP synthase enzyme contains a central stalk embedded in the mitochondrial membrane. What part of this stalk rotates?
A) the 3 3 ring
B) the F1 subunit
C) the d, h, and OSCP subunits
D) the ring of c subunits
A) the 3 3 ring
B) the F1 subunit
C) the d, h, and OSCP subunits
D) the ring of c subunits

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
56
The catalytic headpiece of the ATP synthase enzyme is primarily composed of which subunits?
A) "a hexameric 3 3 ring"
B) "a circle of 10 c subunits"
C) "the F0 subunit"
D) " , , and subunits"
A) "a hexameric 3 3 ring"
B) "a circle of 10 c subunits"
C) "the F0 subunit"
D) " , , and subunits"

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
57
ATP synthesis occurs
A) on the outer mitochondrial membrane.
B) at the ATP synthase complex after ADP and Pi are transported into the mitochondria.
C) as a result of the leakage of H+ back out of the mitochondria.
D) from the electron transfer reactions through complex IV.
A) on the outer mitochondrial membrane.
B) at the ATP synthase complex after ADP and Pi are transported into the mitochondria.
C) as a result of the leakage of H+ back out of the mitochondria.
D) from the electron transfer reactions through complex IV.

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
58
During transfer of ATP, ADP, and Pi, some of the proton gradient is lost in the
A) movement of ATP into the mitochondria by the ATP/ADP translocase.
B) movement of ATP out of the mitochondria.
C) transport of ADP into the mitochondria by the ADP translocase.
D) transport of Pi into the mitochondria by the phosphate translocase.
A) movement of ATP into the mitochondria by the ATP/ADP translocase.
B) movement of ATP out of the mitochondria.
C) transport of ADP into the mitochondria by the ADP translocase.
D) transport of Pi into the mitochondria by the phosphate translocase.

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
59
Which mechanism is the most efficient at moving NADH equivalents from the cytoplasm into the mitochondria?
A) citrate shuttle
B) malate-aspartate shuttle
C) glyoxylate shunt
D) glycerol phosphate shuttle
A) citrate shuttle
B) malate-aspartate shuttle
C) glyoxylate shunt
D) glycerol phosphate shuttle

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
60
What type of transport is illustrated by the mitochondrial ATP/ADP translocase?
A) symporter
B) antiporter
C) facilitated diffusion
D) primary active transporter
A) symporter
B) antiporter
C) facilitated diffusion
D) primary active transporter

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
61
Inhibitors of the electron transport system, such as cyanide (CN - ) and carbon monoxide (CO), inhibit complex IV by binding to the heme iron cofactor. What is the resulting effect on oxidative phosphorylation?
A) Electrons pass through the electron transport system, but no protons are pumped.
B) Protons are pumped, but no electron transport occurs.
C) The electron transport system occurs normally, but no ATP is synthesized.
D) Electron transport is disabled, and no protons are pumped.
A) Electrons pass through the electron transport system, but no protons are pumped.
B) Protons are pumped, but no electron transport occurs.
C) The electron transport system occurs normally, but no ATP is synthesized.
D) Electron transport is disabled, and no protons are pumped.

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
62
The main activator molecule of the ATP synthesis and the electron transport system is
A) ATP.
B) H+.
C) ADP.
D) NAD+.
A) ATP.
B) H+.
C) ADP.
D) NAD+.

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
63
If the mitochondrial ATP synthase were inhibited, but the electron transport chain was allowed to run continuously, the pH of the cytoplasm would
A) decrease.
B) increase.
C) remain unchanged.
D) increase immediately and then decrease.
A) decrease.
B) increase.
C) remain unchanged.
D) increase immediately and then decrease.

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
64
What is the effect of oligomycin, an ATP synthase inhibitor, on the mitochondrial oxidative phosphorylation pathway?
A) The mitochondria cannot reduce NADH or make ATP.
B) No proton gradient is formed, and no ATP is synthesized.
C) Protons leak back into the cytoplasm.
D) The proton gradient is formed, but no ATP synthesis can occur.
A) The mitochondria cannot reduce NADH or make ATP.
B) No proton gradient is formed, and no ATP is synthesized.
C) Protons leak back into the cytoplasm.
D) The proton gradient is formed, but no ATP synthesis can occur.

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
65
Use the table below to (A) write out the balanced reaction between NADH and O2 that drives the electron transport system and (B) compute the standard reduction potential. 


Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
66
Give the chemical reaction between the mitochondrial electron transport system's original electron donor and final electron acceptor.

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
67
On the left hand side of the figure, label the regions above and below the lipid membrane assuming the process occurs in mitochondria. On the right hand side, label the regions assuming the process occurs in chloroplasts.
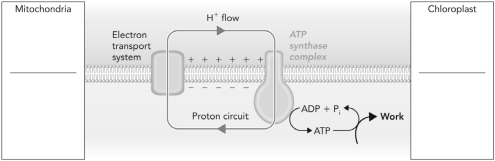


Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
68
Which animal would have the lowest levels of brown adipose tissue?
A) newborn human
B) hibernating squirrel
C) migrating duck
D) polar bear
A) newborn human
B) hibernating squirrel
C) migrating duck
D) polar bear

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
69
The role of brown adipose tissue is to
A) efficiently generate ATP from fat tissue.
B) metabolize excess fat calories.
C) store excess calories as fat.
D) generate heat for the organism.
A) efficiently generate ATP from fat tissue.
B) metabolize excess fat calories.
C) store excess calories as fat.
D) generate heat for the organism.

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
70
Use the following picture of a mitochondrion and label the (A) matrix, (B) crista, (c) inner membrane space, (D) cytoplasm, (E) inner membrane, (F) outer membrane, (G) location of the ATP synthesis, (H) location of elevated pH, and (I) the location of the electron transport system complexes.
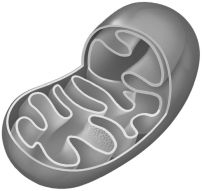


Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
71
How are many mitochondrial diseases passed on from parents to offspring?
A) through the mother only
B) through the father only
C) through an equal distribution from both parents
D) by random mutations in the mitochondrial genome
A) through the mother only
B) through the father only
C) through an equal distribution from both parents
D) by random mutations in the mitochondrial genome

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
72
Which tissue types are affected the most by inherited mitochondrial disorders?
A) those tissues with the highest levels of mitochondria and high levels of activity
B) heart tissue
C) liver tissue
D) those tissues with the lowest levels of mitochondria
A) those tissues with the highest levels of mitochondria and high levels of activity
B) heart tissue
C) liver tissue
D) those tissues with the lowest levels of mitochondria

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
73
Brown adipose tissue is brown in color because of
A) increased levels of thermogenin, or uncoupling protein.
B) increased muscle fiber content.
C) increased numbers of mitochondria.
D) decreased levels of lipid.
A) increased levels of thermogenin, or uncoupling protein.
B) increased muscle fiber content.
C) increased numbers of mitochondria.
D) decreased levels of lipid.

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
74
What happens to patients given the proton gradient uncoupler 2,4-dinitrophenol?
A) They undergo rapid decrease in body temperature as a result of lack of mitochondrial electron transport.
B) They experience lower cellular pH as a result of rapid proton gradient dissipation.
C) Their mitochondrial membranes fail as a result of rapid proton gradient release.
D) Their body temperatures rise as a result of heat release from gradient uncoupling.
A) They undergo rapid decrease in body temperature as a result of lack of mitochondrial electron transport.
B) They experience lower cellular pH as a result of rapid proton gradient dissipation.
C) Their mitochondrial membranes fail as a result of rapid proton gradient release.
D) Their body temperatures rise as a result of heat release from gradient uncoupling.

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
75
What would happen if mitochondria were treated with a proton gradient uncoupler, such as 2,4-dinitrophenol?
A) Electron transfer would stop.
B) Complex I would become reduced, and complexes III and IV would become oxidized.
C) Protons would be pumped by the mitochondrial electron transport chain, although no ATP would be synthesized.
D) Reducing equivalents, in the form of NADH, would no longer be consumed.
A) Electron transfer would stop.
B) Complex I would become reduced, and complexes III and IV would become oxidized.
C) Protons would be pumped by the mitochondrial electron transport chain, although no ATP would be synthesized.
D) Reducing equivalents, in the form of NADH, would no longer be consumed.

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
76
The drug oligomycin inhibits ATP synthase by preventing protons from flowing through the enzyme. Oligomycin must bind to the __________ of ATP synthase.
A) catalytic headpiece
B) F1 subunit
C) ATP binding site
D) F0 subunit of
A) catalytic headpiece
B) F1 subunit
C) ATP binding site
D) F0 subunit of

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
77
What is the cellular location of eukaryotic thermogenin (or uncoupling protein)?
A) mitochondrial inner membrane
B) mitochondrial matrix
C) cytoplasm
D) both cytoplasm and mitochondrial matrix
A) mitochondrial inner membrane
B) mitochondrial matrix
C) cytoplasm
D) both cytoplasm and mitochondrial matrix

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
78
What is the primary fuel metabolized by the mitochondria in brown adipose tissue of hibernating animals?
A) glucose
B) NADH
C) lipid
D) ATP
A) glucose
B) NADH
C) lipid
D) ATP

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
79
Submitochondrial particles are inside-out pieces of the inner mitochondrial membrane formed through the sonication of mitochondria. Draw a diagram of these inside-out particles clearly showing (A) where electron transport from NADH occurs, (B) the direction of proton pumping, (C) the location of ATP synthesis, and (D) where O2 reacts.

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
80
Thermogenin (or uncoupling protein) is not toxic like 2,4-dinitrophenol because the protein
A) is typically expressed in adipose tissues where ample fat calories are found.
B) is less efficient at proton dissipation.
C) cannot leave the mitochondria.
D) is naturally occurring and not synthetic.
A) is typically expressed in adipose tissues where ample fat calories are found.
B) is less efficient at proton dissipation.
C) cannot leave the mitochondria.
D) is naturally occurring and not synthetic.

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck


