Deck 15: Acids and Bases
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/156
Play
Full screen (f)
Deck 15: Acids and Bases
1
What is the conjugate acid of HCO3⁻ ?
A) H3O+
B) H2O
C) CO32-
D) OH⁻
E) H2CO3
A) H3O+
B) H2O
C) CO32-
D) OH⁻
E) H2CO3
H2CO3
2
Which of the following is a general property of a base?
A) Bases have sour taste.
B) Bases turn blue litmus paper red.
C) Bases leave red litmus paper unchanged.
D) Bases turn red litmus paper blue.
E) Bases easily dissolve many metallic elements.
A) Bases have sour taste.
B) Bases turn blue litmus paper red.
C) Bases leave red litmus paper unchanged.
D) Bases turn red litmus paper blue.
E) Bases easily dissolve many metallic elements.
Bases turn red litmus paper blue.
3
Identify the triprotic acid.
A) HNO3
B) H3PO4
C) H2SO3
D) HClO4
E) H2SO4
A) HNO3
B) H3PO4
C) H2SO3
D) HClO4
E) H2SO4
H3PO4
4
Which of the following is an Arrhenius base?
A) CH3CO2H
B) NaOH
C) CH3OH
D) LiCl
E) H2CO3
A) CH3CO2H
B) NaOH
C) CH3OH
D) LiCl
E) H2CO3

Unlock Deck
Unlock for access to all 156 flashcards in this deck.
Unlock Deck
k this deck
5
What is the conjugate base of H2PO4⁻ ?
A) HPO42-
B) PO43-
C) H3PO4
D) H3O+
E) OH⁻
A) HPO42-
B) PO43-
C) H3PO4
D) H3O+
E) OH⁻

Unlock Deck
Unlock for access to all 156 flashcards in this deck.
Unlock Deck
k this deck
6
Which of the following is an Arrhenius acid?
A) H2SO4
B) LiOH
C) NH2CH3
D) CH3CH3
E) NH3
A) H2SO4
B) LiOH
C) NH2CH3
D) CH3CH3
E) NH3

Unlock Deck
Unlock for access to all 156 flashcards in this deck.
Unlock Deck
k this deck
7
Which of the following is the acid found in our stomachs?
A) H2SO4
B) HI
C) HCl
D) HF
E) HBr
A) H2SO4
B) HI
C) HCl
D) HF
E) HBr

Unlock Deck
Unlock for access to all 156 flashcards in this deck.
Unlock Deck
k this deck
8
Identify acid, base, conjugate acid, and conjugate base in the following reaction: H2CO3(aq) + H2O(l) ⇌ HCO3-(aq) + H3O+(aq)
A) H2CO3 is an acid, H2O is a base, HCO3- is a conjugate base, and H3O+ is a conjugate acid.
B) H2CO3 is a conjugate acid, H2O is a conjugate base, HCO3- is a base, and H3O+ is an acid.
C) H2CO3 is an acid, H2O is a conjugate base, HCO3- is a base, and H3O+ is a conjugate acid.
D) H2CO3 is a conjugate acid, H2O is a base, HCO3- is a conjugate base, and H3O+ is an acid.
E) H2CO3 is an acid, H2O is a conjugate acid, HCO3- is a base, and H3O+ is a conjugate base.
A) H2CO3 is an acid, H2O is a base, HCO3- is a conjugate base, and H3O+ is a conjugate acid.
B) H2CO3 is a conjugate acid, H2O is a conjugate base, HCO3- is a base, and H3O+ is an acid.
C) H2CO3 is an acid, H2O is a conjugate base, HCO3- is a base, and H3O+ is a conjugate acid.
D) H2CO3 is a conjugate acid, H2O is a base, HCO3- is a conjugate base, and H3O+ is an acid.
E) H2CO3 is an acid, H2O is a conjugate acid, HCO3- is a base, and H3O+ is a conjugate base.

Unlock Deck
Unlock for access to all 156 flashcards in this deck.
Unlock Deck
k this deck
9
Which of the following is a general property of an acid?
A) Acids have sour taste.
B) Acids are not able to dissolve metals.
C) Acids turn red litmus paper blue.
D) Acids leave blue litmus paper unchanged.
E) All acids are dense liquids.
A) Acids have sour taste.
B) Acids are not able to dissolve metals.
C) Acids turn red litmus paper blue.
D) Acids leave blue litmus paper unchanged.
E) All acids are dense liquids.

Unlock Deck
Unlock for access to all 156 flashcards in this deck.
Unlock Deck
k this deck
10
Identify the monoprotic acid.
A) H2CO3
B) NH3
C) H3PO4
D) H2SO4
E) HBr
A) H2CO3
B) NH3
C) H3PO4
D) H2SO4
E) HBr

Unlock Deck
Unlock for access to all 156 flashcards in this deck.
Unlock Deck
k this deck
11
Which of the following is the correct Arrhenius definition of acids?
A) An acid is a substance that produces H+ ions in aqueous solutions.
B) An acid is a substance that reacts with H+ ions in aqueous solutions.
C) An acid is a substance that produces OH- ions in aqueous solutions.
D) An acid is a substance that decreases the amount of H+ ions in aqueous solutions.
E) An acid is a substance that does not alter the amount of H+ ions in aqueous solutions.
A) An acid is a substance that produces H+ ions in aqueous solutions.
B) An acid is a substance that reacts with H+ ions in aqueous solutions.
C) An acid is a substance that produces OH- ions in aqueous solutions.
D) An acid is a substance that decreases the amount of H+ ions in aqueous solutions.
E) An acid is a substance that does not alter the amount of H+ ions in aqueous solutions.

Unlock Deck
Unlock for access to all 156 flashcards in this deck.
Unlock Deck
k this deck
12
Identify the weak diprotic acid.
A) HNO3
B) H3PO4
C) H2SO3
D) HClO4
E) H2SO4
A) HNO3
B) H3PO4
C) H2SO3
D) HClO4
E) H2SO4

Unlock Deck
Unlock for access to all 156 flashcards in this deck.
Unlock Deck
k this deck
13
Which of the following is a Bronsted-Lowry base?
A) CH4
B) HCN
C) NH3
D) Cl2
E) B2H6
A) CH4
B) HCN
C) NH3
D) Cl2
E) B2H6

Unlock Deck
Unlock for access to all 156 flashcards in this deck.
Unlock Deck
k this deck
14
Identify the diprotic acid.
A) HNO3
B) HCl
C) CH3COOH
D) H2SO4
E) HClO4
A) HNO3
B) HCl
C) CH3COOH
D) H2SO4
E) HClO4

Unlock Deck
Unlock for access to all 156 flashcards in this deck.
Unlock Deck
k this deck
15
Which of the following species is amphoteric?
A) CO32-
B) HCl
C) NH4⁺
D) HPO42-
E) NaOH
A) CO32-
B) HCl
C) NH4⁺
D) HPO42-
E) NaOH

Unlock Deck
Unlock for access to all 156 flashcards in this deck.
Unlock Deck
k this deck
16
Which of the following groups is found in carboxylic acids (like acetic acid)?
A)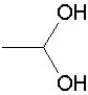
B)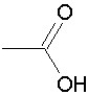
C)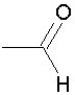
D)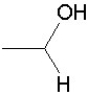
E)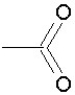
A)

B)

C)

D)

E)


Unlock Deck
Unlock for access to all 156 flashcards in this deck.
Unlock Deck
k this deck
17
Which of the following is a conjugate acid-base pair?
A) NH4+/NH3
B) H3O⁺/OH⁻
C) H2SO3/SO32⁻
D) SO42-/SO32-
E) NaOH/Na+
A) NH4+/NH3
B) H3O⁺/OH⁻
C) H2SO3/SO32⁻
D) SO42-/SO32-
E) NaOH/Na+

Unlock Deck
Unlock for access to all 156 flashcards in this deck.
Unlock Deck
k this deck
18
Which of the following is correct?
A) According to the Bronsted-Lowry theory, an acid is a proton donor while a base is a proton acceptor.
B) According to the Bronsted-Lowry theory, an acid is a proton acceptor while a base is a proton donor.
C) According to the Bronsted-Lowry theory, an acid is a proton donor while a base is a hydroxide ion acceptor.
D) According to the Bronsted-Lowry theory, an acid is a hydroxide donor while a base is a proton acceptor.
E) According to the Bronsted-Lowry theory, an acid is a hydroxide donor while a base is a hydroxide acceptor.
A) According to the Bronsted-Lowry theory, an acid is a proton donor while a base is a proton acceptor.
B) According to the Bronsted-Lowry theory, an acid is a proton acceptor while a base is a proton donor.
C) According to the Bronsted-Lowry theory, an acid is a proton donor while a base is a hydroxide ion acceptor.
D) According to the Bronsted-Lowry theory, an acid is a hydroxide donor while a base is a proton acceptor.
E) According to the Bronsted-Lowry theory, an acid is a hydroxide donor while a base is a hydroxide acceptor.

Unlock Deck
Unlock for access to all 156 flashcards in this deck.
Unlock Deck
k this deck
19
Which of the following is the correct Arrhenius definition of bases?
A) A base is a substance that produces H+ ions in aqueous solutions.
B) A base is a substance that reacts with OH- ions in aqueous solutions.
C) A base is a substance that decreases the amount of OH- ions in aqueous solutions.
D) A base is a substance that produces OH- ions in aqueous solutions.
E) A base is a substance that does not change the concentration of OH- ions in aqueous solutions.
A) A base is a substance that produces H+ ions in aqueous solutions.
B) A base is a substance that reacts with OH- ions in aqueous solutions.
C) A base is a substance that decreases the amount of OH- ions in aqueous solutions.
D) A base is a substance that produces OH- ions in aqueous solutions.
E) A base is a substance that does not change the concentration of OH- ions in aqueous solutions.

Unlock Deck
Unlock for access to all 156 flashcards in this deck.
Unlock Deck
k this deck
20
Which of the following is a Bronsted-Lowry acid?
A) NH4+
B) CH4
C) NH2-
D) NH3
E) Br2
A) NH4+
B) CH4
C) NH2-
D) NH3
E) Br2

Unlock Deck
Unlock for access to all 156 flashcards in this deck.
Unlock Deck
k this deck
21
Find the correct equilibrium constant that shows the ionization constant of a weak acid HA.
A) Ka =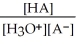
B) Ka =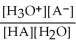
C) Ka =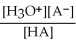
D) Ka =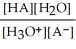
E) Ka =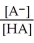
A) Ka =
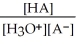
B) Ka =
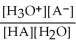
C) Ka =
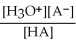
D) Ka =
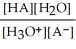
E) Ka =
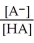

Unlock Deck
Unlock for access to all 156 flashcards in this deck.
Unlock Deck
k this deck
22
Which of the following is TRUE?
A) A neutral solution contains [H2O] = [H3O⁺]
B) A neutral solution does not contain any H3O+ or OH-
C) An acidic solution has [H3O⁺] > [OH⁻]
D) A basic solution does not contain H3O+
E) An acidic solution does not contain OH-
A) A neutral solution contains [H2O] = [H3O⁺]
B) A neutral solution does not contain any H3O+ or OH-
C) An acidic solution has [H3O⁺] > [OH⁻]
D) A basic solution does not contain H3O+
E) An acidic solution does not contain OH-

Unlock Deck
Unlock for access to all 156 flashcards in this deck.
Unlock Deck
k this deck
23
On which equilibrium does the strength of an acid HA depend on?
A) HA(aq) + H2O(l) ⇌ H2A+(aq) + OH-(aq)
B) HA(aq) + H2O(l) ⇌ A-(aq) + H3O+(aq)
C) HA(aq) + H2O(l) ⇌ H(HO)A-(aq) + H+(aq)
D) HA(aq) + OH-(aq) ⇌ A-(aq) + H2O(l)
E) HA(aq) + HA(aq) ⇌ H2A+(aq) + A-(aq)
A) HA(aq) + H2O(l) ⇌ H2A+(aq) + OH-(aq)
B) HA(aq) + H2O(l) ⇌ A-(aq) + H3O+(aq)
C) HA(aq) + H2O(l) ⇌ H(HO)A-(aq) + H+(aq)
D) HA(aq) + OH-(aq) ⇌ A-(aq) + H2O(l)
E) HA(aq) + HA(aq) ⇌ H2A+(aq) + A-(aq)

Unlock Deck
Unlock for access to all 156 flashcards in this deck.
Unlock Deck
k this deck
24
Which of the following is a strong acid?
A) C6H5CO2H
B) HCN
C) HClO4
D) NH4+
E) H2O
A) C6H5CO2H
B) HCN
C) HClO4
D) NH4+
E) H2O

Unlock Deck
Unlock for access to all 156 flashcards in this deck.
Unlock Deck
k this deck
25
What is the Kw of pure water at 50.0 °C, if the pH is 6.630?
A) 2.34 × 10-7
B) 5.50 × 10-14
C) 2.13 × 10-14
D) 1.00 × 10-14
E) 2.34 × 10-14
A) 2.34 × 10-7
B) 5.50 × 10-14
C) 2.13 × 10-14
D) 1.00 × 10-14
E) 2.34 × 10-14

Unlock Deck
Unlock for access to all 156 flashcards in this deck.
Unlock Deck
k this deck
26
Calculate the pH of a solution that contains 3.9 × 10-4 mol L-1 H3O⁺ at 25 °C.
A) 4.59
B) 3.41
C) 10.59
D) 9.41
E) 0.59
A) 4.59
B) 3.41
C) 10.59
D) 9.41
E) 0.59

Unlock Deck
Unlock for access to all 156 flashcards in this deck.
Unlock Deck
k this deck
27
What is the concentration of hydroxide ions in pure water at 30.0 °C if Kw at this temperature is 1.47 × 10-14?
A) 1.00 × 10-7 mol L-1
B) 1.30 × 10-7 mol L-1
C) 1.47 × 10-7 mol L-1
D) 8.93 × 10-8 mol L-1
E) 1.21 × 10-7 mol L-1
A) 1.00 × 10-7 mol L-1
B) 1.30 × 10-7 mol L-1
C) 1.47 × 10-7 mol L-1
D) 8.93 × 10-8 mol L-1
E) 1.21 × 10-7 mol L-1

Unlock Deck
Unlock for access to all 156 flashcards in this deck.
Unlock Deck
k this deck
28
The stronger the acid, ________.
A) the stronger the conjugate acid
B) the stronger the conjugate base
C) the weaker the conjugate base
D) the weaker the conjugate acid
E) the stronger the conjugate acid-base pair
A) the stronger the conjugate acid
B) the stronger the conjugate base
C) the weaker the conjugate base
D) the weaker the conjugate acid
E) the stronger the conjugate acid-base pair

Unlock Deck
Unlock for access to all 156 flashcards in this deck.
Unlock Deck
k this deck
29
Calculate the pOH of a solution that contains 3.9 × 10-4 mol L-1 H3O⁺ at 25 °C.
A) 4.59
B) 3.31
C) 10.59
D) 9.14
E) 0.59
A) 4.59
B) 3.31
C) 10.59
D) 9.14
E) 0.59

Unlock Deck
Unlock for access to all 156 flashcards in this deck.
Unlock Deck
k this deck
30
Which of the following correctly describes the characteristics of a strong acid?
A) ionizes only partially in aqueous solutions
B) has a very electron positive atom attached to the oxygen
C) has a nonpolar bond
D) is completely ionized in aqueous solution
E) is always monoprotic
A) ionizes only partially in aqueous solutions
B) has a very electron positive atom attached to the oxygen
C) has a nonpolar bond
D) is completely ionized in aqueous solution
E) is always monoprotic

Unlock Deck
Unlock for access to all 156 flashcards in this deck.
Unlock Deck
k this deck
31
Which of the following statements is TRUE?
A) A strong acid is composed of a proton and an anion that have a very strong attraction for one another.
B) A weak base is composed of a cation and an anion with a very weak attraction between them.
C) A strong acid has a strong conjugate base.
D) The conjugate base of a very weak acid is stronger than the conjugate base of a strong acid.
E) The dissociation reaction of a weak acid is completely shifted to the right.
A) A strong acid is composed of a proton and an anion that have a very strong attraction for one another.
B) A weak base is composed of a cation and an anion with a very weak attraction between them.
C) A strong acid has a strong conjugate base.
D) The conjugate base of a very weak acid is stronger than the conjugate base of a strong acid.
E) The dissociation reaction of a weak acid is completely shifted to the right.

Unlock Deck
Unlock for access to all 156 flashcards in this deck.
Unlock Deck
k this deck
32
Which of the following solutions would have the highest pH? Assume that they are all 0.10 mol L-1 in acid at 25 °C? The acid is followed by its Ka value.
A) HF, 3.5 × 10-4
B) HCN, 4.9 × 10-10
C) HNO2, 4.6 × 10-4
D) HCHO2, 1.8 × 10-4
E) HClO2, 1.1 × 10-2
A) HF, 3.5 × 10-4
B) HCN, 4.9 × 10-10
C) HNO2, 4.6 × 10-4
D) HCHO2, 1.8 × 10-4
E) HClO2, 1.1 × 10-2

Unlock Deck
Unlock for access to all 156 flashcards in this deck.
Unlock Deck
k this deck
33
Which of the following acids will have the strongest conjugate base?
A) HCl
B) HClO4
C) HNO3
D) HCN
E) HI
A) HCl
B) HClO4
C) HNO3
D) HCN
E) HI

Unlock Deck
Unlock for access to all 156 flashcards in this deck.
Unlock Deck
k this deck
34
Calculate the concentration of OH⁻ in a solution that contains 3.9 × 10-4 mol L-1 H3O⁺ at 25 °C. Also identify the solution as acidic, basic, or neutral.
A) 2.6 × 10-11 mol L-1, acidic
B) 2.6 × 10-11 mol L-1, basic
C) 3.9 × 10-4 mol L-1, neutral
D) 2.7 × 10-2 mol L-1, basic
E) 2.7 × 10-2 mol L-1, acidic
A) 2.6 × 10-11 mol L-1, acidic
B) 2.6 × 10-11 mol L-1, basic
C) 3.9 × 10-4 mol L-1, neutral
D) 2.7 × 10-2 mol L-1, basic
E) 2.7 × 10-2 mol L-1, acidic

Unlock Deck
Unlock for access to all 156 flashcards in this deck.
Unlock Deck
k this deck
35
Calculate the pH of a solution that contains 2.4 × 10-5 mol L-1 H3O⁺ at 25 °C.
A) 2.40
B) 9.38
C) 4.62
D) 11.60
E) 4.17
A) 2.40
B) 9.38
C) 4.62
D) 11.60
E) 4.17

Unlock Deck
Unlock for access to all 156 flashcards in this deck.
Unlock Deck
k this deck
36
What is the pH of pure water at 40.0 °C if the Kw at this temperature is 2.92 × 10-14?
A) 6.767
B) 0.465
C) 7.000
D) 7.233
E) 8.446
A) 6.767
B) 0.465
C) 7.000
D) 7.233
E) 8.446

Unlock Deck
Unlock for access to all 156 flashcards in this deck.
Unlock Deck
k this deck
37
Which of the following equilibria represents the second ionization step of H3PO4?
A) H3PO4(aq) + H2O(l) ⇌ H2PO4-(aq) + H3O+(aq)
B) H3PO4(aq) + 2H2O(l) ⇌ HPO42-(aq) + 2H3O+(aq)
C) H2PO4-(aq) + H2O(l) ⇌ HPO42-(aq) + H3O+(aq)
D) HPO42-(aq) + H2O(aq)⇌ PO43-(aq) + H3O+(aq)
E) H3PO4(aq) + 3H2O(l) ⇌ PO43-(aq) + 3H3O+(aq)
A) H3PO4(aq) + H2O(l) ⇌ H2PO4-(aq) + H3O+(aq)
B) H3PO4(aq) + 2H2O(l) ⇌ HPO42-(aq) + 2H3O+(aq)
C) H2PO4-(aq) + H2O(l) ⇌ HPO42-(aq) + H3O+(aq)
D) HPO42-(aq) + H2O(aq)⇌ PO43-(aq) + H3O+(aq)
E) H3PO4(aq) + 3H2O(l) ⇌ PO43-(aq) + 3H3O+(aq)

Unlock Deck
Unlock for access to all 156 flashcards in this deck.
Unlock Deck
k this deck
38
Which of the following is correct?
A) The smaller the acid ionization constant is, the more the acid ionizes and the stronger the acid.
B) The larger the acid ionization constant is, the less the acid ionizes and the weaker the acid.
C) The smaller the acid ionization constant is, the less the acid ionizes and the weaker the acid.
D) The smaller the acid ionization constant is, the less the acid ionizes and the weaker its conjugate base is.
E) The smaller the acid ionization constant is, the more the acid ionizes and the weaker the acid.
A) The smaller the acid ionization constant is, the more the acid ionizes and the stronger the acid.
B) The larger the acid ionization constant is, the less the acid ionizes and the weaker the acid.
C) The smaller the acid ionization constant is, the less the acid ionizes and the weaker the acid.
D) The smaller the acid ionization constant is, the less the acid ionizes and the weaker its conjugate base is.
E) The smaller the acid ionization constant is, the more the acid ionizes and the weaker the acid.

Unlock Deck
Unlock for access to all 156 flashcards in this deck.
Unlock Deck
k this deck
39
Calculate the concentration of H3O⁺ in a solution that contains 5.5 × 10-5 mol L-1 OH⁻ at 25 °C. Also identify the solution as acidic, basic, or neutral.
A) 1.8 × 10-10 mol L-1, basic
B) 1.8 × 10-10 mol L-1, acidic
C) 5.5 × 10-10 mol L-1, neutral
D) 9.2 × 10-1 mol L-1, acidic
E) 9.2 × 10-1 mol L-1, basic
A) 1.8 × 10-10 mol L-1, basic
B) 1.8 × 10-10 mol L-1, acidic
C) 5.5 × 10-10 mol L-1, neutral
D) 9.2 × 10-1 mol L-1, acidic
E) 9.2 × 10-1 mol L-1, basic

Unlock Deck
Unlock for access to all 156 flashcards in this deck.
Unlock Deck
k this deck
40
Which of the following is a weak acid?
A) HClO4
B) H2SO4
C) HCl
D) HCO2H
E) HNO3
A) HClO4
B) H2SO4
C) HCl
D) HCO2H
E) HNO3

Unlock Deck
Unlock for access to all 156 flashcards in this deck.
Unlock Deck
k this deck
41
Find the percent ionization of a 0.200 mol L-1 solution of formic acid. The Ka of formic acid is 1.8 × 10-4.
A) 3.0%
B) 2.0%
C) 1.0%
D) 3.5%
E) 4.6%
A) 3.0%
B) 2.0%
C) 1.0%
D) 3.5%
E) 4.6%

Unlock Deck
Unlock for access to all 156 flashcards in this deck.
Unlock Deck
k this deck
42
Determine the [H3O⁺] in a 0.265 mol L-1 HClO solution. The Ka of HClO is 2.9 × 10-8.
A) 1.1 × 10-10 mol L-1
B) 7.7 × 10-9 mol L-1
C) 1.3 × 10-6 mol L-1
D) 4.9 × 10-4 mol L-1
E) 8.8 × 10-5 mol L-1
A) 1.1 × 10-10 mol L-1
B) 7.7 × 10-9 mol L-1
C) 1.3 × 10-6 mol L-1
D) 4.9 × 10-4 mol L-1
E) 8.8 × 10-5 mol L-1

Unlock Deck
Unlock for access to all 156 flashcards in this deck.
Unlock Deck
k this deck
43
Find the percent ionization of a 0.265 mol L-1 HClO solution. The Ka of HClO is 2.9 × 10-8.
A) 0.0331%
B) 0.0551%
C) 1.12%
D) 3.65%
E) 3.32%
A) 0.0331%
B) 0.0551%
C) 1.12%
D) 3.65%
E) 3.32%

Unlock Deck
Unlock for access to all 156 flashcards in this deck.
Unlock Deck
k this deck
44
Determine the pH of a 0.461 mol L-1 C6H5CO2H solution if the Ka of C6H5CO2H is 6.5 × 10-5.
A) 2.26
B) 4.52
C) 11.74
D) 9.48
E) 5.48
A) 2.26
B) 4.52
C) 11.74
D) 9.48
E) 5.48

Unlock Deck
Unlock for access to all 156 flashcards in this deck.
Unlock Deck
k this deck
45
Calculate the pOH of an aqueous solution with a pH of 9.85 at 25 °C.
A) 2.15
B) 3.15
C) 4.15
D) 5.15
E) 4.00
A) 2.15
B) 3.15
C) 4.15
D) 5.15
E) 4.00

Unlock Deck
Unlock for access to all 156 flashcards in this deck.
Unlock Deck
k this deck
46
Calculate the hydronium ion concentration in an aqueous solution with a pH of 9.85 at 25 °C.
A) 7.1 × 10-5 mol L-1
B) 4.2 × 10-10 mol L-1
C) 8.7 × 10-10 mol L-1
D) 6.5 × 10-5 mol L-1
E) 1.4 × 10-10 mol L-1
A) 7.1 × 10-5 mol L-1
B) 4.2 × 10-10 mol L-1
C) 8.7 × 10-10 mol L-1
D) 6.5 × 10-5 mol L-1
E) 1.4 × 10-10 mol L-1

Unlock Deck
Unlock for access to all 156 flashcards in this deck.
Unlock Deck
k this deck
47
Which of the following acids is the strongest? The acid is followed by its Ka value.
A) HF, 3.5 × 10-4
B) HCN, 4.9 × 10-10
C) HNO2, 4.6 × 10-4
D) HCHO2, 1.8 × 10-4
E) HClO2, 1.1 × 10-2
A) HF, 3.5 × 10-4
B) HCN, 4.9 × 10-10
C) HNO2, 4.6 × 10-4
D) HCHO2, 1.8 × 10-4
E) HClO2, 1.1 × 10-2

Unlock Deck
Unlock for access to all 156 flashcards in this deck.
Unlock Deck
k this deck
48
The pH of a solution is 5.67 at 25 °C. Calculate the pOH of this solution at 25 °C.
A) 2.40
B) 8.33
C) 4.88
D) 11.4
E) 12.7
A) 2.40
B) 8.33
C) 4.88
D) 11.4
E) 12.7

Unlock Deck
Unlock for access to all 156 flashcards in this deck.
Unlock Deck
k this deck
49
Determine the Ka of an acid whose 0.294 mol L-1 solution has a pH of 2.80.
A) 1.2 × 10-5
B) 8.5 × 10-6
C) 2.7
D) 4.9 × 10-7
E) 5.4 × 10-3
A) 1.2 × 10-5
B) 8.5 × 10-6
C) 2.7
D) 4.9 × 10-7
E) 5.4 × 10-3

Unlock Deck
Unlock for access to all 156 flashcards in this deck.
Unlock Deck
k this deck
50
The pH of a solution is 9.12 at 25 °C. Calculate the pOH of this solution at 25 °C.
A) 2.40
B) 8.33
C) 4.88
D) 11.4
E) 12.7
A) 2.40
B) 8.33
C) 4.88
D) 11.4
E) 12.7

Unlock Deck
Unlock for access to all 156 flashcards in this deck.
Unlock Deck
k this deck
51
Determine the pH of a 0.00598 mol L-1 HClO4 solution.
A) 1.777
B) 6.434
C) 7.566
D) 2.223
E) 3.558
A) 1.777
B) 6.434
C) 7.566
D) 2.223
E) 3.558

Unlock Deck
Unlock for access to all 156 flashcards in this deck.
Unlock Deck
k this deck
52
The pH of a solution is 2.62 at 25 °C. Calculate the pOH of this solution at 25 °C.
A) 2.40
B) 8.33
C) 4.88
D) 11.4
E) 12.7
A) 2.40
B) 8.33
C) 4.88
D) 11.4
E) 12.7

Unlock Deck
Unlock for access to all 156 flashcards in this deck.
Unlock Deck
k this deck
53
Which of the following acids is the weakest? The acid is followed by its Ka value.
A) CH3COOH, 1.8 × 10-5
B) HIO, 2.3 × 10-11
C) HBrO, 2.3 × 10-9
D) HClO, 2.9 × 10-8
E) C6H5COOH, 6.3 × 10-5
A) CH3COOH, 1.8 × 10-5
B) HIO, 2.3 × 10-11
C) HBrO, 2.3 × 10-9
D) HClO, 2.9 × 10-8
E) C6H5COOH, 6.3 × 10-5

Unlock Deck
Unlock for access to all 156 flashcards in this deck.
Unlock Deck
k this deck
54
Determine the pH of a 0.023 mol L-1 HNO3 solution.
A) 12.36
B) 3.68
C) 1.64
D) 2.30
E) 2.49
A) 12.36
B) 3.68
C) 1.64
D) 2.30
E) 2.49

Unlock Deck
Unlock for access to all 156 flashcards in this deck.
Unlock Deck
k this deck
55
Find the percent ionization of a 0.400 mol L-1 solution of formic acid. The Ka of formic acid is 1.8 × 10-4.
A) 3.4%
B) 2.1%
C) 1.1%
D) 3.5%
E) 4.6%
A) 3.4%
B) 2.1%
C) 1.1%
D) 3.5%
E) 4.6%

Unlock Deck
Unlock for access to all 156 flashcards in this deck.
Unlock Deck
k this deck
56
Calculate the pOH of a solution that contains 2.4 × 10-5 mol L-1 H3O⁺ at 25 °C.
A) 2.40
B) 9.38
C) 4.62
D) 11.60
E) 4.17
A) 2.40
B) 9.38
C) 4.62
D) 11.60
E) 4.17

Unlock Deck
Unlock for access to all 156 flashcards in this deck.
Unlock Deck
k this deck
57
Calculate the pH of a solution that contains 7.8 × 10-6 mol L-1 OH⁻ at 25 °C.
A) 1.28
B) 5.11
C) 12.72
D) 8.89
E) 9.64
A) 1.28
B) 5.11
C) 12.72
D) 8.89
E) 9.64

Unlock Deck
Unlock for access to all 156 flashcards in this deck.
Unlock Deck
k this deck
58
Calculate the hydroxide ion concentration in an aqueous solution with a pH of 4.33 at 25 °C.
A) 2.1 × 10-10 mol L-1
B) 9.7 × 10-10 mol L-1
C) 4.7 × 10-5 mol L-1
D) 3.8 × 10-5 mol L-1
E) 6.3 × 10-6 mol L-1
A) 2.1 × 10-10 mol L-1
B) 9.7 × 10-10 mol L-1
C) 4.7 × 10-5 mol L-1
D) 3.8 × 10-5 mol L-1
E) 6.3 × 10-6 mol L-1

Unlock Deck
Unlock for access to all 156 flashcards in this deck.
Unlock Deck
k this deck
59
Calculate the hydroxide ion concentration in an aqueous solution with a pH of 9.85 at 25 °C.
A) 7.1 × 10-5 mol L-1
B) 4.2 × 10-10 mol L-1
C) 8.7 × 10-10 mol L-1
D) 6.5 × 10-5 mol L-1
E) 1.4 × 10-10 mol L-1
A) 7.1 × 10-5 mol L-1
B) 4.2 × 10-10 mol L-1
C) 8.7 × 10-10 mol L-1
D) 6.5 × 10-5 mol L-1
E) 1.4 × 10-10 mol L-1

Unlock Deck
Unlock for access to all 156 flashcards in this deck.
Unlock Deck
k this deck
60
Find the percent ionization of a 0.337 mol L-1 HF solution. The Ka for HF is 3.5 × 10-4.
A) 1.1%
B) 1.2 × 10-2%
C) 3.2%
D) 3.5 × 10-2%
E) 4.7%
A) 1.1%
B) 1.2 × 10-2%
C) 3.2%
D) 3.5 × 10-2%
E) 4.7%

Unlock Deck
Unlock for access to all 156 flashcards in this deck.
Unlock Deck
k this deck
61
Determine the pH of a 0.741 mol L-1 KOH solution at 25 °C.
A) 0.13
B) 13.87
C) 0.17
D) 12.65
E) 11.88
A) 0.13
B) 13.87
C) 0.17
D) 12.65
E) 11.88

Unlock Deck
Unlock for access to all 156 flashcards in this deck.
Unlock Deck
k this deck
62
Determine the pOH of a 0.116 mol L-1 Ba(OH)2 solution at 25 °C.
A) 8.62
B) 13.06
C) 13.37
D) 0.63
E) 12.56
A) 8.62
B) 13.06
C) 13.37
D) 0.63
E) 12.56

Unlock Deck
Unlock for access to all 156 flashcards in this deck.
Unlock Deck
k this deck
63
Which of the following is a weak base?
A) NH(CH3)2
B) N2
C) NaOH
D) CH2CH2
E) Ca(OH)2
A) NH(CH3)2
B) N2
C) NaOH
D) CH2CH2
E) Ca(OH)2

Unlock Deck
Unlock for access to all 156 flashcards in this deck.
Unlock Deck
k this deck
64
Determine the pH of a 0.188 mol L-1 NH3 solution at 25 °C. The Kb of NH3 is 1.76 × 10-5.
A) 5.480
B) 2.740
C) 8.520
D) 11.260
E) 12.656
A) 5.480
B) 2.740
C) 8.520
D) 11.260
E) 12.656

Unlock Deck
Unlock for access to all 156 flashcards in this deck.
Unlock Deck
k this deck
65
Which of the following bases is the strongest? The base is followed by its Kb.
A) (CH3CH2)2NH, 8.6 × 10-4
B) CH3NH2, 4.4 × 10-4
C) C6H5NH2, 4.0 × 10-10
D) NH3, 1.76 × 10-5
E) C5H5N, 1.7 × 10-9
A) (CH3CH2)2NH, 8.6 × 10-4
B) CH3NH2, 4.4 × 10-4
C) C6H5NH2, 4.0 × 10-10
D) NH3, 1.76 × 10-5
E) C5H5N, 1.7 × 10-9

Unlock Deck
Unlock for access to all 156 flashcards in this deck.
Unlock Deck
k this deck
66
Determine the pOH of a 0.188 mol L-1 NH3 solution at 25 °C. The Kb of NH3 is 1.76 × 10-5.
A) 5.480
B) 2.740
C) 8.520
D) 11.260
E) 12.656
A) 5.480
B) 2.740
C) 8.520
D) 11.260
E) 12.656

Unlock Deck
Unlock for access to all 156 flashcards in this deck.
Unlock Deck
k this deck
67
Determine the [OH-] concentration of a 0.741 mol L-1 KOH solution at 25 °C.
A) 1.34 × 10-13 mol L-1
B) 1.34 × 10-14 mol L-1
C) 0.741 mol L-1
D) 7.41 mol L-1
E) 7.41 × 10-13 mol L-1
A) 1.34 × 10-13 mol L-1
B) 1.34 × 10-14 mol L-1
C) 0.741 mol L-1
D) 7.41 mol L-1
E) 7.41 × 10-13 mol L-1

Unlock Deck
Unlock for access to all 156 flashcards in this deck.
Unlock Deck
k this deck
68
Determine the pOH in a 0.235 mol L-1 NaOH solution.
A) 13.76
B) 0.24
C) 13.37
D) 0.63
E) 12
A) 13.76
B) 0.24
C) 13.37
D) 0.63
E) 12

Unlock Deck
Unlock for access to all 156 flashcards in this deck.
Unlock Deck
k this deck
69
Determine the [OH⁻] concentration in a 0.235 mol L-1 NaOH solution.
A) 4.25 × 10-14 mol L-1
B) 0.470 mol L-1
C) 2.13 × 10-14 mol L-1
D) 0.198 mol L-1
E) 0.235 mol L-1
A) 4.25 × 10-14 mol L-1
B) 0.470 mol L-1
C) 2.13 × 10-14 mol L-1
D) 0.198 mol L-1
E) 0.235 mol L-1

Unlock Deck
Unlock for access to all 156 flashcards in this deck.
Unlock Deck
k this deck
70
Calculate the pH of a 0.200 mol L-1 solution of formic acid, Ka = 1.8 × 10-4.
A) 3.84
B) 2.22
C) 4.62
D) 1.97
E) 3.07
A) 3.84
B) 2.22
C) 4.62
D) 1.97
E) 3.07

Unlock Deck
Unlock for access to all 156 flashcards in this deck.
Unlock Deck
k this deck
71
Determine the pOH of a 0.051 mol L-1 Sr(OH)2 solution at 25 °C.
A) 8.62
B) 13.01
C) 13.37
D) 0.991
E) 12.56
A) 8.62
B) 13.01
C) 13.37
D) 0.991
E) 12.56

Unlock Deck
Unlock for access to all 156 flashcards in this deck.
Unlock Deck
k this deck
72
Which of the following bases is the weakest? The base is followed by its Kb value.
A) HOCH2CH2NH2, 3.2 × 10-5
B) NH3, 1.76 × 10-5
C) C5H5N, 1.7 × 10-9
D) (CH3CH2)3N, 5.2 × 10-4
E) CH3CH2NH2, 5.6 × 10-4
A) HOCH2CH2NH2, 3.2 × 10-5
B) NH3, 1.76 × 10-5
C) C5H5N, 1.7 × 10-9
D) (CH3CH2)3N, 5.2 × 10-4
E) CH3CH2NH2, 5.6 × 10-4

Unlock Deck
Unlock for access to all 156 flashcards in this deck.
Unlock Deck
k this deck
73
Determine the [OH⁻] concentration in a 0.169 mol L-1 Ca(OH)2 solution.
A) 0.338 mol L-1
B) 0.169 mol L-1
C) 5.92 × 10-14 mol L-1
D) 2.96 × 10-14 mol L-1
E) 0.298 mol L-1
A) 0.338 mol L-1
B) 0.169 mol L-1
C) 5.92 × 10-14 mol L-1
D) 2.96 × 10-14 mol L-1
E) 0.298 mol L-1

Unlock Deck
Unlock for access to all 156 flashcards in this deck.
Unlock Deck
k this deck
74
Determine the pH of a 0.116 mol L-1 Ba(OH)2 solution at 25 °C.
A) 8.62
B) 13.06
C) 13.37
D) 0.63
E) 12.56
A) 8.62
B) 13.06
C) 13.37
D) 0.63
E) 12.56

Unlock Deck
Unlock for access to all 156 flashcards in this deck.
Unlock Deck
k this deck
75
Which of the following is a strong base?
A) Cl-
B) NH3
C) CH3OH
D) NO3⁻
E) KOH
A) Cl-
B) NH3
C) CH3OH
D) NO3⁻
E) KOH

Unlock Deck
Unlock for access to all 156 flashcards in this deck.
Unlock Deck
k this deck
76
Determine the pH of a 0.051 mol L-1 Sr(OH)2 solution at 25 °C.
A) 8.62
B) 13.01
C) 13.37
D) 0.991
E) 12.56
A) 8.62
B) 13.01
C) 13.37
D) 0.991
E) 12.56

Unlock Deck
Unlock for access to all 156 flashcards in this deck.
Unlock Deck
k this deck
77
Determine the [OH-] concentration of a 0.116 mol L-1 Ba(OH)2 solution at 25 °C.
A) 4.310 × 10-14 mol L-1
B) 0.116 mol L-1
C) 0.232 mol L-1
D) 8.62 × 10-14 mol L-1
E) 0.058 mol L-1
A) 4.310 × 10-14 mol L-1
B) 0.116 mol L-1
C) 0.232 mol L-1
D) 8.62 × 10-14 mol L-1
E) 0.058 mol L-1

Unlock Deck
Unlock for access to all 156 flashcards in this deck.
Unlock Deck
k this deck
78
Determine the pH in a 0.435 mol L-1 LiOH solution.
A) 13.6
B) 0.24
C) 13.37
D) 0.6
E) 12
A) 13.6
B) 0.24
C) 13.37
D) 0.6
E) 12

Unlock Deck
Unlock for access to all 156 flashcards in this deck.
Unlock Deck
k this deck
79
Determine the pH in a 0.235 mol L-1 NaOH solution.
A) 13.76
B) 0.24
C) 13.37
D) 0.63
E) 12
A) 13.76
B) 0.24
C) 13.37
D) 0.63
E) 12

Unlock Deck
Unlock for access to all 156 flashcards in this deck.
Unlock Deck
k this deck
80
Determine the pOH of a 0.741 mol L-1 KOH solution at 25 °C.
A) 0.13
B) 13.87
C) 0.17
D) 12.65
E) 11.88
A) 0.13
B) 13.87
C) 0.17
D) 12.65
E) 11.88

Unlock Deck
Unlock for access to all 156 flashcards in this deck.
Unlock Deck
k this deck


