Deck 1: Units of Measurement for Physical and Chemical Change
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/192
Play
Full screen (f)
Deck 1: Units of Measurement for Physical and Chemical Change
1
Which of the following is an example of physical change?
A) Sugar is dissolved in water.
B) Bread is baked.
C) Fireworks explode.
D) Baking soda decomposes.
A) Sugar is dissolved in water.
B) Bread is baked.
C) Fireworks explode.
D) Baking soda decomposes.
Sugar is dissolved in water.
2
The energy associated with the temperature of an object is known as the ________.
A) potential energy
B) total energy
C) thermal energy
D) gravitational energy
E) magnetic energy
A) potential energy
B) total energy
C) thermal energy
D) gravitational energy
E) magnetic energy
thermal energy
3
________ is the energy associated with motion of an object.
A) Potential energy
B) Kinetic energy
C) Total energy
D) Solar energy
E) Gravitational energy
A) Potential energy
B) Kinetic energy
C) Total energy
D) Solar energy
E) Gravitational energy
Kinetic energy
4
Kilogram is a measure of ________.
A) mass
B) time
C) temperature
D) length
E) volume
A) mass
B) time
C) temperature
D) length
E) volume

Unlock Deck
Unlock for access to all 192 flashcards in this deck.
Unlock Deck
k this deck
5
Which of the following is an example of a chemical change?
A) Copper building materials develop a green patina over time.
B) Solid ice melts.
C) Ethanol evaporates.
D) Brewing tea.
A) Copper building materials develop a green patina over time.
B) Solid ice melts.
C) Ethanol evaporates.
D) Brewing tea.

Unlock Deck
Unlock for access to all 192 flashcards in this deck.
Unlock Deck
k this deck
6
A physical change ________.
A) occurs when iron rusts
B) occurs when sugar is heated into caramel
C) occurs when glucose is converted into energy within your cells
D) occurs when water is evaporated
E) occurs when propane is burned for heat
A) occurs when iron rusts
B) occurs when sugar is heated into caramel
C) occurs when glucose is converted into energy within your cells
D) occurs when water is evaporated
E) occurs when propane is burned for heat

Unlock Deck
Unlock for access to all 192 flashcards in this deck.
Unlock Deck
k this deck
7
Which of the following represents a chemical property of hydrogen gas?
A) It is gaseous at room temperature.
B) It is less dense than air.
C) It reacts explosively with oxygen.
D) It is colourless.
E) It is tasteless.
A) It is gaseous at room temperature.
B) It is less dense than air.
C) It reacts explosively with oxygen.
D) It is colourless.
E) It is tasteless.

Unlock Deck
Unlock for access to all 192 flashcards in this deck.
Unlock Deck
k this deck
8
Kelvin is a measure of ________.
A) mass
B) time
C) temperature
D) length
E) volume
A) mass
B) time
C) temperature
D) length
E) volume

Unlock Deck
Unlock for access to all 192 flashcards in this deck.
Unlock Deck
k this deck
9
Which of the following is an example of a chemical change?
A) Condensation of steam
B) Melting gold
C) Evaporating water
D) Burning wood
E) Sublimation of moth balls
A) Condensation of steam
B) Melting gold
C) Evaporating water
D) Burning wood
E) Sublimation of moth balls

Unlock Deck
Unlock for access to all 192 flashcards in this deck.
Unlock Deck
k this deck
10
Total energy of an object is best defined as the sum of ________.
A) kinetic energy and potential energy
B) kinetic energy and thermal energy
C) chemical energy and potential energy
D) chemical energy and magnetic energy
E) potential energy and magnetic energy
A) kinetic energy and potential energy
B) kinetic energy and thermal energy
C) chemical energy and potential energy
D) chemical energy and magnetic energy
E) potential energy and magnetic energy

Unlock Deck
Unlock for access to all 192 flashcards in this deck.
Unlock Deck
k this deck
11
A chemical change ________.
A) occurs when methane gas is burned
B) occurs when paper is shredded
C) occurs when water is vaporized
D) occurs when sand is mixed with water
E) occurs when powdered lemonade is stirred into water
A) occurs when methane gas is burned
B) occurs when paper is shredded
C) occurs when water is vaporized
D) occurs when sand is mixed with water
E) occurs when powdered lemonade is stirred into water

Unlock Deck
Unlock for access to all 192 flashcards in this deck.
Unlock Deck
k this deck
12
Which of the following represents a physical property?
A) Sodium metal is extremely reactive with chlorine gas.
B) Mercury is a silvery liquid at room temperature.
C) Aluminum has a tendency to "rust."
D) Butane is highly flammable.
E) Argon has an unreactive nature.
A) Sodium metal is extremely reactive with chlorine gas.
B) Mercury is a silvery liquid at room temperature.
C) Aluminum has a tendency to "rust."
D) Butane is highly flammable.
E) Argon has an unreactive nature.

Unlock Deck
Unlock for access to all 192 flashcards in this deck.
Unlock Deck
k this deck
13
________ is the energy associated with the position or composition of an object.
A) Kinetic energy
B) Total energy
C) Solar energy
D) Gravitational energy
E) Potential energy
A) Kinetic energy
B) Total energy
C) Solar energy
D) Gravitational energy
E) Potential energy

Unlock Deck
Unlock for access to all 192 flashcards in this deck.
Unlock Deck
k this deck
14
Which of the following is an SI base unit?
A) volt
B) gram
C) hour
D) kelvin
E) dozen
A) volt
B) gram
C) hour
D) kelvin
E) dozen

Unlock Deck
Unlock for access to all 192 flashcards in this deck.
Unlock Deck
k this deck
15
Identify the unit of measurement that is a SI base unit of measurement.
A) second
B) Celsius
C) cup
D) pound
E) yard
A) second
B) Celsius
C) cup
D) pound
E) yard

Unlock Deck
Unlock for access to all 192 flashcards in this deck.
Unlock Deck
k this deck
16
Metre is a measure of ________.
A) mass
B) time
C) temperature
D) length
E) volume
A) mass
B) time
C) temperature
D) length
E) volume

Unlock Deck
Unlock for access to all 192 flashcards in this deck.
Unlock Deck
k this deck
17
Which of the following is an example of a physical change?
A) Dew forms on a blade of grass.
B) A Halloween light stick glows after shaking.
C) An egg solidifies during cooking.
D) A hydrogen balloon explodes when contacted with a flame.
E) None of the above is a physical change.
A) Dew forms on a blade of grass.
B) A Halloween light stick glows after shaking.
C) An egg solidifies during cooking.
D) A hydrogen balloon explodes when contacted with a flame.
E) None of the above is a physical change.

Unlock Deck
Unlock for access to all 192 flashcards in this deck.
Unlock Deck
k this deck
18
Which of the following is an example of a physical change?
A) Burning wood
B) Melting ice
C) Digesting food
D) Souring milk
E) Making water from hydrogen gas and oxygen gas
A) Burning wood
B) Melting ice
C) Digesting food
D) Souring milk
E) Making water from hydrogen gas and oxygen gas

Unlock Deck
Unlock for access to all 192 flashcards in this deck.
Unlock Deck
k this deck
19
Which of the following statements is FALSE?
A) Energy is neither created nor destroyed.
B) When an object falls from a higher surface, gravitational potential energy converts into kinetic energy.
C) Physical and chemical changes are usually accompanied by energy changes.
D) The molecules that compose gasoline have chemical potential energy.
E) When gasoline undergoes combustion, the molecules that are newly formed have higher potential energy than the molecules that compose gasoline.
A) Energy is neither created nor destroyed.
B) When an object falls from a higher surface, gravitational potential energy converts into kinetic energy.
C) Physical and chemical changes are usually accompanied by energy changes.
D) The molecules that compose gasoline have chemical potential energy.
E) When gasoline undergoes combustion, the molecules that are newly formed have higher potential energy than the molecules that compose gasoline.

Unlock Deck
Unlock for access to all 192 flashcards in this deck.
Unlock Deck
k this deck
20
Second is a measure of ________.
A) mass
B) time
C) temperature
D) length
E) volume
A) mass
B) time
C) temperature
D) length
E) volume

Unlock Deck
Unlock for access to all 192 flashcards in this deck.
Unlock Deck
k this deck
21
Convert 514 nm to m and express it in scientific notation.
A) 514*10-5 m
B) 5.14*10-7 m
C) 51.4*10-7 m
D) 5.14*1011 m
E) 5.14*10-9 m
A) 514*10-5 m
B) 5.14*10-7 m
C) 51.4*10-7 m
D) 5.14*1011 m
E) 5.14*10-9 m

Unlock Deck
Unlock for access to all 192 flashcards in this deck.
Unlock Deck
k this deck
22
Ampere is a measure of ________.
A) luminous intensity
B) amount
C) mass
D) length
E) current
A) luminous intensity
B) amount
C) mass
D) length
E) current

Unlock Deck
Unlock for access to all 192 flashcards in this deck.
Unlock Deck
k this deck
23
Which of the following is an example of intensive properties?
A) density
B) volume
C) mass
D) None of the above is an example of intensive properties.
E) All of the above are examples of intensive properties.
A) density
B) volume
C) mass
D) None of the above is an example of intensive properties.
E) All of the above are examples of intensive properties.

Unlock Deck
Unlock for access to all 192 flashcards in this deck.
Unlock Deck
k this deck
24
If the temperature is 178 °F, what is the temperature in degrees Celsius?
A) 352 °C
B) 451 °C
C) 67 °C
D) 81.1 °C
E) 378 °C
A) 352 °C
B) 451 °C
C) 67 °C
D) 81.1 °C
E) 378 °C

Unlock Deck
Unlock for access to all 192 flashcards in this deck.
Unlock Deck
k this deck
25
Identify the common substance that has the lowest density.
A) ice
B) aluminum
C) copper
D) table salt
E) sugar
A) ice
B) aluminum
C) copper
D) table salt
E) sugar

Unlock Deck
Unlock for access to all 192 flashcards in this deck.
Unlock Deck
k this deck
26
Convert 1.34 km2 to m2.
A) 1340000 m2
B) 134000 m2
C) 1.34 × 107 m2
D) 1.34 × 104 m2
E) 1340 m2
A) 1340000 m2
B) 134000 m2
C) 1.34 × 107 m2
D) 1.34 × 104 m2
E) 1340 m2

Unlock Deck
Unlock for access to all 192 flashcards in this deck.
Unlock Deck
k this deck
27
Convert 8.3 mg to μg.
A) 0.0083 μg
B) 83 μg
C) 830 μg
D) 8300 μg
E) 83000 μg
A) 0.0083 μg
B) 83 μg
C) 830 μg
D) 8300 μg
E) 83000 μg

Unlock Deck
Unlock for access to all 192 flashcards in this deck.
Unlock Deck
k this deck
28
How many dm are in 345 dam?
A) 0.345 dm
B) 3.45 dm
C) 3450 dm
D) 34500 dm
E) 345000 dm
A) 0.345 dm
B) 3.45 dm
C) 3450 dm
D) 34500 dm
E) 345000 dm

Unlock Deck
Unlock for access to all 192 flashcards in this deck.
Unlock Deck
k this deck
29
Convert 12.5 μL to L and express it in scientific notation.
A) 1.25*105 L
B) 1.25*107 L
C) 1.25*10-3 L
D) 1.25*10-7 L
E) 1.25*10-5 L
A) 1.25*105 L
B) 1.25*107 L
C) 1.25*10-3 L
D) 1.25*10-7 L
E) 1.25*10-5 L

Unlock Deck
Unlock for access to all 192 flashcards in this deck.
Unlock Deck
k this deck
30
If a solution has a temperature of 355 K, what is its temperature in degrees Celsius?
A) 165 °C
B) 628 °C
C) 179 °C
D) 279 °C
E) 82 °C
A) 165 °C
B) 628 °C
C) 179 °C
D) 279 °C
E) 82 °C

Unlock Deck
Unlock for access to all 192 flashcards in this deck.
Unlock Deck
k this deck
31
Which of the following is an example of extensive properties?
A) mass
B) colour
C) density
D) temperature
E) taste
A) mass
B) colour
C) density
D) temperature
E) taste

Unlock Deck
Unlock for access to all 192 flashcards in this deck.
Unlock Deck
k this deck
32
Convert 46.9 GHz to THz.
A) 0.0469 THz
B) 46900 THz
C) 0.469 THz
D) 4690 THz
E) 0.000469 THz
A) 0.0469 THz
B) 46900 THz
C) 0.469 THz
D) 4690 THz
E) 0.000469 THz

Unlock Deck
Unlock for access to all 192 flashcards in this deck.
Unlock Deck
k this deck
33
Mole is a measure of ________.
A) luminous intensity
B) amount
C) mass
D) length
E) current
A) luminous intensity
B) amount
C) mass
D) length
E) current

Unlock Deck
Unlock for access to all 192 flashcards in this deck.
Unlock Deck
k this deck
34
Identify the common substance that has the highest density.
A) sugar
B) water
C) glass
D) lead
E) aluminum
A) sugar
B) water
C) glass
D) lead
E) aluminum

Unlock Deck
Unlock for access to all 192 flashcards in this deck.
Unlock Deck
k this deck
35
Convert 4392 m3 to hm3.
A) 4.392 × 10-5 hm3
B) 4.392 × 10-3 hm3
C) 4.392 × 10-1 hm3
D) 4.392 × 101 hm3
E) 4.392 × 102 hm3
A) 4.392 × 10-5 hm3
B) 4.392 × 10-3 hm3
C) 4.392 × 10-1 hm3
D) 4.392 × 101 hm3
E) 4.392 × 102 hm3

Unlock Deck
Unlock for access to all 192 flashcards in this deck.
Unlock Deck
k this deck
36
If the outside temperature is 35 °C, what is the temperature in K?
A) -238 K
B) 308 K
C) 95 K
D) 31 K
E) 63 K
A) -238 K
B) 308 K
C) 95 K
D) 31 K
E) 63 K

Unlock Deck
Unlock for access to all 192 flashcards in this deck.
Unlock Deck
k this deck
37
If the speed limit on a highway is 100 km h-1, what is the speed limit in m h-1?
A) 1 × 107 m h-1
B) 0.1 m h-1
C) 1 × 103 m h-1
D) 1 × 105 m h-1
E) 1 × 101 m h-1
A) 1 × 107 m h-1
B) 0.1 m h-1
C) 1 × 103 m h-1
D) 1 × 105 m h-1
E) 1 × 101 m h-1

Unlock Deck
Unlock for access to all 192 flashcards in this deck.
Unlock Deck
k this deck
38
Determine the density of an object that has a mass of 149.8 g and displaces 12.1 mL of water when placed in a graduated cylinder.
A) 8.08 g mL-1
B) 1.38 g mL-1
C) 12 .4 g mL-1
D) 18.1 g mL-1
E) 11.4 g mL-1
A) 8.08 g mL-1
B) 1.38 g mL-1
C) 12 .4 g mL-1
D) 18.1 g mL-1
E) 11.4 g mL-1

Unlock Deck
Unlock for access to all 192 flashcards in this deck.
Unlock Deck
k this deck
39
How many ns are in 16 ms?
A) 1.6 × 10-2 ns
B) 1.6 × 107 ns
C) 1.6 × 10-5 ns
D) 1.6 × 105 ns
E) 1.6 × 102 ns
A) 1.6 × 10-2 ns
B) 1.6 × 107 ns
C) 1.6 × 10-5 ns
D) 1.6 × 105 ns
E) 1.6 × 102 ns

Unlock Deck
Unlock for access to all 192 flashcards in this deck.
Unlock Deck
k this deck
40
Convert 3000 m h-1 to km h-1.
A) 3 km h-1
B) 0.3 km h-1
C) 30 km h-1
D) 300 km h-1
E) 0.03 km h-1
A) 3 km h-1
B) 0.3 km h-1
C) 30 km h-1
D) 300 km h-1
E) 0.03 km h-1

Unlock Deck
Unlock for access to all 192 flashcards in this deck.
Unlock Deck
k this deck
41
An unknown metal is heated to 850 °F. What is the temperature in K?
A) 1350 K
B) 1120 K
C) 460 K
D) 540 K
E) 730 K
A) 1350 K
B) 1120 K
C) 460 K
D) 540 K
E) 730 K

Unlock Deck
Unlock for access to all 192 flashcards in this deck.
Unlock Deck
k this deck
42
Determine the volume of an object that has a mass of 100 g and a density of 9.55 g mL-1.
A) 10.5 mL
B) 955 mL
C) 0.095 mL
D) 25.6 mL
E) 27.9 mL
A) 10.5 mL
B) 955 mL
C) 0.095 mL
D) 25.6 mL
E) 27.9 mL

Unlock Deck
Unlock for access to all 192 flashcards in this deck.
Unlock Deck
k this deck
43
A solution has a temperature of 18.65 °C. What is the temperature in K?
A) 324.80 K
B) 291.80 K
C) 301.65 K
D) 65.57 K
E) 283.15 K
A) 324.80 K
B) 291.80 K
C) 301.65 K
D) 65.57 K
E) 283.15 K

Unlock Deck
Unlock for access to all 192 flashcards in this deck.
Unlock Deck
k this deck
44
A student performs an experiment to determine the density of a sugar solution. She obtains the following results: 1.11 g mL-1, 1.81 g mL-1, 1.95 g mL-1, 1.75 g mL-1. If the actual value for the density of the sugar solution is 1.75 g mL-1, which statement below best describes her results?
A) Her results are precise, but not accurate.
B) Her results are accurate, but not precise.
C) Her results are both precise and accurate
D) Her results are neither precise nor accurate.
E) It isn't possible to determine from the information given.
A) Her results are precise, but not accurate.
B) Her results are accurate, but not precise.
C) Her results are both precise and accurate
D) Her results are neither precise nor accurate.
E) It isn't possible to determine from the information given.

Unlock Deck
Unlock for access to all 192 flashcards in this deck.
Unlock Deck
k this deck
45
Systematic error is defined as
A) error that tends to be consistently too high or too low.
B) error that has equal probability of being too high or too low.
C) error that averages out with repeated trials.
D) error that is random.
A) error that tends to be consistently too high or too low.
B) error that has equal probability of being too high or too low.
C) error that averages out with repeated trials.
D) error that is random.

Unlock Deck
Unlock for access to all 192 flashcards in this deck.
Unlock Deck
k this deck
46
If the outside temperature is 35 °C, what is the temperature in °F?
A) 95 °F
B) 31 °F
C) 121 °F
D) 5.4 °F
E) 63 °F
A) 95 °F
B) 31 °F
C) 121 °F
D) 5.4 °F
E) 63 °F

Unlock Deck
Unlock for access to all 192 flashcards in this deck.
Unlock Deck
k this deck
47
Ethanol has a boiling point of 351 K. What is the boiling point in °C?
A) 78 °C
B) -195 °C
C) 122 °C
D) 62 °C
E) 86 °C
A) 78 °C
B) -195 °C
C) 122 °C
D) 62 °C
E) 86 °C

Unlock Deck
Unlock for access to all 192 flashcards in this deck.
Unlock Deck
k this deck
48
A student performs an experiment to determine the density of a sugar solution. She obtains the following results: 1.71 g mL-1, 1.73 g mL-1, 1.67 g mL-1, 1.69 g mL-1. If the actual value for the density of the sugar solution is 1.40 g mL-1, which statement below best describes her results?
A) Her results are precise, but not accurate.
B) Her results are accurate, but not precise.
C) Her results are both precise and accurate
D) Her results are neither precise nor accurate.
E) It isn't possible to determine from the information given.
A) Her results are precise, but not accurate.
B) Her results are accurate, but not precise.
C) Her results are both precise and accurate
D) Her results are neither precise nor accurate.
E) It isn't possible to determine from the information given.

Unlock Deck
Unlock for access to all 192 flashcards in this deck.
Unlock Deck
k this deck
49
Determine the volume of an object that has a mass of 455.6 g and a density of 19.3 g mL-1.
A) 87.9 mL
B) 42.4 mL
C) 18.5 mL
D) 23.6 mL
E) 31.2 mL
A) 87.9 mL
B) 42.4 mL
C) 18.5 mL
D) 23.6 mL
E) 31.2 mL

Unlock Deck
Unlock for access to all 192 flashcards in this deck.
Unlock Deck
k this deck
50
A gas phase mixture has a temperature of 643 °C. Calculate the temperature in K.
A) 916 K
B) 873 K
C) 188 K
D) 1391 K
E) 370 K
A) 916 K
B) 873 K
C) 188 K
D) 1391 K
E) 370 K

Unlock Deck
Unlock for access to all 192 flashcards in this deck.
Unlock Deck
k this deck
51
On a cold winter day, the outside temperature is reported as being -35 °C. What is the temperature in K?
A) 98.15 K
B) 238.15 K
C) 308.15 K
D) 400.15 K
E) 283.15 K
A) 98.15 K
B) 238.15 K
C) 308.15 K
D) 400.15 K
E) 283.15 K

Unlock Deck
Unlock for access to all 192 flashcards in this deck.
Unlock Deck
k this deck
52
The melting point of copper is 1984 °F. What is the melting point in K?
A) 1724 K
B) 1631 K
C) 1358 K
D) 2257 K
E) 1286 K
A) 1724 K
B) 1631 K
C) 1358 K
D) 2257 K
E) 1286 K

Unlock Deck
Unlock for access to all 192 flashcards in this deck.
Unlock Deck
k this deck
53
Ethylene glycol is commonly used as antifreeze in the cooling systems of vehicles due to its low freezing point, 8.6 °F. What is the freezing point in °C?
A) 0.0°C
B) -13°C
C) -26°C
D) -45°C
E) -54°C
A) 0.0°C
B) -13°C
C) -26°C
D) -45°C
E) -54°C

Unlock Deck
Unlock for access to all 192 flashcards in this deck.
Unlock Deck
k this deck
54
If the outside air temperature is 30 °F, what is the temperature in kelvin?
A) 303 K
B) 307 K
C) 274 K
D) 272 K
A) 303 K
B) 307 K
C) 274 K
D) 272 K

Unlock Deck
Unlock for access to all 192 flashcards in this deck.
Unlock Deck
k this deck
55
Determine the mass of an object that has a volume of 88.6 mL and a density of 9.77 g mL-1.
A) 298 g
B) 1100 g
C) 907 g
D) 568 g
E) 866 g
A) 298 g
B) 1100 g
C) 907 g
D) 568 g
E) 866 g

Unlock Deck
Unlock for access to all 192 flashcards in this deck.
Unlock Deck
k this deck
56
Ethylene glycol is commonly used as antifreeze in the cooling systems of vehicles due to its low freezing point, 8.6 °F. What is the freezing point in K?
A) 280 K
B) -13 K
C) 260 K
D) 273 K
E) 240 K
A) 280 K
B) -13 K
C) 260 K
D) 273 K
E) 240 K

Unlock Deck
Unlock for access to all 192 flashcards in this deck.
Unlock Deck
k this deck
57
A solution has a temperature of 68 °C. What is the temperature in K?
A) 427 K
B) 20 K
C) 154 K
D) 341 K
E) 296 K
A) 427 K
B) 20 K
C) 154 K
D) 341 K
E) 296 K

Unlock Deck
Unlock for access to all 192 flashcards in this deck.
Unlock Deck
k this deck
58
Ethanol has a freezing point of 159 K. What is the freezing point in °C?
A) -81 °C
B) -78 °C
C) -62 °C
D) -114 °C
E) -195 °C
A) -81 °C
B) -78 °C
C) -62 °C
D) -114 °C
E) -195 °C

Unlock Deck
Unlock for access to all 192 flashcards in this deck.
Unlock Deck
k this deck
59
A student performs an experiment to determine the density of a sugar solution. She obtains the following results: 1.79 g mL-1, 1.81 g mL-1, 1.80 g mL-1, 1.81 g mL-1. If the actual value for the density of the sugar solution is 1.80 g mL-1, which statement below best describes her results?
A) Her results are precise, but not accurate.
B) Her results are accurate, but not precise.
C) Her results are both precise and accurate
D) Her results are neither precise nor accurate.
E) It isn't possible to determine from the information given.
A) Her results are precise, but not accurate.
B) Her results are accurate, but not precise.
C) Her results are both precise and accurate
D) Her results are neither precise nor accurate.
E) It isn't possible to determine from the information given.

Unlock Deck
Unlock for access to all 192 flashcards in this deck.
Unlock Deck
k this deck
60
On a cold winter day, the temperature was -15 °C. What is the temperature in K?
A) 288.15 K
B) 238.15 K
C) 258.15 K
D) 400.15 K
E) 283.15 K
A) 288.15 K
B) 238.15 K
C) 258.15 K
D) 400.15 K
E) 283.15 K

Unlock Deck
Unlock for access to all 192 flashcards in this deck.
Unlock Deck
k this deck
61
Read the length of the metal bar with the correct number of significant figures. 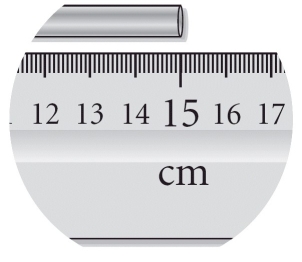
A) 20 cm
B) 15 cm
C) 15.0 cm
D) 15.00 cm
E) 15.000 cm

A) 20 cm
B) 15 cm
C) 15.0 cm
D) 15.00 cm
E) 15.000 cm

Unlock Deck
Unlock for access to all 192 flashcards in this deck.
Unlock Deck
k this deck
62
How many significant figures are in the measurement 0.0005890 g?
A) 4
B) 5
C) 6
D) 7
E) 8
A) 4
B) 5
C) 6
D) 7
E) 8

Unlock Deck
Unlock for access to all 192 flashcards in this deck.
Unlock Deck
k this deck
63
Read the length of the metal bar with the correct number of significant figures. 
A) 20 cm
B) 15 cm
C) 15.0 cm
D) 15.00 cm
E) 15.000 cm

A) 20 cm
B) 15 cm
C) 15.0 cm
D) 15.00 cm
E) 15.000 cm

Unlock Deck
Unlock for access to all 192 flashcards in this deck.
Unlock Deck
k this deck
64
How many significant figures are in the measurement 20.300 m?
A) 3
B) 4
C) 5
D) 1
E) 2
A) 3
B) 4
C) 5
D) 1
E) 2

Unlock Deck
Unlock for access to all 192 flashcards in this deck.
Unlock Deck
k this deck
65
What answer should be reported, with the correct number of significant figures, for the following calculation? 100.0 g × 4.18 J g-1 °C-1 × (26.4 °C - 25.5 °C)
A) 4 × 102 J
B) 3.8 × 102 J
C) 376 J
D) 376.2 J
E) 376.20 J
A) 4 × 102 J
B) 3.8 × 102 J
C) 376 J
D) 376.2 J
E) 376.20 J

Unlock Deck
Unlock for access to all 192 flashcards in this deck.
Unlock Deck
k this deck
66
What answer should be reported, with the correct number of significant figures, for the following calculation? 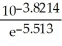
A) 0.037
B) 0.0374
C) 0.03740
D) 0.037399
E) 0.0373995
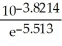
A) 0.037
B) 0.0374
C) 0.03740
D) 0.037399
E) 0.0373995

Unlock Deck
Unlock for access to all 192 flashcards in this deck.
Unlock Deck
k this deck
67
What answer should be reported, with the correct number of significant figures, for the following calculation? (965.43 × 3.911) + 9413.4136
A) 13189
B) 13189.2
C) 1.32 × 104
D) 1.3 × 104
E) 1.319 × 104
A) 13189
B) 13189.2
C) 1.32 × 104
D) 1.3 × 104
E) 1.319 × 104

Unlock Deck
Unlock for access to all 192 flashcards in this deck.
Unlock Deck
k this deck
68
What answer should be reported, with the correct number of significant figures, for the following calculation? 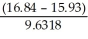 × 12.3
× 12.3
A) 1.16
B) 1
C) 1.162
D) 1.1621
E) 1.2
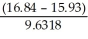 × 12.3
× 12.3A) 1.16
B) 1
C) 1.162
D) 1.1621
E) 1.2

Unlock Deck
Unlock for access to all 192 flashcards in this deck.
Unlock Deck
k this deck
69
What answer should be reported, with the correct number of significant figures, for the following calculation? log(4.28 × 103) - 1
A) 2.63144
B) 2.6314
C) 2.631
D) 2.6
E) 3
A) 2.63144
B) 2.6314
C) 2.631
D) 2.6
E) 3

Unlock Deck
Unlock for access to all 192 flashcards in this deck.
Unlock Deck
k this deck
70
Read the temperature with the correct number of significant figures. 
A) 87 °C
B) 87.2 °C
C) 87.20 °C
D) 87.200 °C
E) 87.2000 °C

A) 87 °C
B) 87.2 °C
C) 87.20 °C
D) 87.200 °C
E) 87.2000 °C

Unlock Deck
Unlock for access to all 192 flashcards in this deck.
Unlock Deck
k this deck
71
How many significant figures are in the measurement 463.090 m?
A) 2
B) 3
C) 4
D) 5
E) 6
A) 2
B) 3
C) 4
D) 5
E) 6

Unlock Deck
Unlock for access to all 192 flashcards in this deck.
Unlock Deck
k this deck
72
How many significant figures are in 3.408 × 104 m?
A) 3
B) 4
C) 5
D) 7
E) 8
A) 3
B) 4
C) 5
D) 7
E) 8

Unlock Deck
Unlock for access to all 192 flashcards in this deck.
Unlock Deck
k this deck
73
What answer should be reported, with the correct number of significant figures, for the following calculation? (433.621 - 333.9) × 11.900
A) 1.19 × 103
B) 1.187 × 103
C) 1.1868 × 103
D) 1.18680 × 103
E) 1.186799 × 103
A) 1.19 × 103
B) 1.187 × 103
C) 1.1868 × 103
D) 1.18680 × 103
E) 1.186799 × 103

Unlock Deck
Unlock for access to all 192 flashcards in this deck.
Unlock Deck
k this deck
74
How many significant figures are in 1009.630 mL?
A) 3
B) 4
C) 5
D) 6
E) 7
A) 3
B) 4
C) 5
D) 6
E) 7

Unlock Deck
Unlock for access to all 192 flashcards in this deck.
Unlock Deck
k this deck
75
What answer should be reported, with the correct number of significant figures, for the following calculation?
10-4.68
A) 2.1 × 10-5
B) 2 09 × 10-5
C) 2 × 10-5
D) 2.089 × 10-5
E) 2.0893 × 10-5
10-4.68
A) 2.1 × 10-5
B) 2 09 × 10-5
C) 2 × 10-5
D) 2.089 × 10-5
E) 2.0893 × 10-5

Unlock Deck
Unlock for access to all 192 flashcards in this deck.
Unlock Deck
k this deck
76
What answer should be reported, with the correct number of significant figures, for the following calculation? (249.362 + 42) / 63.498
A) 4.6
B) 4.59
C) 4.589
D) 4.5885
E) 4.58852
A) 4.6
B) 4.59
C) 4.589
D) 4.5885
E) 4.58852

Unlock Deck
Unlock for access to all 192 flashcards in this deck.
Unlock Deck
k this deck
77
Read the water level with the correct number of significant figures. 
A) 5 mL
B) 5.3 mL
C) 5.32 mL
D) 5.320 mL
E) 5.3200 mL

A) 5 mL
B) 5.3 mL
C) 5.32 mL
D) 5.320 mL
E) 5.3200 mL

Unlock Deck
Unlock for access to all 192 flashcards in this deck.
Unlock Deck
k this deck
78
What answer should be reported, with the correct number of significant figures, for the following calculation? 100 × 10-1.4 + log(1.6 × 102)
A) 6.2
B) 6.19
C) 6.18
D) 6
E) 6.185
A) 6.2
B) 6.19
C) 6.18
D) 6
E) 6.185

Unlock Deck
Unlock for access to all 192 flashcards in this deck.
Unlock Deck
k this deck
79
What answer should be reported, with the correct number of significant figures, for the following calculation? 6.83192458 + log(6.7831 × 104)
A) 11.66335
B) 11.663353
C) 11.6634
D) 11.6633528
E) 11.663
A) 11.66335
B) 11.663353
C) 11.6634
D) 11.6633528
E) 11.663

Unlock Deck
Unlock for access to all 192 flashcards in this deck.
Unlock Deck
k this deck
80
What answer should be reported, with the correct number of significant figures, for the following calculation? -log(3.28 × 10-3)
A) 2
B) 2.484
C) 2.4841
D) 2.48
E) 2.5
A) 2
B) 2.484
C) 2.4841
D) 2.48
E) 2.5

Unlock Deck
Unlock for access to all 192 flashcards in this deck.
Unlock Deck
k this deck


