Deck 5: Light: the Cosmic Messenger
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/148
Play
Full screen (f)
Deck 5: Light: the Cosmic Messenger
1
What is the angular resolution of the human eye?
A)about 1 arcminute, or 1/60 of a degree
B)about 1 degree
C)about 1 milliarcsecond
D)about 1 arcsecond (1/3600 of a degree)
A)about 1 arcminute, or 1/60 of a degree
B)about 1 degree
C)about 1 milliarcsecond
D)about 1 arcsecond (1/3600 of a degree)
A
2
If you heat a gas so that collisions are continually bumping electrons to higher energy levels, when the electrons fall back to lower energy levels the gas produces
A)radio waves.
B)thermal radiation.
C)an emission line spectrum.
D)X- rays.
E)an absorption line spectrum.
A)radio waves.
B)thermal radiation.
C)an emission line spectrum.
D)X- rays.
E)an absorption line spectrum.
C
3
An atomic nucleus has a size of about
A)10- 15 meters.
B)10- 12 meters.
C)10- 6 meters.
D)10- 9 meters.
A)10- 15 meters.
B)10- 12 meters.
C)10- 6 meters.
D)10- 9 meters.
A
4
The wavelength of a wave is
A)how strong the wave is.
B)the distance between where the wave is emitted and where it is absorbed.
C)equal to the speed of the wave times the wave's frequency.
D)the distance between a peak of the wave and the next trough.
E)the distance between two adjacent peaks of the wave.
A)how strong the wave is.
B)the distance between where the wave is emitted and where it is absorbed.
C)equal to the speed of the wave times the wave's frequency.
D)the distance between a peak of the wave and the next trough.
E)the distance between two adjacent peaks of the wave.

Unlock Deck
Unlock for access to all 148 flashcards in this deck.
Unlock Deck
k this deck
5
The simplified spectra for four stars is shown here. Which star has the lowest temperature? 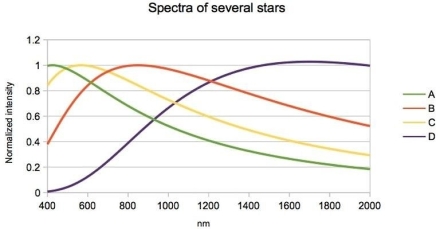
A)star B (orange line)
B)star D (purple line)
C)star C (yellow line)
D)star A (green line)

A)star B (orange line)
B)star D (purple line)
C)star C (yellow line)
D)star A (green line)

Unlock Deck
Unlock for access to all 148 flashcards in this deck.
Unlock Deck
k this deck
6
Laboratory measurements show hydrogen produces a spectral line at a wavelength of 486.1 nanometers (nm). A particular star's spectrum shows the same hydrogen line at a wavelength of 486.0 nm. What can we conclude?
A)The star is getting colder.
B)The star is getting hotter.
C)The star is moving toward us.
D)The star is moving away from us.
A)The star is getting colder.
B)The star is getting hotter.
C)The star is moving toward us.
D)The star is moving away from us.

Unlock Deck
Unlock for access to all 148 flashcards in this deck.
Unlock Deck
k this deck
7
The diagram represents energy levels in a hydrogen atom. The labeled transitions (A through
A)The electron remains in level 2 until it absorbs an additional 10.2 eV of energy.
B)The electron returns to level 1 by emitting an ultraviolet photon with 10.2 eV of energy.
C)The electron jumps to level 3 as soon as it absorbs any additional energy.
D)A different electron drops into level 1, since it is now unoccupied.
E)represent an electron moving between energy levels. Suppose that an electron in a hydrogen atom absorbs 10.2 eV of energy, so that it moves from level 1 to level 2. What typically happens next?
A)The electron remains in level 2 until it absorbs an additional 10.2 eV of energy.
B)The electron returns to level 1 by emitting an ultraviolet photon with 10.2 eV of energy.
C)The electron jumps to level 3 as soon as it absorbs any additional energy.
D)A different electron drops into level 1, since it is now unoccupied.
E)represent an electron moving between energy levels. Suppose that an electron in a hydrogen atom absorbs 10.2 eV of energy, so that it moves from level 1 to level 2. What typically happens next?

Unlock Deck
Unlock for access to all 148 flashcards in this deck.
Unlock Deck
k this deck
8
Which of the following objects would be most likely to produce an emission- line spectrum?
A)a star like our Sun
B)the Earth
C)a neon light
D)a light bulb
A)a star like our Sun
B)the Earth
C)a neon light
D)a light bulb

Unlock Deck
Unlock for access to all 148 flashcards in this deck.
Unlock Deck
k this deck
9
Which of the following best describes the fundamental difference between two different chemical elements (such as oxygen and carbon)?
A)They have different numbers of protons in their nucleus.
B)They have different names.
C)They have different atomic mass numbers.
D)They have different numbers of electrons.
A)They have different numbers of protons in their nucleus.
B)They have different names.
C)They have different atomic mass numbers.
D)They have different numbers of electrons.

Unlock Deck
Unlock for access to all 148 flashcards in this deck.
Unlock Deck
k this deck
10
The largest effective telescope, created by radio interferometry, is the diameter of
A)Earth.
B)tens of miles across, in the deserts of New Mexico.
C)several football fields, in a natural depression in Puerto Rico.
D)the state of New Mexico.
E)the continental United States.
A)Earth.
B)tens of miles across, in the deserts of New Mexico.
C)several football fields, in a natural depression in Puerto Rico.
D)the state of New Mexico.
E)the continental United States.

Unlock Deck
Unlock for access to all 148 flashcards in this deck.
Unlock Deck
k this deck
11
Which of the following telescopes is best suited for studying the hottest intergalactic gas (10 million K)in a cluster of galaxies?
A)Hubble Space Telescope (UV and optical and some infrared)
B)Very Large Array Radio Telescope
C)Chandra X- ray Telescope
D)Herschel Infrared Telescope
A)Hubble Space Telescope (UV and optical and some infrared)
B)Very Large Array Radio Telescope
C)Chandra X- ray Telescope
D)Herschel Infrared Telescope

Unlock Deck
Unlock for access to all 148 flashcards in this deck.
Unlock Deck
k this deck
12
Betelgeuse is the bright red star representing the left shoulder of the constellation Orion. All the following statements about Betelgeuse are true. Which one can you infer from its red color?
A)It is much brighter than the Sun.
B)It is much more massive than the Sun.
C)It is moving away from us.
D)Its surface is cooler than the surface of the Sun.
A)It is much brighter than the Sun.
B)It is much more massive than the Sun.
C)It is moving away from us.
D)Its surface is cooler than the surface of the Sun.

Unlock Deck
Unlock for access to all 148 flashcards in this deck.
Unlock Deck
k this deck
13
If a material is transparent, then it
A)scatters light well.
B)reflects light well.
C)absorbs light well.
D)emits light well.
E)transmits light well.
A)scatters light well.
B)reflects light well.
C)absorbs light well.
D)emits light well.
E)transmits light well.

Unlock Deck
Unlock for access to all 148 flashcards in this deck.
Unlock Deck
k this deck
14
Doppler shifted hydrogen absorption lines are seen in the spectrum of a star. The hydrogen line at 656.28 nm is seen to be shifted to 656.08 nm. The star is moving at which fraction of the speed of light?
A)about 0.03 (3 × 10-2)
B)about 0.2
C)about 0.000003 (3 × 10-6)
D)about 0.0003 (3 ×10-4)
A)about 0.03 (3 × 10-2)
B)about 0.2
C)about 0.000003 (3 × 10-6)
D)about 0.0003 (3 ×10-4)

Unlock Deck
Unlock for access to all 148 flashcards in this deck.
Unlock Deck
k this deck
15
Suppose you know the frequency of a photon and the speed of light. What else can you determine about the photon?
A)the chemical composition of the object that emitted it
B)its wavelength and energy
C)its acceleration
D)its temperature
A)the chemical composition of the object that emitted it
B)its wavelength and energy
C)its acceleration
D)its temperature

Unlock Deck
Unlock for access to all 148 flashcards in this deck.
Unlock Deck
k this deck
16
Which of the following objects is not a close approximation of a thermal emitter?
A)you
B)a planet
C)a star
D)a filament in a light bulb
E)hot, thin gas
A)you
B)a planet
C)a star
D)a filament in a light bulb
E)hot, thin gas

Unlock Deck
Unlock for access to all 148 flashcards in this deck.
Unlock Deck
k this deck
17
What is the purpose of interferometry?
A)It allows two or more small telescopes to achieve the angular resolution of a much larger telescope.
B)It is designed to prevent light pollution from interfering with astronomical observations.
C)It allows two or more small telescopes to achieve a larger light- collecting area than they would have independently.
D)It reduces the twinkling of stars caused by atmospheric turbulence.
A)It allows two or more small telescopes to achieve the angular resolution of a much larger telescope.
B)It is designed to prevent light pollution from interfering with astronomical observations.
C)It allows two or more small telescopes to achieve a larger light- collecting area than they would have independently.
D)It reduces the twinkling of stars caused by atmospheric turbulence.

Unlock Deck
Unlock for access to all 148 flashcards in this deck.
Unlock Deck
k this deck
18
Visible light from a distant star can be spread into a spectrum by using a glass prism or .
A)a diffraction grating
B)a flat glass mirror
C)adaptive optics
D)a telescope
A)a diffraction grating
B)a flat glass mirror
C)adaptive optics
D)a telescope

Unlock Deck
Unlock for access to all 148 flashcards in this deck.
Unlock Deck
k this deck
19
How many atoms fit across the period at the end of this sentence?
A)hundreds
B)billions
C)thousands
D)millions
E)more than you could count in a lifetime
A)hundreds
B)billions
C)thousands
D)millions
E)more than you could count in a lifetime

Unlock Deck
Unlock for access to all 148 flashcards in this deck.
Unlock Deck
k this deck
20
When an atom loses an electron, it becomes
A)sublimated.
B)a plasma.
C)dissociated.
D)an isotope.
E)ionized.
A)sublimated.
B)a plasma.
C)dissociated.
D)an isotope.
E)ionized.

Unlock Deck
Unlock for access to all 148 flashcards in this deck.
Unlock Deck
k this deck
21
One of the absorption lines of hydrogen has a rest wavelength of 656 nm (at rest means with respect to the observer, like in a lab). You observe several stars and measure the wavelength of this same hydrogen absorption line in each star. Based on the measured wavelength of this line, which star is moving towards the Earth the fastest?
A)630 nm
B)670 nm
C)640 nm
D)680 nm
E)656 nm
A)630 nm
B)670 nm
C)640 nm
D)680 nm
E)656 nm

Unlock Deck
Unlock for access to all 148 flashcards in this deck.
Unlock Deck
k this deck
22
A typical atom has a size of about
A)1 picometer (10- 12 meters).
B)1 Angstrom (10- 10 meters).
C)1 millimeter (10- 3 meters).
D)1 nanometer (10- 9 meters).
A)1 picometer (10- 12 meters).
B)1 Angstrom (10- 10 meters).
C)1 millimeter (10- 3 meters).
D)1 nanometer (10- 9 meters).

Unlock Deck
Unlock for access to all 148 flashcards in this deck.
Unlock Deck
k this deck
23
Doppler shifted hydrogen absorption lines are seen in the spectrum of a star. The hydrogen line at 656.28 nm is seen to be shifted to 656.08 nm. How large is the fractional shift in wavelength?
A)about 0.03 (3 × 10-2)
B)about 0.0003 (3 × 10-4)
C)about 0.2
D)about 0.000003 (3 × 10-6)
A)about 0.03 (3 × 10-2)
B)about 0.0003 (3 × 10-4)
C)about 0.2
D)about 0.000003 (3 × 10-6)

Unlock Deck
Unlock for access to all 148 flashcards in this deck.
Unlock Deck
k this deck
24
Blue light hitting a red sweatshirt is an example of
A)absorption.
B)emission.
C)reflection or scattering.
D)transmission.
A)absorption.
B)emission.
C)reflection or scattering.
D)transmission.

Unlock Deck
Unlock for access to all 148 flashcards in this deck.
Unlock Deck
k this deck
25
Which of the following statements about electrons is not true?
A)Electrons orbit the nucleus rather like planets orbiting the Sun.
B)An electron has a negative electrical charge.
C)Electrons can jump between energy levels in an atom only if they receive or give up an amount of energy equal to the difference in energy between the energy levels.
D)Electrons have very little mass compared to protons or neutrons.
E)Within an atom, an electron can have only particular energies.
A)Electrons orbit the nucleus rather like planets orbiting the Sun.
B)An electron has a negative electrical charge.
C)Electrons can jump between energy levels in an atom only if they receive or give up an amount of energy equal to the difference in energy between the energy levels.
D)Electrons have very little mass compared to protons or neutrons.
E)Within an atom, an electron can have only particular energies.

Unlock Deck
Unlock for access to all 148 flashcards in this deck.
Unlock Deck
k this deck
26
An atom that has fewer electrons than protons is called a(n).
A)ion
B)solid
C)plasma
D)molecule
A)ion
B)solid
C)plasma
D)molecule

Unlock Deck
Unlock for access to all 148 flashcards in this deck.
Unlock Deck
k this deck
27
The Chandra X- ray Observatory must operate in space because
A)X- rays are too dangerous to be allowed on the ground.
B)X- ray telescopes require the use of grazing incidence mirrors.
C)It was built by NASA.
D)X- rays do not penetrate Earth's atmosphere.
A)X- rays are too dangerous to be allowed on the ground.
B)X- ray telescopes require the use of grazing incidence mirrors.
C)It was built by NASA.
D)X- rays do not penetrate Earth's atmosphere.

Unlock Deck
Unlock for access to all 148 flashcards in this deck.
Unlock Deck
k this deck
28
In what wavelength range was interferometry first routinely used?
A)radio
B)X- ray
C)infrared
D)optical
E)ultraviolet
A)radio
B)X- ray
C)infrared
D)optical
E)ultraviolet

Unlock Deck
Unlock for access to all 148 flashcards in this deck.
Unlock Deck
k this deck
29
How does the light- collecting area of an 8- meter telescope compare to that of a 2- meter telescope?
A)The 8- meter telescope has 8 times the light- collecting area of the 2- meter telescope.
B)The 8- meter telescope has 4 times the light- collecting area of the 2- meter telescope.
C)The answer cannot be determined from the information given in the question.
D)The 8- meter telescope has 16 times the light- collecting area of the 2- meter telescope.
A)The 8- meter telescope has 8 times the light- collecting area of the 2- meter telescope.
B)The 8- meter telescope has 4 times the light- collecting area of the 2- meter telescope.
C)The answer cannot be determined from the information given in the question.
D)The 8- meter telescope has 16 times the light- collecting area of the 2- meter telescope.

Unlock Deck
Unlock for access to all 148 flashcards in this deck.
Unlock Deck
k this deck
30
How are wavelength, frequency, and energy related for photons of light?
A)Longer wavelength means higher frequency and lower energy.
B)Longer wavelength means lower frequency and higher energy.
C)Longer wavelength means lower frequency and lower energy.
D)Longer wavelength means higher frequency and higher energy.
E)There is no simple relationship because different photons travel at different speeds.
A)Longer wavelength means higher frequency and lower energy.
B)Longer wavelength means lower frequency and higher energy.
C)Longer wavelength means lower frequency and lower energy.
D)Longer wavelength means higher frequency and higher energy.
E)There is no simple relationship because different photons travel at different speeds.

Unlock Deck
Unlock for access to all 148 flashcards in this deck.
Unlock Deck
k this deck
31
Doppler shifted hydrogen absorption lines are seen in the spectrum of a star. The hydrogen line at 656.28 nm is seen to be shifted to 656.08 nm. Is the star moving towards or away from us, or can we not tell?
A)moving towards us
B)moving away from us
C)There is not enough information to determine the answer.
A)moving towards us
B)moving away from us
C)There is not enough information to determine the answer.

Unlock Deck
Unlock for access to all 148 flashcards in this deck.
Unlock Deck
k this deck
32
If a hydrogen emission line appears at 656.2 nm in the laboratory, what can we say about a cloud of hydrogen gas with this same emission line at 680.2 nm?
A)It is neither receding nor approaching us.
B)The location of the emission line has nothing to do with speed.
C)It is approaching us.
D)It is receding away from us.
A)It is neither receding nor approaching us.
B)The location of the emission line has nothing to do with speed.
C)It is approaching us.
D)It is receding away from us.

Unlock Deck
Unlock for access to all 148 flashcards in this deck.
Unlock Deck
k this deck
33
Which of the following observational techniques is most appropriate for measuring Doppler shifts?
A)timing (measuring how the amount of light changes with time)
B)imaging (taking a picture)
C)spectroscopy (taking a spectrum)
A)timing (measuring how the amount of light changes with time)
B)imaging (taking a picture)
C)spectroscopy (taking a spectrum)

Unlock Deck
Unlock for access to all 148 flashcards in this deck.
Unlock Deck
k this deck
34
When considering light as made up of individual "pieces," each characterized by a particular amount of energy, the pieces are called .
A)photons
B)gamma rays
C)frequencies
D)wavicles
A)photons
B)gamma rays
C)frequencies
D)wavicles

Unlock Deck
Unlock for access to all 148 flashcards in this deck.
Unlock Deck
k this deck
35
A gas heated to millions of degrees would emit
A)an equal amount of all wavelengths of light.
B)no light, because it is too hot.
C)mostly ultraviolet light.
D)mostly X- rays.
E)mostly radio waves.
A)an equal amount of all wavelengths of light.
B)no light, because it is too hot.
C)mostly ultraviolet light.
D)mostly X- rays.
E)mostly radio waves.

Unlock Deck
Unlock for access to all 148 flashcards in this deck.
Unlock Deck
k this deck
36
Studying a spectrum from a star can tell us a lot. All of the following statements are true except one. Which statement is not true?
A)The total amount of light in the spectrum tells us the star's radius.
B)We can identify chemical elements present in the star by recognizing patterns of spectral lines that correspond to particular chemicals.
C)The peak of the star's thermal emission tells us its temperature: hotter stars peak at shorter (bluer)wavelengths.
D)Shifts in the wavelengths of spectral lines compared to the wavelengths of those same lines measured in a laboratory on Earth can tell us the star's speed toward or away from us.
A)The total amount of light in the spectrum tells us the star's radius.
B)We can identify chemical elements present in the star by recognizing patterns of spectral lines that correspond to particular chemicals.
C)The peak of the star's thermal emission tells us its temperature: hotter stars peak at shorter (bluer)wavelengths.
D)Shifts in the wavelengths of spectral lines compared to the wavelengths of those same lines measured in a laboratory on Earth can tell us the star's speed toward or away from us.

Unlock Deck
Unlock for access to all 148 flashcards in this deck.
Unlock Deck
k this deck
37
Everything looks red through a red filter because
A)the filter reflects red light and transmits other colors.
B)the filter absorbs red light and emits other colors.
C)the filter emits red light and absorbs other colors.
D)the filter transmits red light and absorbs other colors.
A)the filter reflects red light and transmits other colors.
B)the filter absorbs red light and emits other colors.
C)the filter emits red light and absorbs other colors.
D)the filter transmits red light and absorbs other colors.

Unlock Deck
Unlock for access to all 148 flashcards in this deck.
Unlock Deck
k this deck
38
Suppose you look at a spectrum of visible light by looking through a prism or diffraction grating. How can you decide whether it is an emission line spectrum or an absorption line spectrum?
A)An emission line spectrum consists of bright lines on a dark background, while an absorption line spectrum consists of dark lines on a rainbow background.
B)The only way to decide is to make a graph of the intensity of the light at every wavelength, and then analyze the graph carefully.
C)An emission line spectrum consists of a long bright line, while an absorption line spectrum consists of a long dark line.
D)The emission line spectrum is produced by electrons jumping up in energy level, while the absorption line spectrum is produced by electrons jumping down in energy level.
A)An emission line spectrum consists of bright lines on a dark background, while an absorption line spectrum consists of dark lines on a rainbow background.
B)The only way to decide is to make a graph of the intensity of the light at every wavelength, and then analyze the graph carefully.
C)An emission line spectrum consists of a long bright line, while an absorption line spectrum consists of a long dark line.
D)The emission line spectrum is produced by electrons jumping up in energy level, while the absorption line spectrum is produced by electrons jumping down in energy level.

Unlock Deck
Unlock for access to all 148 flashcards in this deck.
Unlock Deck
k this deck
39
Without telescopes or other aid, we can look up and see the Moon in the night sky because it
A)emits thermal radiation.
B)reflects infrared light.
C)glows through radioactive decay.
D)emits visible light.
E)reflects visible light.
A)emits thermal radiation.
B)reflects infrared light.
C)glows through radioactive decay.
D)emits visible light.
E)reflects visible light.

Unlock Deck
Unlock for access to all 148 flashcards in this deck.
Unlock Deck
k this deck
40
How can an electron in an atom lose energy to go from a higher energy level to a lower energy level?
A)It releases a photon equal in energy to its own energy drop.
B)It loses gravitational potential energy.
C)It absorbs a photon equal in energy to its own energy drop.
D)It exchanges gravitational potential energy for kinetic energy.
E)It loses kinetic energy.
A)It releases a photon equal in energy to its own energy drop.
B)It loses gravitational potential energy.
C)It absorbs a photon equal in energy to its own energy drop.
D)It exchanges gravitational potential energy for kinetic energy.
E)It loses kinetic energy.

Unlock Deck
Unlock for access to all 148 flashcards in this deck.
Unlock Deck
k this deck
41
You and I are emitting primarily
A)radio radiation.
B)infrared radiation.
C)visual radiation.
D)X- ray radiation.
A)radio radiation.
B)infrared radiation.
C)visual radiation.
D)X- ray radiation.

Unlock Deck
Unlock for access to all 148 flashcards in this deck.
Unlock Deck
k this deck
42
Cell phone signals passing through walls is an example of
A)emission.
B)transmission.
C)absorption.
D)reflection or scattering.
A)emission.
B)transmission.
C)absorption.
D)reflection or scattering.

Unlock Deck
Unlock for access to all 148 flashcards in this deck.
Unlock Deck
k this deck
43
Suppose you built a scale- model atom in which the nucleus is the size of a tennis ball. About how far would the cloud of electrons extend?
A)a few meters
B)to the Sun
C)several centimeters
D)several kilometers
A)a few meters
B)to the Sun
C)several centimeters
D)several kilometers

Unlock Deck
Unlock for access to all 148 flashcards in this deck.
Unlock Deck
k this deck
44
Which of the following statements about electrical charge is true?
A)Two positive charges will attract each other.
B)A positive charge and a negative charge will repel each other.
C)Two negative charges will attract each other.
D)A positive charge and a negative charge will attract each other.
A)Two positive charges will attract each other.
B)A positive charge and a negative charge will repel each other.
C)Two negative charges will attract each other.
D)A positive charge and a negative charge will attract each other.

Unlock Deck
Unlock for access to all 148 flashcards in this deck.
Unlock Deck
k this deck
45
The energies of two photons you might detect emitted by hydrogen atoms are 10.2 and 2.1 eV. Which photon has the longest wavelength?
A)the 2.1 eV photon
B)the 10 eV photon
C)They both have the same wavelength.
A)the 2.1 eV photon
B)the 10 eV photon
C)They both have the same wavelength.

Unlock Deck
Unlock for access to all 148 flashcards in this deck.
Unlock Deck
k this deck
46
We are measuring the spectra of two hydrogen gas clouds. The laboratory frame wavelength of one hydrogen line is 656.2 nm. Cloud A's emission line wavelength is 660.1 nm and Cloud B's emission line wavelength is 670.1 nm. What can we conclude about these clouds?
A)They are both approaching us, and Cloud A is approaching faster than Cloud B.
B)They are both approaching us, and Cloud B is approaching faster than Cloud A.
C)They are both receding from us, and Cloud A is receding faster than Cloud B.
D)They are both receding from us, and Cloud B is receding faster than Cloud A.
A)They are both approaching us, and Cloud A is approaching faster than Cloud B.
B)They are both approaching us, and Cloud B is approaching faster than Cloud A.
C)They are both receding from us, and Cloud A is receding faster than Cloud B.
D)They are both receding from us, and Cloud B is receding faster than Cloud A.

Unlock Deck
Unlock for access to all 148 flashcards in this deck.
Unlock Deck
k this deck
47
The spectra of most galaxies show redshifts. This means that their spectral lines .
A)have wavelengths that are shorter than normal
B)have a higher intensity in the red part of the spectrum
C)always are in the red part of the visible spectrum
D)have wavelengths that are longer than normal
A)have wavelengths that are shorter than normal
B)have a higher intensity in the red part of the spectrum
C)always are in the red part of the visible spectrum
D)have wavelengths that are longer than normal

Unlock Deck
Unlock for access to all 148 flashcards in this deck.
Unlock Deck
k this deck
48
Which of the following is not an advantage of the Hubble Space Telescope over ground- based telescopes?
A)It can observe infrared and ultraviolet light, as well as visible light.
B)It is closer to the stars.
C)It never has to close because of cloudy skies.
D)Stars do not twinkle when observed from space.
A)It can observe infrared and ultraviolet light, as well as visible light.
B)It is closer to the stars.
C)It never has to close because of cloudy skies.
D)Stars do not twinkle when observed from space.

Unlock Deck
Unlock for access to all 148 flashcards in this deck.
Unlock Deck
k this deck
49
Which of the following best describes why we say that light is an electromagnetic wave?
A)Light can be produced only by electric or magnetic appliances.
B)The term electromagnetic wave arose for historical reasons, but we now know that light has nothing to do with either electricity or magnetism.
C)The passage of a light wave can cause electrically charged particles to move up and down.
D)Light is produced only when massive fields of electric and magnetic energy collide with one another.
A)Light can be produced only by electric or magnetic appliances.
B)The term electromagnetic wave arose for historical reasons, but we now know that light has nothing to do with either electricity or magnetism.
C)The passage of a light wave can cause electrically charged particles to move up and down.
D)Light is produced only when massive fields of electric and magnetic energy collide with one another.

Unlock Deck
Unlock for access to all 148 flashcards in this deck.
Unlock Deck
k this deck
50
Which of the following is not a good reason to place observatories on remote mountain tops?
A)to be able to observe at radio wavelengths
B)to reduce light absorption
C)to reduce light pollution
D)to reduce light distortion
A)to be able to observe at radio wavelengths
B)to reduce light absorption
C)to reduce light pollution
D)to reduce light distortion

Unlock Deck
Unlock for access to all 148 flashcards in this deck.
Unlock Deck
k this deck
51
Which of the following statements about thermal radiation is always true?
A)A hot object emits photons with a higher average energy than a cool object.
B)A hot object emits more radio waves than a cool object.
C)A hot object emits more X- rays than a cool object.
D)A hot object emits photons with a longer wavelength than a cool object.
A)A hot object emits photons with a higher average energy than a cool object.
B)A hot object emits more radio waves than a cool object.
C)A hot object emits more X- rays than a cool object.
D)A hot object emits photons with a longer wavelength than a cool object.

Unlock Deck
Unlock for access to all 148 flashcards in this deck.
Unlock Deck
k this deck
52
In what part of the electromagnetic spectrum do the biggest telescopes on Earth operate?
A)X- ray
B)visible
C)infrared
D)ultraviolet
E)radio
A)X- ray
B)visible
C)infrared
D)ultraviolet
E)radio

Unlock Deck
Unlock for access to all 148 flashcards in this deck.
Unlock Deck
k this deck
53
When an electron drops to a lower energy level in an atom,
A)the extra energy disappears.
B)the atom moves more slowly.
C)light at a wavelength specific to the change in energy levels is emitted.
D)the electron becomes more massive.
A)the extra energy disappears.
B)the atom moves more slowly.
C)light at a wavelength specific to the change in energy levels is emitted.
D)the electron becomes more massive.

Unlock Deck
Unlock for access to all 148 flashcards in this deck.
Unlock Deck
k this deck
54
Compared to the size of its nucleus, the size of an atom is about
A)the same.
B)one hundred thousand times greater.
C)ten times greater.
D)a thousand times greater.
E)a hundred times greater.
A)the same.
B)one hundred thousand times greater.
C)ten times greater.
D)a thousand times greater.
E)a hundred times greater.

Unlock Deck
Unlock for access to all 148 flashcards in this deck.
Unlock Deck
k this deck
55
Which forms of light are lower in energy and frequency than the light that our eyes can see?
A)visible light
B)infrared and radio
C)ultraviolet and X- rays
D)infrared and ultraviolet
A)visible light
B)infrared and radio
C)ultraviolet and X- rays
D)infrared and ultraviolet

Unlock Deck
Unlock for access to all 148 flashcards in this deck.
Unlock Deck
k this deck
56
In order for an atom to absorb a photon (a particle of light),
A)the photon must have enough energy to remove an electron from the atom.
B)the atom must have lost all of its electrons.
C)the photon must have energy matching the difference in energy between energy levels in the atom.
D)A or C
E)B or C
A)the photon must have enough energy to remove an electron from the atom.
B)the atom must have lost all of its electrons.
C)the photon must have energy matching the difference in energy between energy levels in the atom.
D)A or C
E)B or C

Unlock Deck
Unlock for access to all 148 flashcards in this deck.
Unlock Deck
k this deck
57
The frequency of a wave is
A)the number of peaks passing by any point each second.
B)equal to the speed of the wave divided by the wavelength of the wave.
C)measured in cycles per second.
D)measured in hertz (Hz).
E)all of the above
A)the number of peaks passing by any point each second.
B)equal to the speed of the wave divided by the wavelength of the wave.
C)measured in cycles per second.
D)measured in hertz (Hz).
E)all of the above

Unlock Deck
Unlock for access to all 148 flashcards in this deck.
Unlock Deck
k this deck
58
One star is emitting primarily visible light and another star is emitting primarily infrared light. Which star is hotter?
A)the star emitting infrared light
B)the star emitting visible light
C)Both stars are the same temperature.
D)The temperature also depends on the radius of the star, so one can't decide based on the information provided.
A)the star emitting infrared light
B)the star emitting visible light
C)Both stars are the same temperature.
D)The temperature also depends on the radius of the star, so one can't decide based on the information provided.

Unlock Deck
Unlock for access to all 148 flashcards in this deck.
Unlock Deck
k this deck
59
Doppler shifted hydrogen absorption lines are seen in the spectrum of a star. The hydrogen line at 656.28 nm is seen to be shifted to 656.08 nm. How large is the shift in wavelength?
A)0)2 nm
B)2 nm
C)0)02 nm
A)0)2 nm
B)2 nm
C)0)02 nm

Unlock Deck
Unlock for access to all 148 flashcards in this deck.
Unlock Deck
k this deck
60
Consider an atom of gold in which the nucleus contains 79 protons and 118 neutrons. What is its atomic number and atomic mass number?
A)The atomic number is 118, and the atomic mass number is 79.
B)The atomic number is 79, and the atomic mass number is 197.
C)The atomic number is 79, and the atomic mass number is 118.
D)The atomic number is 118, and the atomic mass number is 197.
A)The atomic number is 118, and the atomic mass number is 79.
B)The atomic number is 79, and the atomic mass number is 197.
C)The atomic number is 79, and the atomic mass number is 118.
D)The atomic number is 118, and the atomic mass number is 197.

Unlock Deck
Unlock for access to all 148 flashcards in this deck.
Unlock Deck
k this deck
61
An atom of the element iron has an atomic number of 26 and an atomic mass number of 56. If it is neutral, how many protons, neutrons, and electrons does it have?
A)26 protons, 30 neutrons, 30 electrons
B)26 protons, 30 neutrons, 26 electrons
C)13 protons, 56 neutrons, 13 electrons
D)13 protons, 43 neutrons, 13 electrons
E)26 protons, 56 neutrons, 26 electrons
A)26 protons, 30 neutrons, 30 electrons
B)26 protons, 30 neutrons, 26 electrons
C)13 protons, 56 neutrons, 13 electrons
D)13 protons, 43 neutrons, 13 electrons
E)26 protons, 56 neutrons, 26 electrons

Unlock Deck
Unlock for access to all 148 flashcards in this deck.
Unlock Deck
k this deck
62
From shortest to longest wavelength, which of the following correctly orders the different categories of electromagnetic radiation?
A)gamma rays, X- rays, visible light, ultraviolet, infrared, radio
B)gamma rays, X- rays, ultraviolet, visible light, infrared, radio
C)radio, infrared, visible light, ultraviolet, X- rays, gamma rays
D)infrared, visible light, ultraviolet, X- rays, gamma rays, radio
A)gamma rays, X- rays, visible light, ultraviolet, infrared, radio
B)gamma rays, X- rays, ultraviolet, visible light, infrared, radio
C)radio, infrared, visible light, ultraviolet, X- rays, gamma rays
D)infrared, visible light, ultraviolet, X- rays, gamma rays, radio

Unlock Deck
Unlock for access to all 148 flashcards in this deck.
Unlock Deck
k this deck
63
Which of the following properties of a star can be measured directly, during a single night at the telescope?
A)parallax
B)apparent brightness
C)luminosity
D)distance
A)parallax
B)apparent brightness
C)luminosity
D)distance

Unlock Deck
Unlock for access to all 148 flashcards in this deck.
Unlock Deck
k this deck
64
Suppose you want to know the chemical composition of a distant star. Which piece of information is most useful to you?
A)the Doppler shift of the star's spectrum
B)the wavelengths of spectral lines in the star's spectrum
C)whether the star's spectrum has more emission lines or more absorption lines
D)the peak energy of the star's thermal radiation
A)the Doppler shift of the star's spectrum
B)the wavelengths of spectral lines in the star's spectrum
C)whether the star's spectrum has more emission lines or more absorption lines
D)the peak energy of the star's thermal radiation

Unlock Deck
Unlock for access to all 148 flashcards in this deck.
Unlock Deck
k this deck
65
Suppose you see two stars: a blue star and a red star. Which of the following can you conclude about the two stars? Assume that no Doppler shifts are involved. (Hint: Think about the laws of thermal radiation.)
A)The red star has a hotter surface temperature than the blue star.
B)The blue star is farther away than the red star.
C)The blue star is more massive than the red star.
D)The blue star has a hotter surface temperature than the red star.
E)The red star is more massive than the blue star.
A)The red star has a hotter surface temperature than the blue star.
B)The blue star is farther away than the red star.
C)The blue star is more massive than the red star.
D)The blue star has a hotter surface temperature than the red star.
E)The red star is more massive than the blue star.

Unlock Deck
Unlock for access to all 148 flashcards in this deck.
Unlock Deck
k this deck
66
Why is the Earth's daytime sky blue?
A)The sky reflects the blue of the oceans and lakes.
B)Air molecules are blue in color.
C)Molecules absorb more red light than blue light.
D)Molecules scatter blue light more than red light.
A)The sky reflects the blue of the oceans and lakes.
B)Air molecules are blue in color.
C)Molecules absorb more red light than blue light.
D)Molecules scatter blue light more than red light.

Unlock Deck
Unlock for access to all 148 flashcards in this deck.
Unlock Deck
k this deck
67
Doppler shifted hydrogen absorption lines are seen in the spectrum of a star. The hydrogen line at 656.28 nm is seen to be shifted to 656.08 nm. How fast is the star moving (Note: The speed of light is approximately 300,000 km/s, or 3 × 105 km/s.)?
A)about 1,000 km/s
B)about 10,000 km/s
C)about 100 km/s
D)about 1,000,000 km/s
A)about 1,000 km/s
B)about 10,000 km/s
C)about 100 km/s
D)about 1,000,000 km/s

Unlock Deck
Unlock for access to all 148 flashcards in this deck.
Unlock Deck
k this deck
68
Currently, the largest optical telescope mirrors have a diameter of
A)5 m.
B)1 m.
C)10 m.
D)100 m.
E)2 m.
A)5 m.
B)1 m.
C)10 m.
D)100 m.
E)2 m.

Unlock Deck
Unlock for access to all 148 flashcards in this deck.
Unlock Deck
k this deck
69
Each of the following describes an "Atom 1" and an "Atom 2." In which case are the two atoms different isotopes of the same element?
A)Atom 1: nucleus with 4 protons and 5 neutrons, surrounded by 4 electrons; Atom 2: nucleus with 5 protons and 5 neutrons, surrounded by 4 electrons
B)Atom 1: nucleus with 6 protons and 8 neutrons, surrounded by 6 electrons; Atom 2: nucleus with 7 protons and 8 neutrons, surrounded by 7 electrons
C)Atom 1: nucleus with 8 protons and 8 neutrons, surrounded by 8 electrons; Atom 2: nucleus with 8 protons and 8 neutrons, surrounded by 7 electrons
D)Atom 1: nucleus with 7 protons and 8 neutrons, surrounded by 7 electrons; Atom 2: nucleus with 7 protons and 7 neutrons, surrounded by 7 electrons
A)Atom 1: nucleus with 4 protons and 5 neutrons, surrounded by 4 electrons; Atom 2: nucleus with 5 protons and 5 neutrons, surrounded by 4 electrons
B)Atom 1: nucleus with 6 protons and 8 neutrons, surrounded by 6 electrons; Atom 2: nucleus with 7 protons and 8 neutrons, surrounded by 7 electrons
C)Atom 1: nucleus with 8 protons and 8 neutrons, surrounded by 8 electrons; Atom 2: nucleus with 8 protons and 8 neutrons, surrounded by 7 electrons
D)Atom 1: nucleus with 7 protons and 8 neutrons, surrounded by 7 electrons; Atom 2: nucleus with 7 protons and 7 neutrons, surrounded by 7 electrons

Unlock Deck
Unlock for access to all 148 flashcards in this deck.
Unlock Deck
k this deck
70
Which of the following describes the light that can be detected from a person?
A)The person reflects many wavelengths of visible light and emits a continuum of wavelengths of infrared light.
B)The person emits many wavelengths of visible light and reflects a continuum of wavelengths of infrared light.
C)The person absorbs a few narrow wavelengths of visible light according to their composition.
D)The person emits a few narrow wavelengths of visible light according to their composition.
A)The person reflects many wavelengths of visible light and emits a continuum of wavelengths of infrared light.
B)The person emits many wavelengths of visible light and reflects a continuum of wavelengths of infrared light.
C)The person absorbs a few narrow wavelengths of visible light according to their composition.
D)The person emits a few narrow wavelengths of visible light according to their composition.

Unlock Deck
Unlock for access to all 148 flashcards in this deck.
Unlock Deck
k this deck
71
Why do astronomers need different telescope designs to observe across the electromagnetic spectrum?
A)Telescopes have to adapt to the greater distortion of the atmosphere at shorter wavelengths.
B)Because light pollution is worse at radio wavelengths than visible wavelengths
C)Photons of different energy behave differently and require different collection strategies.
D)New telescopes incorporate new technology to increase their efficiency.
E)Astronomers and engineers enjoy the challenge of making new telescope designs.
A)Telescopes have to adapt to the greater distortion of the atmosphere at shorter wavelengths.
B)Because light pollution is worse at radio wavelengths than visible wavelengths
C)Photons of different energy behave differently and require different collection strategies.
D)New telescopes incorporate new technology to increase their efficiency.
E)Astronomers and engineers enjoy the challenge of making new telescope designs.

Unlock Deck
Unlock for access to all 148 flashcards in this deck.
Unlock Deck
k this deck
72
Which of the following statements about X- rays and radio waves is not true?
A)Neither X- rays nor radio waves can penetrate Earth's atmosphere.
B)X- rays have higher energy than radio waves.
C)X- rays and radio waves are both forms of light, or electromagnetic radiation.
D)X- rays have shorter wavelengths than radio waves.
E)X- rays have higher frequency than radio waves.
A)Neither X- rays nor radio waves can penetrate Earth's atmosphere.
B)X- rays have higher energy than radio waves.
C)X- rays and radio waves are both forms of light, or electromagnetic radiation.
D)X- rays have shorter wavelengths than radio waves.
E)X- rays have higher frequency than radio waves.

Unlock Deck
Unlock for access to all 148 flashcards in this deck.
Unlock Deck
k this deck
73
We can see each other in the classroom right now because we
A)reflect visible light.
B)emit thermal radiation.
C)emit visible light.
D)emit infrared light.
E)reflect infrared light.
A)reflect visible light.
B)emit thermal radiation.
C)emit visible light.
D)emit infrared light.
E)reflect infrared light.

Unlock Deck
Unlock for access to all 148 flashcards in this deck.
Unlock Deck
k this deck
74
Thermal radiation is defined as .
A)radiation produced by an extremely hot object
B)radiation with a spectrum whose shape depends only on the temperature of the emitting object
C)radiation that is felt as heat radiation in the form of emission lines from an object
A)radiation produced by an extremely hot object
B)radiation with a spectrum whose shape depends only on the temperature of the emitting object
C)radiation that is felt as heat radiation in the form of emission lines from an object

Unlock Deck
Unlock for access to all 148 flashcards in this deck.
Unlock Deck
k this deck
75
Suppose you watch a leaf bobbing up and down as ripples pass it by in a pond. You notice that it does two full up and down bobs each second. Which statement is true of the ripples on the pond?
A)They have a wavelength of two cycles per second.
B)We can calculate the wavelength of the ripples from their frequency.
C)They have a frequency of 2 hertz.
D)They have a frequency of 4 hertz.
A)They have a wavelength of two cycles per second.
B)We can calculate the wavelength of the ripples from their frequency.
C)They have a frequency of 2 hertz.
D)They have a frequency of 4 hertz.

Unlock Deck
Unlock for access to all 148 flashcards in this deck.
Unlock Deck
k this deck
76
What is an artificial star?
A)a meteor
B)a satellite orbiting Earth
C)a point of light in Earth's atmosphere created by a laser for the purpose of monitoring atmospheric fluctuations
D)the unseen member of a binary star system
E)a possible source of dark matter in the universe
A)a meteor
B)a satellite orbiting Earth
C)a point of light in Earth's atmosphere created by a laser for the purpose of monitoring atmospheric fluctuations
D)the unseen member of a binary star system
E)a possible source of dark matter in the universe

Unlock Deck
Unlock for access to all 148 flashcards in this deck.
Unlock Deck
k this deck
77
If we say that a material is opaque to ultraviolet light, we mean that it .
A)emits ultraviolet light
B)absorbs ultraviolet light
C)transmits ultraviolet light
D)reflects ultraviolet light
A)emits ultraviolet light
B)absorbs ultraviolet light
C)transmits ultraviolet light
D)reflects ultraviolet light

Unlock Deck
Unlock for access to all 148 flashcards in this deck.
Unlock Deck
k this deck
78
When an electron in an atom goes from a higher energy state to a lower energy state, the atom
A)absorbs a photon of a specific frequency.
B)absorbs several photons of a specific frequency.
C)can absorb a photon of any frequency.
D)can emit a photon of any frequency.
E)emits a photon of a specific frequency.
A)absorbs a photon of a specific frequency.
B)absorbs several photons of a specific frequency.
C)can absorb a photon of any frequency.
D)can emit a photon of any frequency.
E)emits a photon of a specific frequency.

Unlock Deck
Unlock for access to all 148 flashcards in this deck.
Unlock Deck
k this deck
79
Spectra from neutral atoms compared with spectra from ionized atoms of the same element
A)are slightly blueshifted.
B)have different sets of spectral lines.
C)have the same sets of spectral lines but different widths for those lines.
D)are slightly redshifted.
E)are the same.
A)are slightly blueshifted.
B)have different sets of spectral lines.
C)have the same sets of spectral lines but different widths for those lines.
D)are slightly redshifted.
E)are the same.

Unlock Deck
Unlock for access to all 148 flashcards in this deck.
Unlock Deck
k this deck
80
From lowest energy to highest energy, which of the following correctly orders the different categories of electromagnetic radiation?
A)infrared, visible light, ultraviolet, X- rays, gamma rays, radio
B)gamma rays, X- rays, visible light, ultraviolet, infrared, radio
C)visible light, infrared, X- rays, ultraviolet, gamma rays, radio
D)radio, infrared, visible light, ultraviolet, X- rays, gamma rays
E)radio, X- rays, visible light, ultraviolet, infrared, gamma rays
A)infrared, visible light, ultraviolet, X- rays, gamma rays, radio
B)gamma rays, X- rays, visible light, ultraviolet, infrared, radio
C)visible light, infrared, X- rays, ultraviolet, gamma rays, radio
D)radio, infrared, visible light, ultraviolet, X- rays, gamma rays
E)radio, X- rays, visible light, ultraviolet, infrared, gamma rays

Unlock Deck
Unlock for access to all 148 flashcards in this deck.
Unlock Deck
k this deck


