Deck 13: Properties of Solutions
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/121
Play
Full screen (f)
Deck 13: Properties of Solutions
1
Which of the following liquids will have the lowest freezing point?
A) pure H2O
B) aqueous CoI2 (0.030 m)
C) aqueous FeI3 (0.030 m)
D) aqueous glucose (0.050 m)
E) aqueous NaI (0.030 m)
A) pure H2O
B) aqueous CoI2 (0.030 m)
C) aqueous FeI3 (0.030 m)
D) aqueous glucose (0.050 m)
E) aqueous NaI (0.030 m)
aqueous FeI3 (0.030 m)
2
A solution is prepared by dissolving calcium chloride in water and diluting to 500.0 mL. If this solution contains 44 ppm chloride ions, the concentration of calcium ions is ppm.
A) 11
B) 44
C) 88
D) 500
E) 22
A) 11
B) 44
C) 88
D) 500
E) 22
22
3
A supersaturated solution _ _.
A) is one with a higher concentration than the solubility
B) exists only in theory and cannot actually be prepared
C) is one that has been heated
D) must be in contact with undissolved solid
E) is one with more than one solute
A) is one with a higher concentration than the solubility
B) exists only in theory and cannot actually be prepared
C) is one that has been heated
D) must be in contact with undissolved solid
E) is one with more than one solute
is one with a higher concentration than the solubility
4
The dissolution of water in octane (C8H18) is prevented by _ _.
A) hydrogen bonding between water molecules
B) repulsion between like- charged water and octane molecules
C) dipole- dipole attraction between octane molecules
D) London dispersion forces between octane molecules
E) ion- dipole attraction between water and octane molecules
A) hydrogen bonding between water molecules
B) repulsion between like- charged water and octane molecules
C) dipole- dipole attraction between octane molecules
D) London dispersion forces between octane molecules
E) ion- dipole attraction between water and octane molecules

Unlock Deck
Unlock for access to all 121 flashcards in this deck.
Unlock Deck
k this deck
5
When solutions of strong electrolytes in water are formed, the ions are surrounded by water molecules. These interactions are best described as a case of _.
A) hydration
B) crystallization
C) supersaturation
D) dehydration
E) solvation
A) hydration
B) crystallization
C) supersaturation
D) dehydration
E) solvation

Unlock Deck
Unlock for access to all 121 flashcards in this deck.
Unlock Deck
k this deck
6
The dissolution of gases in water is virtually always exothermic because _.
A) neither of the two endothermic steps in the solution- formation process is necessary
B) one of the two endothermic steps (separation of solute particles) in the solution- formation process is unnecessary
C) gases react exothermically with water
D) the exothermic step in the solution- formation process is unnecessary
E) all three steps in the solution- formation process are exothermic
A) neither of the two endothermic steps in the solution- formation process is necessary
B) one of the two endothermic steps (separation of solute particles) in the solution- formation process is unnecessary
C) gases react exothermically with water
D) the exothermic step in the solution- formation process is unnecessary
E) all three steps in the solution- formation process are exothermic

Unlock Deck
Unlock for access to all 121 flashcards in this deck.
Unlock Deck
k this deck
7
Which of the following is not a colloid?
A) whipped cream
B) smoke
C) air
D) homogenized milk
E) fog
A) whipped cream
B) smoke
C) air
D) homogenized milk
E) fog

Unlock Deck
Unlock for access to all 121 flashcards in this deck.
Unlock Deck
k this deck
8
A solution is prepared by adding 30.00 g of lactose (milk sugar) to 110.0 g of water at 55 °C. The partial pressure of water above the solution is torr. The vapor pressure of pure water at 55 °C is 118 torr. The MW of lactose is 342.3 g/mol.
A) 169.4
B) 1.670
C) 92.7
D) 94.1
E) 116.3
A) 169.4
B) 1.670
C) 92.7
D) 94.1
E) 116.3

Unlock Deck
Unlock for access to all 121 flashcards in this deck.
Unlock Deck
k this deck
9
An unsaturated solution is one that _.
A) contains the maximum concentration of solute possible, and is in equilibrium with undissolved solute
B) has a concentration lower than the solubility
C) contains no solute
D) has no double bonds
E) contains more dissolved solute than the solubility allows
A) contains the maximum concentration of solute possible, and is in equilibrium with undissolved solute
B) has a concentration lower than the solubility
C) contains no solute
D) has no double bonds
E) contains more dissolved solute than the solubility allows

Unlock Deck
Unlock for access to all 121 flashcards in this deck.
Unlock Deck
k this deck
10
Ammonium nitrate (NH4NO3) dissolves readily in water even though the dissolution is endothermic by 26.4 kJ/mol. The solution process is spontaneous because _.
A) of the increase in disorder upon dissolution of this strong electrolyte
B) the vapor pressure of the water decreases upon addition of the solute
C) of the increase in enthalpy upon dissolution of this strong electrolyte
D) of the decrease in enthalpy upon addition of the solute
E) osmotic properties predict this behavior
A) of the increase in disorder upon dissolution of this strong electrolyte
B) the vapor pressure of the water decreases upon addition of the solute
C) of the increase in enthalpy upon dissolution of this strong electrolyte
D) of the decrease in enthalpy upon addition of the solute
E) osmotic properties predict this behavior

Unlock Deck
Unlock for access to all 121 flashcards in this deck.
Unlock Deck
k this deck
11
A solution contains 28% phosphoric acid by mass. This means that .
A) 1 mL of this solution contains 28 g of phosphoric acid
B) the density of this solution is 2.8 g/mL
C) 1 L of this solution has a mass of 28 g
D) 100 g of this solution contains 28 g of phosphoric acid
E) 1 L of this solution contains 28 mL of phosphoric acid
A) 1 mL of this solution contains 28 g of phosphoric acid
B) the density of this solution is 2.8 g/mL
C) 1 L of this solution has a mass of 28 g
D) 100 g of this solution contains 28 g of phosphoric acid
E) 1 L of this solution contains 28 mL of phosphoric acid

Unlock Deck
Unlock for access to all 121 flashcards in this deck.
Unlock Deck
k this deck
12
Which of the following cannot be a colloid?
A) a foam
B) an aerosol
C) an emulsion
D) a homogenous mixture
E) All of the above are colloids.
A) a foam
B) an aerosol
C) an emulsion
D) a homogenous mixture
E) All of the above are colloids.

Unlock Deck
Unlock for access to all 121 flashcards in this deck.
Unlock Deck
k this deck
13
Calculate the freezing point of a solution containing 40.0 grams of KCl and 4400.0 grams of water. The molal- freezing- point- depression constant (Kf) for water is 1.86 ° C/m.
A) +0.45 oC
B) - 0.45 oC
C) - 0.23 oC
D) 1.23 oC
E) +0.23 oC
A) +0.45 oC
B) - 0.45 oC
C) - 0.23 oC
D) 1.23 oC
E) +0.23 oC

Unlock Deck
Unlock for access to all 121 flashcards in this deck.
Unlock Deck
k this deck
14
When argon is placed in a container of neon, the argon spontaneously disperses throughout the neon because .
A) of solvent- solute interactions
B) the dispersion of argon atoms produces an increase in disorder
C) of hydrogen bonding
D) of the large attractive forces between argon and neon atoms
E) a decrease in energy occurs when the two mix
A) of solvent- solute interactions
B) the dispersion of argon atoms produces an increase in disorder
C) of hydrogen bonding
D) of the large attractive forces between argon and neon atoms
E) a decrease in energy occurs when the two mix

Unlock Deck
Unlock for access to all 121 flashcards in this deck.
Unlock Deck
k this deck
15
A saturated solution .
A) contains as much solvent as it can hold
B) cannot be attained
C) contains dissolved solute in equilibrium with undissolved solid
D) will rapidly precipitate if a seed crystal is added
E) contains no double bonds
A) contains as much solvent as it can hold
B) cannot be attained
C) contains dissolved solute in equilibrium with undissolved solid
D) will rapidly precipitate if a seed crystal is added
E) contains no double bonds

Unlock Deck
Unlock for access to all 121 flashcards in this deck.
Unlock Deck
k this deck
16
The phrase "like dissolves like" refers to the fact that _.
A) gases can only dissolve other gases
B) condensed phases can only dissolve other condensed phases
C) polar solvents dissolve polar solutes and nonpolar solvents dissolve nonpolar solutes
D) polar solvents dissolve nonpolar solutes and vice versa
E) solvents can only dissolve solutes of similar molar mass
A) gases can only dissolve other gases
B) condensed phases can only dissolve other condensed phases
C) polar solvents dissolve polar solutes and nonpolar solvents dissolve nonpolar solutes
D) polar solvents dissolve nonpolar solutes and vice versa
E) solvents can only dissolve solutes of similar molar mass

Unlock Deck
Unlock for access to all 121 flashcards in this deck.
Unlock Deck
k this deck
17
In a saturated solution of a salt in water, .
A) seed crystal addition may cause massive crystallization
B) the rate of crystallization > the rate of dissolution
C) addition of more water causes massive crystallization
D) the rate of dissolution > the rate of crystallization
E) the rate of crystallization = the rate of dissolution
A) seed crystal addition may cause massive crystallization
B) the rate of crystallization > the rate of dissolution
C) addition of more water causes massive crystallization
D) the rate of dissolution > the rate of crystallization
E) the rate of crystallization = the rate of dissolution

Unlock Deck
Unlock for access to all 121 flashcards in this deck.
Unlock Deck
k this deck
18
A 0.100 m solution of which one of the following solutes will have the lowest vapor pressure?
A) Ca(ClO4)2
B) Al(ClO4)3
C) KClO4
D) sucrose
E) NaCl
A) Ca(ClO4)2
B) Al(ClO4)3
C) KClO4
D) sucrose
E) NaCl

Unlock Deck
Unlock for access to all 121 flashcards in this deck.
Unlock Deck
k this deck
19
The Henry's law constant for helium gas in water at 30 °C is 3.70 × 10- 4 M/atm. When the partial pressure of helium above a sample of water is 0.650 atm, the concentration of helium in the water is M
A) 1.76 × 103
B) 2.41 × 10- 4
C) 5.69 × 10- 4
D) 3.70 × 10- 4
E) 1.30
A) 1.76 × 103
B) 2.41 × 10- 4
C) 5.69 × 10- 4
D) 3.70 × 10- 4
E) 1.30

Unlock Deck
Unlock for access to all 121 flashcards in this deck.
Unlock Deck
k this deck
20
Which one of the following is most soluble in hexane (C6H14)?
A) CH3CH2CH2OH
B) CH3OH
C) CH3CH2CH2CH2CH2OH
D) CH3CH2CH2CH2OH
E) CH3CH2OH
A) CH3CH2CH2OH
B) CH3OH
C) CH3CH2CH2CH2CH2OH
D) CH3CH2CH2CH2OH
E) CH3CH2OH

Unlock Deck
Unlock for access to all 121 flashcards in this deck.
Unlock Deck
k this deck
21
A solution is prepared by dissolving 16.2 g of benzene (C6H6) in 282 g of carbon tetrachloride (CCl4). The concentration of benzene in this solution is molal. The molar masses of C6H6 and CCl4 are 78.1 g/mol and 154 g/mol, respectively.
A) 0.102
B) 7.36 × 10- 4
C) 5.43
D) 0.736
E) 0.0543
A) 0.102
B) 7.36 × 10- 4
C) 5.43
D) 0.736
E) 0.0543

Unlock Deck
Unlock for access to all 121 flashcards in this deck.
Unlock Deck
k this deck
22
At 20°C, a 2.32 M aqueous solution of ammonium chloride has a density of 1.0344 g/mL. What is the molality of ammonium chloride in the solution? The formula weight of NH4Cl is 53.50 g/mol.
A) 0.0449
B) 2.55
C) 12.00
D) 0.446
E) 2.32
A) 0.0449
B) 2.55
C) 12.00
D) 0.446
E) 2.32

Unlock Deck
Unlock for access to all 121 flashcards in this deck.
Unlock Deck
k this deck
23
A solution is prepared by dissolving 7.00 g of glycerin (C3H8O3) in 201 g of ethanol (C2H5OH). The freezing point of the solution is °C. The freezing point of pure ethanol is - 114.6 °C at 1 atm. The molal- freezing- point- depression constant (Kf) for ethanol is 1.99 ° C/m. The molar masses of glycerin and of ethanol are 92.1 g/mol and 46.1 g/mol, respectively.
A) - 121.3
B) - 107.9
C) - 113.8
D) 0.752
E) - 115.4
A) - 121.3
B) - 107.9
C) - 113.8
D) 0.752
E) - 115.4

Unlock Deck
Unlock for access to all 121 flashcards in this deck.
Unlock Deck
k this deck
24
The concentration of chloride ion in a solution that contains 35.0 ppm chloride is % by mass.
A) 3.50 × 101
B) 3.50 × 10- 3
C) 3.50 × 10- 6
D) 3.50 × 102
E) 3.50 × 10- 2
A) 3.50 × 101
B) 3.50 × 10- 3
C) 3.50 × 10- 6
D) 3.50 × 102
E) 3.50 × 10- 2

Unlock Deck
Unlock for access to all 121 flashcards in this deck.
Unlock Deck
k this deck
25
The most likely van't Hoff factor for an 0.01 m CaI2 solution is .
A) 1.27
B) 1.00
C) 3.29
D) 2.69
E) 3.00
A) 1.27
B) 1.00
C) 3.29
D) 2.69
E) 3.00

Unlock Deck
Unlock for access to all 121 flashcards in this deck.
Unlock Deck
k this deck
26
Which one of the following substances is more likely to dissolve in CCl4?
A) CBr4
B) NaCl
C) HBr
D) HCl
E) CH3CH2OH
A) CBr4
B) NaCl
C) HBr
D) HCl
E) CH3CH2OH

Unlock Deck
Unlock for access to all 121 flashcards in this deck.
Unlock Deck
k this deck
27
At 20°C, a 3.54 M aqueous solution of ammonium chloride has a density of 1.0512 g/mL. What is the mass % of ammonium chloride in the solution? The formula weight of NH4Cl is 53.50 g/mol.
A) 6.95
B) 4.10
C) 3.36
D) 18.00
E) 0.297
A) 6.95
B) 4.10
C) 3.36
D) 18.00
E) 0.297

Unlock Deck
Unlock for access to all 121 flashcards in this deck.
Unlock Deck
k this deck
28
Hydrophobic colloids .
A) can be stabilized by coagulation
B) can be stabilized by adsorption of ions
C) will separate into two phases if they are stabilized
D) are those that do not contain water
E) are those that contain water
A) can be stabilized by coagulation
B) can be stabilized by adsorption of ions
C) will separate into two phases if they are stabilized
D) are those that do not contain water
E) are those that contain water

Unlock Deck
Unlock for access to all 121 flashcards in this deck.
Unlock Deck
k this deck
29
Molality is defined as the .
A) moles solute/kg solution
B) moles solute/Liters solution
C) moles solute/moles solvent
D) moles solute/kg solvent
E) none (dimensionless)
A) moles solute/kg solution
B) moles solute/Liters solution
C) moles solute/moles solvent
D) moles solute/kg solvent
E) none (dimensionless)

Unlock Deck
Unlock for access to all 121 flashcards in this deck.
Unlock Deck
k this deck
30
The osmotic pressure of a solution formed by dissolving 25.0 mg of aspirin (C9H8O4) in 0.250 L of water at 25 °C is atm.
A) 1.14 × 10- 3
B) 0.0136
C) 1.38
D) 13.6
E) 2.45
A) 1.14 × 10- 3
B) 0.0136
C) 1.38
D) 13.6
E) 2.45

Unlock Deck
Unlock for access to all 121 flashcards in this deck.
Unlock Deck
k this deck
31
Of the concentration units below, only _ _ is temperature dependent.
A) mass %
B) ppb
C) ppm
D) molality
E) molarity
A) mass %
B) ppb
C) ppm
D) molality
E) molarity

Unlock Deck
Unlock for access to all 121 flashcards in this deck.
Unlock Deck
k this deck
32
Which of the following choices has the compounds correctly arranged in order of increasing solubility in water? (least soluble to most soluble
A) LiF < NaNO3 < CHCl3
B) CH4 < NaNO3 < CHCl3
C) CH3OH < CH4 < LiF
D) CCl4 < CHCl3 < NaNO3
E) CH3OH < Cl4 < CHCl3
A) LiF < NaNO3 < CHCl3
B) CH4 < NaNO3 < CHCl3
C) CH3OH < CH4 < LiF
D) CCl4 < CHCl3 < NaNO3
E) CH3OH < Cl4 < CHCl3

Unlock Deck
Unlock for access to all 121 flashcards in this deck.
Unlock Deck
k this deck
33
Colligative properties of solutions include all of the following except .
A) the increase of reaction rates with increase in temperature
B) depression of vapor pressure upon addition of a solute to a solvent
C) depression of the freezing point of a solution upon addition of a solute to a solvent
D) an increase in the osmotic pressure of a solution upon the addition of more solute
E) elevation of the boiling point of a solution upon addition of a solute to a solvent
A) the increase of reaction rates with increase in temperature
B) depression of vapor pressure upon addition of a solute to a solvent
C) depression of the freezing point of a solution upon addition of a solute to a solvent
D) an increase in the osmotic pressure of a solution upon the addition of more solute
E) elevation of the boiling point of a solution upon addition of a solute to a solvent

Unlock Deck
Unlock for access to all 121 flashcards in this deck.
Unlock Deck
k this deck
34
If the partial pressure of oxygen in the air a diver breathes is too great, _.
A) respiratory tissue is damaged by oxidation
B) hyperventilation results
C) the urge to breathe is reduced and not enough CO2 is removed from the body
D) the urge to breathe is increased and excessive CO2 is removed from the body
E) No problems result from this situation.
A) respiratory tissue is damaged by oxidation
B) hyperventilation results
C) the urge to breathe is reduced and not enough CO2 is removed from the body
D) the urge to breathe is increased and excessive CO2 is removed from the body
E) No problems result from this situation.

Unlock Deck
Unlock for access to all 121 flashcards in this deck.
Unlock Deck
k this deck
35
A solution contains 11% by mass of sodium chloride. This means that .
A) 100 mL of the solution contains 11 g of sodium chloride
B) the molality of the solution is 11
C) there are 11 g of sodium chloride in in 1.0 mL of this solution
D) the density of the solution is 11 g/mL
E) 100 g of the solution contains 11 g of sodium chloride
A) 100 mL of the solution contains 11 g of sodium chloride
B) the molality of the solution is 11
C) there are 11 g of sodium chloride in in 1.0 mL of this solution
D) the density of the solution is 11 g/mL
E) 100 g of the solution contains 11 g of sodium chloride

Unlock Deck
Unlock for access to all 121 flashcards in this deck.
Unlock Deck
k this deck
36
Of the following, a 0.1 M aqueous solution of will have the lowest freezing point.
A) Al(NO3)3
B) NaCl
C) K2CrO4
D) Na2SO4
E) sucrose
A) Al(NO3)3
B) NaCl
C) K2CrO4
D) Na2SO4
E) sucrose

Unlock Deck
Unlock for access to all 121 flashcards in this deck.
Unlock Deck
k this deck
37
A solution is prepared by adding 1.43 mol of KCl to 889 g of water. The concentration of KCl is
Molal.
A) 622
B) 0.622
C) 1.61 × 10- 3
D) 1.27 × 103
E) 1.61
Molal.
A) 622
B) 0.622
C) 1.61 × 10- 3
D) 1.27 × 103
E) 1.61

Unlock Deck
Unlock for access to all 121 flashcards in this deck.
Unlock Deck
k this deck
38
The ideal value of i (van't Hoff factor) for (NH4)3PO4.
A) 5
B) 1
C) 4
D) 3
E) 2
A) 5
B) 1
C) 4
D) 3
E) 2

Unlock Deck
Unlock for access to all 121 flashcards in this deck.
Unlock Deck
k this deck
39
Which one of the following substances is more likely to dissolve in benzene (C6H6)?
A) CCl4
B) CH3CH2OH
C) HBr
D) NH3
E) NaCl
A) CCl4
B) CH3CH2OH
C) HBr
D) NH3
E) NaCl

Unlock Deck
Unlock for access to all 121 flashcards in this deck.
Unlock Deck
k this deck
40
A 1.35 m aqueous solution of compound X had a boiling point of 101.4°C. Which one of the following could be compound X? The boiling point elevation constant for water is 0.52°C/m.
A) Na3PO4
B) CH3CH2OH
C) KCl
D) CaCl2
E) C6H12O6
A) Na3PO4
B) CH3CH2OH
C) KCl
D) CaCl2
E) C6H12O6

Unlock Deck
Unlock for access to all 121 flashcards in this deck.
Unlock Deck
k this deck
41
The Procter & Gamble Company product called olestraTM is formed by combining a sugar molecule with .
A) alcohols
B) cholesterol
C) vitamin A
D) fatty acids
E) protein
A) alcohols
B) cholesterol
C) vitamin A
D) fatty acids
E) protein

Unlock Deck
Unlock for access to all 121 flashcards in this deck.
Unlock Deck
k this deck
42
A solution contains 15 ppm of benzene. The density of the solution is 1.00 g/mL. This means that
A) there are 15 mg of benzene in 1.0 L of this solution
B) 100 g of the solution contains 15 g of benzene
C) 100 g of the solution contains 15 mg of benzene
D) the molarity of the solution is 15
E) the solution is 15% by mass of benzene
A) there are 15 mg of benzene in 1.0 L of this solution
B) 100 g of the solution contains 15 g of benzene
C) 100 g of the solution contains 15 mg of benzene
D) the molarity of the solution is 15
E) the solution is 15% by mass of benzene

Unlock Deck
Unlock for access to all 121 flashcards in this deck.
Unlock Deck
k this deck
43
Which produces the greatest number of ions when one mole dissolves in water?
A) NH4NO3
B) NH4Cl
C) Na2SO4
D) NaCl
E) sucrose
A) NH4NO3
B) NH4Cl
C) Na2SO4
D) NaCl
E) sucrose

Unlock Deck
Unlock for access to all 121 flashcards in this deck.
Unlock Deck
k this deck
44
Formation of solutions where the process is endothermic can be spontaneous provided that
)
A) they are accompanied by an increase in order
B) the solvent is a gas and the solute is a solid
C) they are accompanied by an increase in disorder
D) the solvent is water and the solute is a gas
E) they are accompanied by another process that is exothermic
)
A) they are accompanied by an increase in order
B) the solvent is a gas and the solute is a solid
C) they are accompanied by an increase in disorder
D) the solvent is water and the solute is a gas
E) they are accompanied by another process that is exothermic

Unlock Deck
Unlock for access to all 121 flashcards in this deck.
Unlock Deck
k this deck
45
Hydration is a specific example of the phenomenon known generally as .
A) condensation
B) disordering
C) dilution
D) salutation
E) solvation
A) condensation
B) disordering
C) dilution
D) salutation
E) solvation

Unlock Deck
Unlock for access to all 121 flashcards in this deck.
Unlock Deck
k this deck
46
The principal reason for the extremely low solubility of NaCl in benzene (C6H6) is the _ .
A) strong solvent- solvent interactions
B) increased disorder due to mixing of solute and solvent
C) hydrogen bonding in C6H6
D) strength of the covalent bond in NaCl
E) weak solvation of Na+ and Cl- by C6H6
A) strong solvent- solvent interactions
B) increased disorder due to mixing of solute and solvent
C) hydrogen bonding in C6H6
D) strength of the covalent bond in NaCl
E) weak solvation of Na+ and Cl- by C6H6

Unlock Deck
Unlock for access to all 121 flashcards in this deck.
Unlock Deck
k this deck
47
A solution with a concentration higher than the solubility is _.
A) is unsaturated
B) is supercritical
C) is saturated
D) is supersaturated
E) is not possible
A) is unsaturated
B) is supercritical
C) is saturated
D) is supersaturated
E) is not possible

Unlock Deck
Unlock for access to all 121 flashcards in this deck.
Unlock Deck
k this deck
48
The process of a substance sticking to the surface of another is called
A) coagulation
B) adsorption
C) absorption
D) diffusion
E) effusion
A) coagulation
B) adsorption
C) absorption
D) diffusion
E) effusion

Unlock Deck
Unlock for access to all 121 flashcards in this deck.
Unlock Deck
k this deck
49
The largest value of the Henry's Law constant for the liquid solvent H2O will be obtained with Gas as the solute and a temperature of °C.
A) Ar, 11
B) C2H4, 45
C) CO2, 32
D) HCl, 49
E) N2, 15
A) Ar, 11
B) C2H4, 45
C) CO2, 32
D) HCl, 49
E) N2, 15

Unlock Deck
Unlock for access to all 121 flashcards in this deck.
Unlock Deck
k this deck
50
Which one of the following concentration units varies with temperature?
A) mole fraction
B) mass percent
C) molarity
D) molality
E) all of the above
A) mole fraction
B) mass percent
C) molarity
D) molality
E) all of the above

Unlock Deck
Unlock for access to all 121 flashcards in this deck.
Unlock Deck
k this deck
51
The magnitudes of Kf and of Kb depend on the identity of the .
A) solvent and on temperature
B) solute and solvent
C) solvent
D) solute
E) solution
A) solvent and on temperature
B) solute and solvent
C) solvent
D) solute
E) solution

Unlock Deck
Unlock for access to all 121 flashcards in this deck.
Unlock Deck
k this deck
52
At 20°C, an aqueous solution that is 24.00% by mass in ammonium chloride has a density of 1.0674 g/mL. What is the molarity of ammonium chloride in the solution? The formula weight of NH4Cl is 53.50 g/mol.
A) 22.5
B) 4.79
C) 0.479
D) 5.90
E) 0.0445
A) 22.5
B) 4.79
C) 0.479
D) 5.90
E) 0.0445

Unlock Deck
Unlock for access to all 121 flashcards in this deck.
Unlock Deck
k this deck
53
Which one of the following substances would be the most soluble in CCl4?
A) C10H22
B) CH3CH2OH
C) H2O
D) NaCl
E) NH3
A) C10H22
B) CH3CH2OH
C) H2O
D) NaCl
E) NH3

Unlock Deck
Unlock for access to all 121 flashcards in this deck.
Unlock Deck
k this deck
54
Which one of the following is most soluble in water?
A) CH3CH2OH
B) CH3CH2CH2CH2OH
C) CH3OH
D) CH3CH2CH2OH
E) CH3CH2CH2CH2CH2OH
A) CH3CH2OH
B) CH3CH2CH2CH2OH
C) CH3OH
D) CH3CH2CH2OH
E) CH3CH2CH2CH2CH2OH

Unlock Deck
Unlock for access to all 121 flashcards in this deck.
Unlock Deck
k this deck
55
Which of the following substances is more likely to dissolve in CH3OH?
A) N2
B) Kr
C) CCl4
D) H2
E) CH3CH2OH
A) N2
B) Kr
C) CCl4
D) H2
E) CH3CH2OH

Unlock Deck
Unlock for access to all 121 flashcards in this deck.
Unlock Deck
k this deck
56
Which of the following aqueous solutions will have the highest boiling point?
A) 0.10 m SrSO4
B) 0.20 m glucose
C) 0.25 m sucrose
D) 0.10 m NaCl
E) 0.10 m Na2SO4
A) 0.10 m SrSO4
B) 0.20 m glucose
C) 0.25 m sucrose
D) 0.10 m NaCl
E) 0.10 m Na2SO4

Unlock Deck
Unlock for access to all 121 flashcards in this deck.
Unlock Deck
k this deck
57
Which of the following substances is more likely to dissolve in water?
A) HOCH2CH2OH
B) CH3(CH2)8CH2OH
C) CCl4
D) CHCl3
E)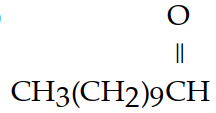
A) HOCH2CH2OH
B) CH3(CH2)8CH2OH
C) CCl4
D) CHCl3
E)
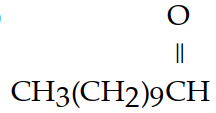

Unlock Deck
Unlock for access to all 121 flashcards in this deck.
Unlock Deck
k this deck
58
A solution contains 15 ppm of benzene. The density of the solution is 1.00 g/mL. This means that
)
A) there are 15 mg of benzene in 1.0 g of this solution
B) 1.0 L of the solution contains 15 g of benzene
C) the solution is 15% by mass of benzene
D) 1.0 g of the solution contains 15 × 10- 6 g of benzene
E) 100 g of the solution contains 15 g of benzene
)
A) there are 15 mg of benzene in 1.0 g of this solution
B) 1.0 L of the solution contains 15 g of benzene
C) the solution is 15% by mass of benzene
D) 1.0 g of the solution contains 15 × 10- 6 g of benzene
E) 100 g of the solution contains 15 g of benzene

Unlock Deck
Unlock for access to all 121 flashcards in this deck.
Unlock Deck
k this deck
59
As the concentration of a solute in a solution increases, the freezing point of the solution
And the vapor pressure of the solution .
A) decreases, increases
B) decreases, is unaffected
C) increases, increases
D) decreases, decreases
E) increases, decreases
And the vapor pressure of the solution .
A) decreases, increases
B) decreases, is unaffected
C) increases, increases
D) decreases, decreases
E) increases, decreases

Unlock Deck
Unlock for access to all 121 flashcards in this deck.
Unlock Deck
k this deck
60
Which of the following liquids will have the lowest freezing point?
A) pure H2O
B) aqueous FeI3 (0.24 m)
C) aqueous sucrose (0.60 m)
D) aqueous glucose (0.60 m)
E) aqueous KF (0.50 m)
A) pure H2O
B) aqueous FeI3 (0.24 m)
C) aqueous sucrose (0.60 m)
D) aqueous glucose (0.60 m)
E) aqueous KF (0.50 m)

Unlock Deck
Unlock for access to all 121 flashcards in this deck.
Unlock Deck
k this deck
61
Water (H2O) and the alcohol methanol (CH3OH) are infinitely soluble in each other. The primary intermolecular force responsible for this is .

Unlock Deck
Unlock for access to all 121 flashcards in this deck.
Unlock Deck
k this deck
62
A solution contains 150.8 grams of NaCl in 678.3 grams of water. Calculate the vapor pressure of water (in torr) over the solution at 25.0 oC. (Note:
the vapor pressure of pure water at 25.0 oC is 23.76 torr.)
the vapor pressure of pure water at 25.0 oC is 23.76 torr.)

Unlock Deck
Unlock for access to all 121 flashcards in this deck.
Unlock Deck
k this deck
63
What is the osmotic pressure (in atm) of a 0.040 M solution of a non- electrolyte at 30.0 oC?

Unlock Deck
Unlock for access to all 121 flashcards in this deck.
Unlock Deck
k this deck
64
Pressure has an appreciable effect on the solubility of in liquids.
A) solids
B) solids and liquids
C) liquids
D) salts
E) gases
A) solids
B) solids and liquids
C) liquids
D) salts
E) gases

Unlock Deck
Unlock for access to all 121 flashcards in this deck.
Unlock Deck
k this deck
65
A sample of potassium nitrate (49.0 g) is dissolved in 101 g of water at 100 °C, with precautions taken to avoid evaporation of any water. The solution is cooled to 30.0 °C and no precipitate is observed. This solution is .
A) saturated
B) unsaturated
C) hydrated
D) placated
E) supersaturated
A) saturated
B) unsaturated
C) hydrated
D) placated
E) supersaturated

Unlock Deck
Unlock for access to all 121 flashcards in this deck.
Unlock Deck
k this deck
66
The vapor pressure of pure water at 25 °C is 23.8 torr. What is the vapor pressure (torr) of water above a solution prepared by dissolving 18.0 g of glucose (anonelectrolyte, MW = 180.0 g/mol) in
95.0 g of water?
A) 0.443
B) 23.8
C) 24.3
D) 0.451
E) 23.4
95.0 g of water?
A) 0.443
B) 23.8
C) 24.3
D) 0.451
E) 23.4

Unlock Deck
Unlock for access to all 121 flashcards in this deck.
Unlock Deck
k this deck
67
Which component of air is the primary problem in a condition known as "the bends?"
A) N2
B) He
C) CO2
D) CO
E) O2
A) N2
B) He
C) CO2
D) CO
E) O2

Unlock Deck
Unlock for access to all 121 flashcards in this deck.
Unlock Deck
k this deck
68
Determine the fraction of ionization of HX if a solution prepared by dissolving 0.020 mol of HX in 115 g of water freezes at - 0.47 °C. The molal freezing- point- depression constant of water is
1)86 °C/m.
A) 1.45
B) 0.45
C) 0.044
D) 0.348
E) 0.30
1)86 °C/m.
A) 1.45
B) 0.45
C) 0.044
D) 0.348
E) 0.30

Unlock Deck
Unlock for access to all 121 flashcards in this deck.
Unlock Deck
k this deck
69
The concentration of HCl in a solution that is prepared by dissolving 5.5 g of HCl in 200 g of C2H6O is _ molal.
A) 1.3
B) 0.75
C) 27.5
D) 7.5 × 10- 4
E) 3.3 × 10- 2
A) 1.3
B) 0.75
C) 27.5
D) 7.5 × 10- 4
E) 3.3 × 10- 2

Unlock Deck
Unlock for access to all 121 flashcards in this deck.
Unlock Deck
k this deck
70
When two nonpolar organic liquids are mixed, a solution forms and the enthalpy of solution is quite small. Label the two organic liquids as A (solvent) and B (solute) The formation of solution is favored by .
A) the highly negative enthalpy of the solution process
B) an increase in disorder, since A- A, B- B, and A- B interactions are similar
C) solvation of the solvent, A
D) hydration of the solute, B
E) the equal enthalpy of the solvent and solute
A) the highly negative enthalpy of the solution process
B) an increase in disorder, since A- A, B- B, and A- B interactions are similar
C) solvation of the solvent, A
D) hydration of the solute, B
E) the equal enthalpy of the solvent and solute

Unlock Deck
Unlock for access to all 121 flashcards in this deck.
Unlock Deck
k this deck
71
The mole fraction of urea (MW = 60.0 g/mol) in a solution prepared by dissolving 16 g of urea in 39 g of H2O is _ .
A) 0.37
B) 0.58
C) 9.1
D) 0.13
E) 0.11
A) 0.37
B) 0.58
C) 9.1
D) 0.13
E) 0.11

Unlock Deck
Unlock for access to all 121 flashcards in this deck.
Unlock Deck
k this deck
72
Physical properties of a solution that depend on the quantity of the solute particles present, but not the kind or identity of the particles, are termed properties.

Unlock Deck
Unlock for access to all 121 flashcards in this deck.
Unlock Deck
k this deck
73
A solution is prepared by dissolving 6.00 g of an unknown nonelectrolyte in enough water to make
1)00 L of solution. The osmotic pressure of this solution is 0.750 atm at 25.0 °C. What is the molecular weight (g/mol) of the unknown solute?
A) 16.4
B) 110
C) 5.12 × 10- 3
D) 30.6
E) 195
1)00 L of solution. The osmotic pressure of this solution is 0.750 atm at 25.0 °C. What is the molecular weight (g/mol) of the unknown solute?
A) 16.4
B) 110
C) 5.12 × 10- 3
D) 30.6
E) 195

Unlock Deck
Unlock for access to all 121 flashcards in this deck.
Unlock Deck
k this deck
74
A sample of potassium chlorate (15.0 g) is dissolved in 201 g of water at 70 °C, with precautions taken to avoid evaporation of any water. The solution is cooled to 30.0 °C and no precipitate is observed. This solution is .
A) unsaturated
B) saturated
C) miscible
D) supersaturated
E) hydrated
A) unsaturated
B) saturated
C) miscible
D) supersaturated
E) hydrated

Unlock Deck
Unlock for access to all 121 flashcards in this deck.
Unlock Deck
k this deck
75
The phenomenon used to differentiate colloids and true solutions is called the effect.

Unlock Deck
Unlock for access to all 121 flashcards in this deck.
Unlock Deck
k this deck
76
A solution is prepared by dissolving 0.60 g of nicotine (a nonelectrolyte) in water to make 12 mL of solution. The osmotic pressure of the solution is 7.55 atm at 25 °C. The molecular weight of nicotine is g/mol.
A) 43
B) 0.60
C) 160
D) 28
E) 50
A) 43
B) 0.60
C) 160
D) 28
E) 50

Unlock Deck
Unlock for access to all 121 flashcards in this deck.
Unlock Deck
k this deck
77
Calculate the freezing point (0°C) of a 0.05500 m aqueous solution of NaNO3. The molal freezing- point- depression constant of water is 1.86 °C/m.
A) 0.1023
B) 0.0286
C) - 0.05627
D) - 0.1023
E) - 0.2046
A) 0.1023
B) 0.0286
C) - 0.05627
D) - 0.1023
E) - 0.2046

Unlock Deck
Unlock for access to all 121 flashcards in this deck.
Unlock Deck
k this deck
78
Which one of the following solutes has a limiting van't Hoff factor (i) of 3 when dissolved in water?
A) CCl4
B) Na2SO4
C) sucrose
D) KNO3
E) CH3OH
A) CCl4
B) Na2SO4
C) sucrose
D) KNO3
E) CH3OH

Unlock Deck
Unlock for access to all 121 flashcards in this deck.
Unlock Deck
k this deck
79
A solution contains 150.8 grams of NaCl in 678.3 grams of water. Calculate the vapor pressure lowering (in torr) of the solution at 25.0 oC. (Note:
the vapor pressure of pure water at 25.0 oC is 23.76 torr.)
the vapor pressure of pure water at 25.0 oC is 23.76 torr.)

Unlock Deck
Unlock for access to all 121 flashcards in this deck.
Unlock Deck
k this deck
80
The concentration of KCl in a solution prepared by adding 0.0660 mol of KCl to 1.00 mol of water is
% by mass.
A) 0.733
B) 0.0130
C) 0.0519
D) 7.33 × 10- 4
E) 5.19
% by mass.
A) 0.733
B) 0.0130
C) 0.0519
D) 7.33 × 10- 4
E) 5.19

Unlock Deck
Unlock for access to all 121 flashcards in this deck.
Unlock Deck
k this deck


