Deck 12: Modern Materials
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/47
Play
Full screen (f)
Deck 12: Modern Materials
1
The formation of a condensation polymer generally involves _ .
A) the addition of a plasticizer
B) the formation of significant crosslinking
C) the elimination of a small molecule
D) the mixing of sulfur with an addition polymer
E) the vaporization of a plasticizer
A) the addition of a plasticizer
B) the formation of significant crosslinking
C) the elimination of a small molecule
D) the mixing of sulfur with an addition polymer
E) the vaporization of a plasticizer
the elimination of a small molecule
2
In the context of biopolymers, the opposite of the polymerization process is .
A) addition
B) condensation
C) cross- linking
D) hydrolysis
E) sublimation
A) addition
B) condensation
C) cross- linking
D) hydrolysis
E) sublimation
hydrolysis
3
In initial steps of the sol- gel process, a reactive metal is treated with an alcohol to form a metal alkoxide. The metal alkoxide is then combined with water to form the metal hydroxide. The metal hydroxide is not formed directly by reaction of the metal with water because .
A) the direct reaction of a reactive metal with water will give a complex mixture of metal oxides and hydroxide
B) finer particles are obtained in the two- step process
C) the two- step process prevents oxidation of the metal to an unstable oxidation state
D) the metal hydroxide formed in the two- step process is more soluble and is more easily utilized
E) the alcohol stabilizes the metal hydroxide making it less susceptible to attack by the base added later
A) the direct reaction of a reactive metal with water will give a complex mixture of metal oxides and hydroxide
B) finer particles are obtained in the two- step process
C) the two- step process prevents oxidation of the metal to an unstable oxidation state
D) the metal hydroxide formed in the two- step process is more soluble and is more easily utilized
E) the alcohol stabilizes the metal hydroxide making it less susceptible to attack by the base added later
the direct reaction of a reactive metal with water will give a complex mixture of metal oxides and hydroxide
4
Biocompatibility means that material is .
A) made by biological methods
B) integratable into living organisms
C) made from biological material
D) biodegradable
E) none of the above
A) made by biological methods
B) integratable into living organisms
C) made from biological material
D) biodegradable
E) none of the above

Unlock Deck
Unlock for access to all 47 flashcards in this deck.
Unlock Deck
k this deck
5
Materials that eventually break down in the body .
A) are always toxic
B) cannot be used as biomaterials
C) can be used to construct "scaffoldings" on which natural cells grow
D) can be used to construct bone- replacements
E) can be used to construct long- term replacements for heart valves
A) are always toxic
B) cannot be used as biomaterials
C) can be used to construct "scaffoldings" on which natural cells grow
D) can be used to construct bone- replacements
E) can be used to construct long- term replacements for heart valves

Unlock Deck
Unlock for access to all 47 flashcards in this deck.
Unlock Deck
k this deck
6
Molecules with only single bonds do not generally exhibit liquid- crystalline properties because
)
A) molecules without multiple bonds lack the flexibility necessary for alignment
B) molecules with only single bonds are gases
C) molecules without multiple bonds lack the rigidity necessary for alignment
D) molecules with only single bonds are too big to exhibit liquid- crystalline properties
E) molecules without multiple bonds are too small to exhibit liquid- crystalline properties
)
A) molecules without multiple bonds lack the flexibility necessary for alignment
B) molecules with only single bonds are gases
C) molecules without multiple bonds lack the rigidity necessary for alignment
D) molecules with only single bonds are too big to exhibit liquid- crystalline properties
E) molecules without multiple bonds are too small to exhibit liquid- crystalline properties

Unlock Deck
Unlock for access to all 47 flashcards in this deck.
Unlock Deck
k this deck
7
A biomaterial intended for use as a long- term replacement of a blood vessel _ .
A) must be rigid and chemically inert
B) must be flexible and have an open porous structure
C) should be designed such that it encourages coagulation of blood
D) must be rigid and must not degrade over time
E) must be rigid and have rough surfaces
A) must be rigid and chemically inert
B) must be flexible and have an open porous structure
C) should be designed such that it encourages coagulation of blood
D) must be rigid and must not degrade over time
E) must be rigid and have rough surfaces

Unlock Deck
Unlock for access to all 47 flashcards in this deck.
Unlock Deck
k this deck
8
Natural rubber is too soft and chemically reactive for practical applications. Vulcanization of natural rubber entails _ .
A) crosslinking reactive polymer chains with sulfur atoms
B) increasing the average molecular weight of a condensation polymer
C) decreasing the average molecular weight of an addition polymer
D) conversion of an addition polymer to a condensation polymer
E) conversion of a condensation polymer to an addition polymer
A) crosslinking reactive polymer chains with sulfur atoms
B) increasing the average molecular weight of a condensation polymer
C) decreasing the average molecular weight of an addition polymer
D) conversion of an addition polymer to a condensation polymer
E) conversion of a condensation polymer to an addition polymer

Unlock Deck
Unlock for access to all 47 flashcards in this deck.
Unlock Deck
k this deck
9
For a given substance that exhibits liquid- crystalline properties, the liquid- crystalline state exists
)
A) in a range of temperatures above the melting point of the solid
B) in a range of temperatures from below the melting point to above the melting point
C) at one particular temperature below the melting point of the solid
D) in a range of temperatures below the melting point of the solid
E) at one particular temperature above the melting point of the solid
)
A) in a range of temperatures above the melting point of the solid
B) in a range of temperatures from below the melting point to above the melting point
C) at one particular temperature below the melting point of the solid
D) in a range of temperatures below the melting point of the solid
E) at one particular temperature above the melting point of the solid

Unlock Deck
Unlock for access to all 47 flashcards in this deck.
Unlock Deck
k this deck
10
Which of the following is most likely to exhibit liquid- crystalline behavior?
A)
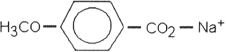
B) CH3CH2CH2CH2CH2CH2CH2CH3
C) CH3CH2CH2CH2CH2- Na+
D) CH3CH2- C(CH3)2- CH2CH3
E)
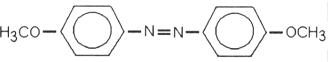
A)

B) CH3CH2CH2CH2CH2CH2CH2CH3
C) CH3CH2CH2CH2CH2- Na+
D) CH3CH2- C(CH3)2- CH2CH3
E)
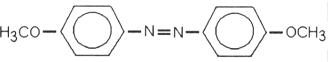

Unlock Deck
Unlock for access to all 47 flashcards in this deck.
Unlock Deck
k this deck
11
As a polymer becomes more crystalline, _.
A) its density decreases
B) its stiffness decreases
C) its melting point decreases
D) its yield stress decreases
E) none of the above are correct
A) its density decreases
B) its stiffness decreases
C) its melting point decreases
D) its yield stress decreases
E) none of the above are correct

Unlock Deck
Unlock for access to all 47 flashcards in this deck.
Unlock Deck
k this deck
12
A biomaterial intended for use as a long- term replacement of a bone must .
A) have sufficient rigidity
B) be chemically inert
C) possess all of these qualities
D) not degrade over time
E) not cause an immune response
A) have sufficient rigidity
B) be chemically inert
C) possess all of these qualities
D) not degrade over time
E) not cause an immune response

Unlock Deck
Unlock for access to all 47 flashcards in this deck.
Unlock Deck
k this deck
13
In what year was the first systematic work involving liquid crystals reported?
A) 1776
B) 1954
C) 1943
D) 1978
E) 1888
A) 1776
B) 1954
C) 1943
D) 1978
E) 1888

Unlock Deck
Unlock for access to all 47 flashcards in this deck.
Unlock Deck
k this deck
14
Advantages to replacement of metal parts used in high- temperature applications with ceramics include:
1) Ceramics are easily manufactured free of defects.
2) Ceramics are less dense than metals.
3) Ceramics are less brittle than metals.
4) Ceramics are more resistant to corrosion than metals.
A) 2, 3, 4
B) 1, 2, 4
C) 2, 4
D) 1, 2, 3, 4
E) 1, 3, 4
1) Ceramics are easily manufactured free of defects.
2) Ceramics are less dense than metals.
3) Ceramics are less brittle than metals.
4) Ceramics are more resistant to corrosion than metals.
A) 2, 3, 4
B) 1, 2, 4
C) 2, 4
D) 1, 2, 3, 4
E) 1, 3, 4

Unlock Deck
Unlock for access to all 47 flashcards in this deck.
Unlock Deck
k this deck
15
An elastomer will fail to regain its original dimensions following a distortion beyond its
A) phase boundary
B) glass transition
C) London force
D) crystallinity
E) elastic limit
A) phase boundary
B) glass transition
C) London force
D) crystallinity
E) elastic limit

Unlock Deck
Unlock for access to all 47 flashcards in this deck.
Unlock Deck
k this deck
16
Cholesteric liquid crystals are colored because .
A) of the slight twist between layers
B) All of the molecules contain multiple benzene rings.
C) of the large spacing between layers
D) each molecule is a chromophore
E) of the large number of conjugated bonds
A) of the slight twist between layers
B) All of the molecules contain multiple benzene rings.
C) of the large spacing between layers
D) each molecule is a chromophore
E) of the large number of conjugated bonds

Unlock Deck
Unlock for access to all 47 flashcards in this deck.
Unlock Deck
k this deck
17
Cholesteric liquid crystals have not been used for monitoring of .
A) skin temperature
B) all of the above
C) hot spots in microelectronic circuits
D) temperature of light
E) temperature of cookware
A) skin temperature
B) all of the above
C) hot spots in microelectronic circuits
D) temperature of light
E) temperature of cookware

Unlock Deck
Unlock for access to all 47 flashcards in this deck.
Unlock Deck
k this deck
18
Of the following chemical forms, are not commonly found in ceramics.
A) silicates
B) carbides
C) nitrates
D) oxides
E) aluminates
A) silicates
B) carbides
C) nitrates
D) oxides
E) aluminates

Unlock Deck
Unlock for access to all 47 flashcards in this deck.
Unlock Deck
k this deck
19
Superconductivity means that .
A) electrons move faster
B) electrons move through a shorter path
C) electrons move without resistance
D) super expensive materials were used
E) diamagnetic atoms become elongated
A) electrons move faster
B) electrons move through a shorter path
C) electrons move without resistance
D) super expensive materials were used
E) diamagnetic atoms become elongated

Unlock Deck
Unlock for access to all 47 flashcards in this deck.
Unlock Deck
k this deck
20
The material first shown to exhibit what we now call superconductivity was _ .
A) a metal
B) a composite
C) a ceramic
D) a polymer
E) a thin film
A) a metal
B) a composite
C) a ceramic
D) a polymer
E) a thin film

Unlock Deck
Unlock for access to all 47 flashcards in this deck.
Unlock Deck
k this deck
21
are solid- state materials that can be made either semiconducting or metallic without any doping.

Unlock Deck
Unlock for access to all 47 flashcards in this deck.
Unlock Deck
k this deck
22
are materials characterized by an energy gap between a filled valence band and an empty conduction band.

Unlock Deck
Unlock for access to all 47 flashcards in this deck.
Unlock Deck
k this deck
23
The process of adding controlled amounts of impurity atoms to a material is known as
.
.

Unlock Deck
Unlock for access to all 47 flashcards in this deck.
Unlock Deck
k this deck
24
Nylon is formed by the reaction of a with a .

Unlock Deck
Unlock for access to all 47 flashcards in this deck.
Unlock Deck
k this deck
25
Which of the following is not a natural polymer? silk
Starch protein cellulose nylon
A) cellulose
B) protein
C) starch
D) silk
E) nylon
Starch protein cellulose nylon
A) cellulose
B) protein
C) starch
D) silk
E) nylon

Unlock Deck
Unlock for access to all 47 flashcards in this deck.
Unlock Deck
k this deck
26
The empirical formula of an addition polymer .
A) is the same as that of the monomer from which it is formed except that 2 H and 1 C have been added
B) is the same as that of the monomer from which it is formed except that 2 H and 1 O have been subtracted
C) is the same as that of the monomer from which it is formed except that 2 H and 1 C have been subtracted
D) is the same as that of the monomer from which it is formed
E) is the same as that of the monomer from which it is formed except that 2 H and 1 O have been added
A) is the same as that of the monomer from which it is formed except that 2 H and 1 C have been added
B) is the same as that of the monomer from which it is formed except that 2 H and 1 O have been subtracted
C) is the same as that of the monomer from which it is formed except that 2 H and 1 C have been subtracted
D) is the same as that of the monomer from which it is formed
E) is the same as that of the monomer from which it is formed except that 2 H and 1 O have been added

Unlock Deck
Unlock for access to all 47 flashcards in this deck.
Unlock Deck
k this deck
27
For a given substance that exhibits liquid- crystalline properties, the transition from solid to liquid- crystal state occurs .
A) over a range of temperatures between the melting point of the solid and the boiling point of the liquid
B) at a well defined temperature above the melting point of the solid
C) at a well defined temperature below the melting point of the solid
D) at the melting point of the solid
E) over a range of temperatures that includes the melting point of the solid
A) over a range of temperatures between the melting point of the solid and the boiling point of the liquid
B) at a well defined temperature above the melting point of the solid
C) at a well defined temperature below the melting point of the solid
D) at the melting point of the solid
E) over a range of temperatures that includes the melting point of the solid

Unlock Deck
Unlock for access to all 47 flashcards in this deck.
Unlock Deck
k this deck
28
A sample of natural rubber (200.0 g) is vulcanized, with the complete consumption of 4.8 g of sulfur. Natural rubber is a polymer of isoprene (C5H8). Four sulfur atoms are used in each crosslink connection. What percent of the isoprene units will be crosslinked?
A) 1.3
B) 9.4
C) 2.5
D) 7.6
E) 5.1
A) 1.3
B) 9.4
C) 2.5
D) 7.6
E) 5.1

Unlock Deck
Unlock for access to all 47 flashcards in this deck.
Unlock Deck
k this deck
29
A material is sintered by .
A) sieving to achieve uniform particle size
B) finely dividing the solid
C) heating the finely divided solid to a high temperature under pressure
D) placing in the middle
E) heating with sulfur
A) sieving to achieve uniform particle size
B) finely dividing the solid
C) heating the finely divided solid to a high temperature under pressure
D) placing in the middle
E) heating with sulfur

Unlock Deck
Unlock for access to all 47 flashcards in this deck.
Unlock Deck
k this deck
30
The monomer that is polymerized to make natural rubber is .
A) ethylene
B) adipic acid
C) formaldehyde
D) melamine
E) isoprene
A) ethylene
B) adipic acid
C) formaldehyde
D) melamine
E) isoprene

Unlock Deck
Unlock for access to all 47 flashcards in this deck.
Unlock Deck
k this deck
31
Short, high purity fibers reinforced with aluminum borosilicate fibers are used to manufacture the space shuttle tiles.

Unlock Deck
Unlock for access to all 47 flashcards in this deck.
Unlock Deck
k this deck
32
Which of the following is not a biopolymer? protein
Polysaccharide polyurethane RNA
DNA
A) RNA
B) DNA
C) protein
D) polyurethane
E) polysaccharide
Polysaccharide polyurethane RNA
DNA
A) RNA
B) DNA
C) protein
D) polyurethane
E) polysaccharide

Unlock Deck
Unlock for access to all 47 flashcards in this deck.
Unlock Deck
k this deck
33
MRI stands for .
A) Magnetic Resonance Imaging
B) Meissner Resonance Inductance
C) Material Research Instrument
D) Material Resistive Impedance
E) Me Really Imaginative
A) Magnetic Resonance Imaging
B) Meissner Resonance Inductance
C) Material Research Instrument
D) Material Resistive Impedance
E) Me Really Imaginative

Unlock Deck
Unlock for access to all 47 flashcards in this deck.
Unlock Deck
k this deck
34
A _ _ liquid crystal has the least order and is the most liquid- like.
A) smectic B
B) cholesteric
C) nematic
D) smectic C
E) smectic
A) smectic B
B) cholesteric
C) nematic
D) smectic C
E) smectic

Unlock Deck
Unlock for access to all 47 flashcards in this deck.
Unlock Deck
k this deck
35
In the smectic A liquid- crystalline phase, _ .
A) the molecules are oriented in a totally random fashion
B) the molecules are aligned with their long axes tilted with respect to a line perpendicular to the plane in which the molecules are stacked
C) disk- shaped molecules are aligned through a stacking of the disks in layers
D) the molecules are aligned along their long axes, with no ordering with respect to the ends of the molecules
E) the molecules are arranged in sheets, with their long axes parallel and their ends aligned as well
A) the molecules are oriented in a totally random fashion
B) the molecules are aligned with their long axes tilted with respect to a line perpendicular to the plane in which the molecules are stacked
C) disk- shaped molecules are aligned through a stacking of the disks in layers
D) the molecules are aligned along their long axes, with no ordering with respect to the ends of the molecules
E) the molecules are arranged in sheets, with their long axes parallel and their ends aligned as well

Unlock Deck
Unlock for access to all 47 flashcards in this deck.
Unlock Deck
k this deck
36
Write the chemical formulas for both polyethylene and the monomer from which it is formed.

Unlock Deck
Unlock for access to all 47 flashcards in this deck.
Unlock Deck
k this deck
37
A category plastic container will generally be the most easily recycled.
A) 1
B) 2
C) 3
D) 4
E) 22
A) 1
B) 2
C) 3
D) 4
E) 22

Unlock Deck
Unlock for access to all 47 flashcards in this deck.
Unlock Deck
k this deck
38
Which one of the following is an addition polymer with the same structure as polyethylene except that one hydrogen on every other carbon is replaced by a benzene ring?
Polyvinyl chloride polypropylene polystyrene polyurethane nylon 6,6
A) polystyrene
B) polypropylene
C) nylon 6, 6
D) polyvinyl chloride
E) polyurethane
Polyvinyl chloride polypropylene polystyrene polyurethane nylon 6,6
A) polystyrene
B) polypropylene
C) nylon 6, 6
D) polyvinyl chloride
E) polyurethane

Unlock Deck
Unlock for access to all 47 flashcards in this deck.
Unlock Deck
k this deck
39
Of the following, only is not a polymer. cellulose
Nylon starch protein
Stainless steel
A) cellulose
B) nylon
C) starch
D) protein
E) stainless steel
Nylon starch protein
Stainless steel
A) cellulose
B) nylon
C) starch
D) protein
E) stainless steel

Unlock Deck
Unlock for access to all 47 flashcards in this deck.
Unlock Deck
k this deck
40
Which type of liquid crystal is colored and changes color with temperature?
A) smectic A
B) smectic B
C) smectic C
D) nematic
E) cholesteric
A) smectic A
B) smectic B
C) smectic C
D) nematic
E) cholesteric

Unlock Deck
Unlock for access to all 47 flashcards in this deck.
Unlock Deck
k this deck
41
There are different amino acids present in most proteins.
A) 1
B) 2
C) 99
D) 16
E) 20
A) 1
B) 2
C) 99
D) 16
E) 20

Unlock Deck
Unlock for access to all 47 flashcards in this deck.
Unlock Deck
k this deck
42
Molecules containing only single bonds do not exhibit liquid- crystal behavior because free rotation can occur around single bonds making these molecules flexible.

Unlock Deck
Unlock for access to all 47 flashcards in this deck.
Unlock Deck
k this deck
43
A plasticizer makes a polymer more pliable by reducing the interactions between polymer chains.

Unlock Deck
Unlock for access to all 47 flashcards in this deck.
Unlock Deck
k this deck
44
Silicon technology is based on the fact that silicon oxide is a chemically stable conductor.

Unlock Deck
Unlock for access to all 47 flashcards in this deck.
Unlock Deck
k this deck
45
Superconductors exhibit the Meissner effect.

Unlock Deck
Unlock for access to all 47 flashcards in this deck.
Unlock Deck
k this deck
46
Vulcanization involves heating rubber with sulfur dioxide to produce a thermosetting polymer.

Unlock Deck
Unlock for access to all 47 flashcards in this deck.
Unlock Deck
k this deck
47
Polyethylene is formed by a condensation reaction.

Unlock Deck
Unlock for access to all 47 flashcards in this deck.
Unlock Deck
k this deck


