Deck 1: Introduction: Matter and Measurement
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/118
Play
Full screen (f)
Deck 1: Introduction: Matter and Measurement
1
Which of the following has the same number of significant figures as the number 1.00310?
A) 5.119
B) 1 × 106
C) 199.791
D) 100
E) 8.66
A) 5.119
B) 1 × 106
C) 199.791
D) 100
E) 8.66
199.791
2
Which of the following is the same as 0.001 cm?
A) 100 mm
B) 1 mm
C) 0.01 m
D) 0.01 dm
E) 0.01 mm
A) 100 mm
B) 1 mm
C) 0.01 m
D) 0.01 dm
E) 0.01 mm
0.01 mm
3
Express the temperature, 422.35 K, in degrees Celsius.
A) 149.20°C
B) 792.23°C
C) 50.89°C
D) 695.50°C
E) 22.78°C
A) 149.20°C
B) 792.23°C
C) 50.89°C
D) 695.50°C
E) 22.78°C
149.20°C
4
Which calculation clearly shows a conversion between temperatures in degrees Celsius, t(°C), and temperature in Kelvins, T(K)?
A) T(K) = t(°C)
B) T(K) = [t(°C) - 32] / 1.8
C) T(K) = 273 - t(°C)
D) T(K) = [t(°C) + 32] × 1.8
E) T(K) = t(°C) + 273
A) T(K) = t(°C)
B) T(K) = [t(°C) - 32] / 1.8
C) T(K) = 273 - t(°C)
D) T(K) = [t(°C) + 32] × 1.8
E) T(K) = t(°C) + 273

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
5
Which one of the following elements has a symbol that is not derived from its foreign name?
A) aluminum
B) tin
C) lead
D) copper
E) mercury
A) aluminum
B) tin
C) lead
D) copper
E) mercury

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
6
Which states of matter are significantly compressible?
A) solids and liquids
B) liquids only
C) solids only
D) gases only
E) liquids and gases
A) solids and liquids
B) liquids only
C) solids only
D) gases only
E) liquids and gases

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
7
Homogeneous mixtures are also known as _.
A) solids
B) solutions
C) substances
D) compounds
E) elements
A) solids
B) solutions
C) substances
D) compounds
E) elements

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
8
Which one of the following has the element name and symbol correctly matched?
A) P, potassium
B) Sn, silicon
C) Mg, manganese
D) Ag, silver
E) C, copper
A) P, potassium
B) Sn, silicon
C) Mg, manganese
D) Ag, silver
E) C, copper

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
9
The number with the most significant zeros is .
A) 250000001
B) 0.02500001
C) 2.501 × 10- 7
D) 0.00002510
E) 2.5100000
A) 250000001
B) 0.02500001
C) 2.501 × 10- 7
D) 0.00002510
E) 2.5100000

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
10
What is the volume (in cm3) of a 63.4 g piece of metal with a density of 12.86 g/cm3?
A) .425
B) 4.93
C) 19.5
D) 6.65
E) none of the above
A) .425
B) 4.93
C) 19.5
D) 6.65
E) none of the above

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
11
If an object is accelerating at a rate of 25 m/s2, how fast will it be moving (in m/s) after 1.50 min? (Assume an initial velocity of zero.)
A) 3.6
B) 38
C) 2.3 × 103
D) 17
E) 0.060
A) 3.6
B) 38
C) 2.3 × 103
D) 17
E) 0.060

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
12
Gases and liquids share the property of .
A) definite shape
B) compressibility
C) indefinite shape
D) incompressibility
E) definite volume
A) definite shape
B) compressibility
C) indefinite shape
D) incompressibility
E) definite volume

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
13
Which of the following are chemical processes?
1) rusting of a nail
2) freezing of water
3) decomposition of water into hydrogen and oxygen gases
4) compression of oxygen gas
A) 1, 3, 4
B) 1, 4
C) 2, 3, 4
D) 1, 2
E) 1, 3
1) rusting of a nail
2) freezing of water
3) decomposition of water into hydrogen and oxygen gases
4) compression of oxygen gas
A) 1, 3, 4
B) 1, 4
C) 2, 3, 4
D) 1, 2
E) 1, 3

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
14
Of the objects below, is the most dense.
A) an object with a volume of 2.5 L and a mass of 12.5 kg
B) an object with a volume of 13 dm3 and a mass of 1.29 × 103 g
C) an object with a volume of 0.00212 m3 and a mass of 4.22 × 104 mg
D) an object with a volume of 3.91 × 10- 24 nm3 and a mass of 7.93 × 10- 1 ng
E) an object with a volume of 139 mL and a mass of 93 g
A) an object with a volume of 2.5 L and a mass of 12.5 kg
B) an object with a volume of 13 dm3 and a mass of 1.29 × 103 g
C) an object with a volume of 0.00212 m3 and a mass of 4.22 × 104 mg
D) an object with a volume of 3.91 × 10- 24 nm3 and a mass of 7.93 × 10- 1 ng
E) an object with a volume of 139 mL and a mass of 93 g

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
15
Which one of the following is a pure substance?
A) wood
B) concrete
C) salt water
D) milk
E) elemental copper
A) wood
B) concrete
C) salt water
D) milk
E) elemental copper

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
16
Which of the following liquids has the greatest density?
A) 13 cm3 with a mass of 23 g
B) 210 cm3 with a mass of 12 g
C) 3.5 cm3 with a mass of 10 g
D) 54 cm3 with a mass of 45 g
E) 0.022 cm3 with a mass of 0.10 g
A) 13 cm3 with a mass of 23 g
B) 210 cm3 with a mass of 12 g
C) 3.5 cm3 with a mass of 10 g
D) 54 cm3 with a mass of 45 g
E) 0.022 cm3 with a mass of 0.10 g

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
17
Which one of the following is the highest temperature?
A) 302 K
B) 38°C
C) 96°F
D) none of the above
E) the freezing point of water
A) 302 K
B) 38°C
C) 96°F
D) none of the above
E) the freezing point of water

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
18
Expressing a number in scientific notation .
A) removes significant zeros
B) allows to increase the number's precision
C) changes its value
D) removes ambiguity as to the significant figures
E) all of the above
A) removes significant zeros
B) allows to increase the number's precision
C) changes its value
D) removes ambiguity as to the significant figures
E) all of the above

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
19
What is the physical state in which matter has no specific shape but does have a specific volume?
A) solid
B) salts
C) gas
D) liquid
E) ice
A) solid
B) salts
C) gas
D) liquid
E) ice

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
20
If an object is accelerating at a rate of 25 m/s2, how long (in seconds) will it take to reach a speed of 550 m/s? (Assume an initial velocity of zero.)
A) 22
B) 1.4 × 104
C) 2.3 × 102
D) 0.045
E) 1.2 × 104
A) 22
B) 1.4 × 104
C) 2.3 × 102
D) 0.045
E) 1.2 × 104

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
21
The law of constant composition applies to .
A) homogeneous mixtures
B) solids
C) heterogeneous mixtures
D) solutions
E) compounds
A) homogeneous mixtures
B) solids
C) heterogeneous mixtures
D) solutions
E) compounds

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
22
If matter is uniform throughout and cannot be separated into other substances by physical means, it is .
A) a homogeneous mixture
B) either an element or a compound
C) an element
D) a heterogeneous mixture
E) a compound
A) a homogeneous mixture
B) either an element or a compound
C) an element
D) a heterogeneous mixture
E) a compound

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
23
In the following list, only is not an example of matter.
A) table salt
B) dust
C) planets
D) light
E) elemental phosphorus
A) table salt
B) dust
C) planets
D) light
E) elemental phosphorus

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
24
You have to calculate the volume of a gas sample with mass of 1.000 × 103 g and density of 1.027 g/L , but you have forgotten the formula. Which way of reasoning would help you in finding the correct mass?
A) If 1.027 L of gas has a mass of 1 g, then L has the mass of 1.000 × 103 g.
B) If 1.027 g of a gas takes up a volume of 1 L, then 1.000 × 103 g of the same gas takes up a volume of .
A) If 1.027 L of gas has a mass of 1 g, then L has the mass of 1.000 × 103 g.
B) If 1.027 g of a gas takes up a volume of 1 L, then 1.000 × 103 g of the same gas takes up a volume of .

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
25
What decimal power does the abbreviation Milli represent?
A) 1 × 109
B) 1 × 106
C) 1 × 10- 6
D) 1 × 103
E) 1 × 10- 3
A) 1 × 109
B) 1 × 106
C) 1 × 10- 6
D) 1 × 103
E) 1 × 10- 3

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
26
Which one of the following is often easily separated into its components by simple techniques such as filtering or decanting?
A) homogeneous mixture
B) elements
C) solutions
D) compounds
E) heterogeneous mixture
A) homogeneous mixture
B) elements
C) solutions
D) compounds
E) heterogeneous mixture

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
27
Which of the following is an illustration of the law of constant composition?
A) Water and salt have different boiling points.
B) Water boils at 100°C at 1 atm pressure.
C) Water is a compound.
D) Water can be separated into other substances by a chemical process.
E) Water is 11% hydrogen and 89% oxygen by mass.
A) Water and salt have different boiling points.
B) Water boils at 100°C at 1 atm pressure.
C) Water is a compound.
D) Water can be separated into other substances by a chemical process.
E) Water is 11% hydrogen and 89% oxygen by mass.

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
28
Of the following, only is a chemical reaction.
A) dropping a penny into a glass of water
B) melting of lead
C) crushing of stone
D) dissolving sugar in water
E) tarnishing of silver
A) dropping a penny into a glass of water
B) melting of lead
C) crushing of stone
D) dissolving sugar in water
E) tarnishing of silver

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
29
How many liters of air are in a room that measures 10.0 ft × 11.0 ft and has an 8.00 ft ceiling? 1 in. = 2.54 cm (exactly); 1 L = 103 cm3
A) 92.8
B) 8.84 × 105
C) 2.68 × 107
D) 2.49 × 104
E) 26.8
A) 92.8
B) 8.84 × 105
C) 2.68 × 107
D) 2.49 × 104
E) 26.8

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
30
A wooden object has a mass of 10.782 g and occupies a volume of 13.72 mL. What is the density of the object determined to an appropriate number of significant figures?
A) 8 × 10- 1 g/mL
B) 7.9 × 10- 1 g/mL
C) 7.86 × 10- 1 g/mL
D) 7.859 × 10- 1 g/mL
E) 7.8586 × 10- 1 g/mL
A) 8 × 10- 1 g/mL
B) 7.9 × 10- 1 g/mL
C) 7.86 × 10- 1 g/mL
D) 7.859 × 10- 1 g/mL
E) 7.8586 × 10- 1 g/mL

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
31
Round the number 0.08535 to two significant figures.
A) 0.086
B) 0.08535
C) 0.09
D) 0.085
E) 0.0854
A) 0.086
B) 0.08535
C) 0.09
D) 0.085
E) 0.0854

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
32
The density of air under ordinary conditions at 25°C is 1.19 g/L. How many kilograms of air is in a room that measures 11.0 ft × 11.0 ft and has an 10.0 ft ceiling? 1 in. = 2.54 cm (exactly);
1 L = 103 cm3
A) 0.0962
B) 4.08 × 104
C) 40.8
D) 3.66
E) 0.152
1 L = 103 cm3
A) 0.0962
B) 4.08 × 104
C) 40.8
D) 3.66
E) 0.152

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
33
The estimated costs for remodelling the interior of an apartment are:
Three 1- gallon cans of paint at
$13)22 each (including tax), two paint brushes at $9.53 each (including tax), and $135 for a helper. The total estimated cost with the appropriate significant figures is $ .
A) 2 × 102
B) 194
C) 193.72
D) 1.9 × 102
E) 193.7
Three 1- gallon cans of paint at
$13)22 each (including tax), two paint brushes at $9.53 each (including tax), and $135 for a helper. The total estimated cost with the appropriate significant figures is $ .
A) 2 × 102
B) 194
C) 193.72
D) 1.9 × 102
E) 193.7

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
34
Osmium has a density of 22.6 g/cm3. What volume (in cm3) would be occupied by a 21.8 g sample of osmium?
A) 2.03 × 10- 3
B) 1.04
C) 493
D) 0.965
E) 2.03 × 103
A) 2.03 × 10- 3
B) 1.04
C) 493
D) 0.965
E) 2.03 × 103

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
35
How many significant figures are in the measurement 5.34 g?
A) 4
B) 2
C) 3
D) 1
E) 5
A) 4
B) 2
C) 3
D) 1
E) 5

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
36
Acceleration due to gravity of a free- falling object is 9.8 m/s2. Express this in millimeters/millisecond2.
A) 9.8 × 10- 6
B) 9.8 × 106
C) 9.8 × 10- 9
D) 9.8 × 10- 3
E) 9.8 × 103
A) 9.8 × 10- 6
B) 9.8 × 106
C) 9.8 × 10- 9
D) 9.8 × 10- 3
E) 9.8 × 103

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
37
Round the number 0.007222 to three significant figures.
A) 0.00723
B) 0.007
C) 0.0072
D) 0.00722
E) 0.007225
A) 0.00723
B) 0.007
C) 0.0072
D) 0.00722
E) 0.007225

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
38
An element cannot .
A) be part of a heterogeneous mixture
B) be a pure substance
C) be part of a homogeneous mixture
D) interact with other elements to form compounds
E) be separated into other substances by chemical means
A) be part of a heterogeneous mixture
B) be a pure substance
C) be part of a homogeneous mixture
D) interact with other elements to form compounds
E) be separated into other substances by chemical means

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
39
In which one of the following numbers are all of the zeros significant?
A) 0.1000
B) 100.090090
C) 0.143290
D) 00.0030020
E) 0.05843
A) 0.1000
B) 100.090090
C) 0.143290
D) 00.0030020
E) 0.05843

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
40
One angstrom, symbolized Å, is 10- 10 m. 1 cm3 = Å3.
A) 10- 30
B) 10- 24
C) 1030
D) 10- 9
E) 1024
A) 10- 30
B) 10- 24
C) 1030
D) 10- 9
E) 1024

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
41
Of the following, only is an extensive property.
A) mass
B) density
C) freezing point
D) temperature
E) boiling point
A) mass
B) density
C) freezing point
D) temperature
E) boiling point

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
42
The symbol for the element phosphorous is _.

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
43
Which one of the following is not an intensive property?
A) boiling point
B) density
C) mass
D) melting point
E) temperature
A) boiling point
B) density
C) mass
D) melting point
E) temperature

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
44
A combination of sand, salt, and water is an example of a _.
A) pure substance
B) solid
C) homogeneous mixture
D) heterogeneous mixture
E) compound
A) pure substance
B) solid
C) homogeneous mixture
D) heterogeneous mixture
E) compound

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
45
A cube of an unknown metal measures 1.61 mm on one side. The mass of the cube is 36 mg. Which of the following is most likely the unknown metal? 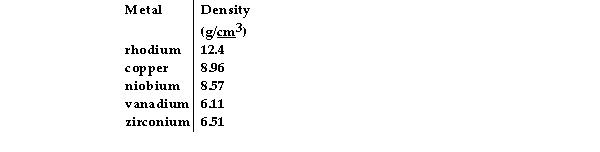
A) vanadium
B) zirconium
C) copper
D) niobium
E) rhodium
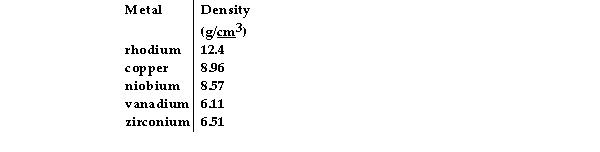
A) vanadium
B) zirconium
C) copper
D) niobium
E) rhodium

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
46
Accuracy refers to _ _.
A) how close a measured number is to other measured numbers
B) how close a measured number is to zero
C) how close a measured number is to infinity
D) how close a measured number is to the calculated value
E) how close a measured number is to the true value
A) how close a measured number is to other measured numbers
B) how close a measured number is to zero
C) how close a measured number is to infinity
D) how close a measured number is to the calculated value
E) how close a measured number is to the true value

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
47
Which one of the following has the element name and symbol correctly matched?
A) Fe, iron
B) N, neon
C) B, bromine
D) S, sodium
E) Tn, tin
A) Fe, iron
B) N, neon
C) B, bromine
D) S, sodium
E) Tn, tin

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
48
How many liters of wine can be held in a wine barrel whose capacity is 26.0 gal? 1 gal = 4 qt = 3.7854 L.
A) 0.146
B) 6.87
C) 1.46 × 10- 4
D) 6.87 × 103
E) 98.4
A) 0.146
B) 6.87
C) 1.46 × 10- 4
D) 6.87 × 103
E) 98.4

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
49
Which one of the following is an intensive property?
A) temperature
B) mass
C) volume
D) amount
E) heat content
A) temperature
B) mass
C) volume
D) amount
E) heat content

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
50
The width, length, and height of a large, custom- made shipping crate are 1.22 m, 3.22 m, and 0.83 m, respectively. The volume of the box using the correct number of significant figures is m3.
A) 3.3
B) 3.261
C) 3.26057
D) 3.26
E) 3.2606
A) 3.3
B) 3.261
C) 3.26057
D) 3.26
E) 3.2606

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
51
Of the following, is the smallest mass.
A) 2.5 × 109 fg
B) 2.5 × 1010 ng
C) 2.5 × 10- 2 mg
D) 25 kg
E) 2.5 × 1015 pg
A) 2.5 × 109 fg
B) 2.5 × 1010 ng
C) 2.5 × 10- 2 mg
D) 25 kg
E) 2.5 × 1015 pg

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
52
In the following list, only is not an example of a chemical reaction.
A) the formation of polyethylene from ethylene
B) dissolution of a penny in nitric acid
C) a burning candle
D) the rusting of iron
E) the condensation of water vapor
A) the formation of polyethylene from ethylene
B) dissolution of a penny in nitric acid
C) a burning candle
D) the rusting of iron
E) the condensation of water vapor

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
53
Round the following number to four significant figures and express the result in standard exponential notation:
229.613
A) 2.296 × 10- 2
B) 22.96 × 10- 1
C) 0.2296 × 103
D) 2.296 × 102
E) 229.6
229.613
A) 2.296 × 10- 2
B) 22.96 × 10- 1
C) 0.2296 × 103
D) 2.296 × 102
E) 229.6

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
54
The law of constant composition says .
A) that all substances have the same composition
B) that the composition of a compound is always the same
C) that the composition of a heterogeneous mixture is always the same
D) that the composition of a homogeneous mixture is always the same
E) that the composition of an element is always the same
A) that all substances have the same composition
B) that the composition of a compound is always the same
C) that the composition of a heterogeneous mixture is always the same
D) that the composition of a homogeneous mixture is always the same
E) that the composition of an element is always the same

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
55
What decimal power does the abbreviation f represent?
A) 1 × 106
B) 1 × 10- 15
C) 1 × 10- 12
D) 1 × 10- 1
E) 1 × 103
A) 1 × 106
B) 1 × 10- 15
C) 1 × 10- 12
D) 1 × 10- 1
E) 1 × 103

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
56
Which one of the following is true about the liter?
A) It is the SI base unit for volume.
B) It is equivalent to a cubic decimeter.
C) It is slightly smaller than a gallon.
D) It contains 106 cubic centimeters.
E) It is slightly smaller than a quart.
A) It is the SI base unit for volume.
B) It is equivalent to a cubic decimeter.
C) It is slightly smaller than a gallon.
D) It contains 106 cubic centimeters.
E) It is slightly smaller than a quart.

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
57
For which of the following can the composition vary?
A) both homogeneous and heterogeneous mixtures
B) pure substance
C) homogeneous mixture
D) heterogeneous mixture
E) element
A) both homogeneous and heterogeneous mixtures
B) pure substance
C) homogeneous mixture
D) heterogeneous mixture
E) element

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
58
How many significant figures should be retained in the result of the following calculation?
12.00000 × 0.9893 + 13.00335 × 0.0107
A) 2
B) 3
C) 4
D) 5
E) 6
12.00000 × 0.9893 + 13.00335 × 0.0107
A) 2
B) 3
C) 4
D) 5
E) 6

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
59
Precision refers to _.
A) how close a measured number is to other measured numbers
B) how close a measured number is to infinity
C) how close a measured number is to zero
D) how close a measured number is to the calculated value
E) how close a measured number is to the true value
A) how close a measured number is to other measured numbers
B) how close a measured number is to infinity
C) how close a measured number is to zero
D) how close a measured number is to the calculated value
E) how close a measured number is to the true value

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
60
The correct answer (reported to the proper number of significant figures) to the following is
.
11.5 × 8.78 = __________
.
11.5 × 8.78 = __________

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
61
A one degree of temperature difference is the smallest on the temperature scale.
A) Kelvin
B) Celsius
C) Kelvin and Celsius
D) Fahrenheit and Celsius
E) Fahrenheit
A) Kelvin
B) Celsius
C) Kelvin and Celsius
D) Fahrenheit and Celsius
E) Fahrenheit

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
62
38.325 lbs = _____________grams.

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
63
There are _ ng in a pg.
A) 0.01
B) 1000
C) 10
D) 0.001
E) 100
A) 0.01
B) 1000
C) 10
D) 0.001
E) 100

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
64
1 milligram =_______________ micrograms

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
65
There should be _ significant figures in the answer to the following computation.
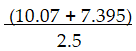
A) 1
B) 2
C) 3
D) 4
E) 5
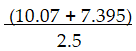
A) 1
B) 2
C) 3
D) 4
E) 5

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
66
Gases do not have a fixed as they are able to be .

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
67
A certain liquid has a density of 2.67 g/cm3. 30.5 mL of this liquid would have a mass of __________ Kg.
A) 0.0114
B) 81.4
C) 11.4
D) 0.0814
E) 0.0875
A) 0.0114
B) 81.4
C) 11.4
D) 0.0814
E) 0.0875

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
68
1.035 × 10- 4 L = _ mL

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
69
Momentum is defined as the product of mass and velocity. The SI unit for momentum is ?
A)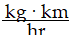
B)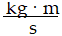
C)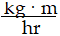
D)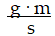
E)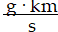
A)
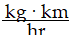
B)
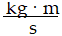
C)
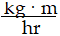
D)
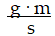
E)
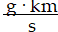

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
70
The correct answer (reported to the proper number of significant figures) to the following is
.
(1815- 1806) × (9.11 × 7.92) = __________
.
(1815- 1806) × (9.11 × 7.92) = __________

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
71
Mass and volume are often referred to as properties of substances.

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
72
1 picometer = centimeters
A) 1 × 1010
B) 1 × 10- 10
C) 1 × 10- 8
D) 1 × 10- 12
E) 1 × 108
A) 1 × 1010
B) 1 × 10- 10
C) 1 × 10- 8
D) 1 × 10- 12
E) 1 × 108

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
73
"Absolute zero" refers to .
A) °C + 9/5(°F - 32)
B) 273.15°C
C) 0 Kelvin
D) 0° Celsius
E) 0° Fahrenheit
A) °C + 9/5(°F - 32)
B) 273.15°C
C) 0 Kelvin
D) 0° Celsius
E) 0° Fahrenheit

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
74
The number 1.00430 has significant figures.
A) 2
B) 3
C) 4
D) 5
E) 6
A) 2
B) 3
C) 4
D) 5
E) 6

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
75
A certain liquid has a density of 2.67 g/cm3. 1340 g of this liquid would occupy a volume of__________L.
A) 35.8
B) 50.2
C) 3.58
D) 1.99 × 10- 3
E) 0.502
A) 35.8
B) 50.2
C) 3.58
D) 1.99 × 10- 3
E) 0.502

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
76
A concise verbal statement or mathematical equation that summarizes a broad variety of observations and experiences is called a(n) .
A) experiment
B) hypothesis
C) test
D) law
E) theory
A) experiment
B) hypothesis
C) test
D) law
E) theory

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
77
If matter is uniform throughout, cannot be separated into other substances by physical processes, but can be decomposed into other substances by chemical processes, it is called a (an) .
A) element
B) compound
C) heterogeneous mixture
D) homogeneous mixture
E) mixture of elements
A) element
B) compound
C) heterogeneous mixture
D) homogeneous mixture
E) mixture of elements

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
78
The quantity _ m is the same as 3 km.
A) 0.003
B) 3000
C) 0.03
D) 30
E) 300
A) 0.003
B) 3000
C) 0.03
D) 30
E) 300

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
79
A temperature of _ K is the same as 63°F.
A) 290
B) 336
C) 276
D) 17
E) 29
A) 290
B) 336
C) 276
D) 17
E) 29

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
80
Sn is the symbol for the element _.

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck


