Deck 24: Chemistry of Coordination Compounds
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/124
Play
Full screen (f)
Deck 24: Chemistry of Coordination Compounds
1
Which one of the following species is a potential polydentate ligand (chelating agent)?
A) Cl-
B) NH3
C) H2O
D) C2O42-
E) CN-
A) Cl-
B) NH3
C) H2O
D) C2O42-
E) CN-
C2O42-
2
A complex of correctly written formula [Pt(NH3)3Br]Br·H2O has which set of ligands in its inner coordination sphere?
A) 3 NH3, 1 Br- - , and 1 H2O
B) 3 NH3, 2 Br- , and 1 H2O
C) 3 NH3 and 2 Br-
D) 3 NH3
E) 3 NH3 and 1 Br-
A) 3 NH3, 1 Br- - , and 1 H2O
B) 3 NH3, 2 Br- , and 1 H2O
C) 3 NH3 and 2 Br-
D) 3 NH3
E) 3 NH3 and 1 Br-
3 NH3 and 1 Br-
3
What is the charge on the complex ion in Ca2[Fe(CN)6]?
A) 3-
B) 2+
C) 1-
D) 2-
E) 4-
A) 3-
B) 2+
C) 1-
D) 2-
E) 4-
4-
4
What purpose would sodium tripolyphosphate serve in a detergent formulation?
A) to aid in removal of rust stains from surfaces and from clothes
B) to improve the flow characteristics of the detergent in the box
C) to aid in keeping the inside of washing machines clean and free from corrosion
D) to complex and hence sequester metal ions in hard water
E) to reduce bacterial growth in the detergent upon storage
A) to aid in removal of rust stains from surfaces and from clothes
B) to improve the flow characteristics of the detergent in the box
C) to aid in keeping the inside of washing machines clean and free from corrosion
D) to complex and hence sequester metal ions in hard water
E) to reduce bacterial growth in the detergent upon storage

Unlock Deck
Unlock for access to all 124 flashcards in this deck.
Unlock Deck
k this deck
5
A ligand with a single donor atom is called .
A) bidentate
B) polydentate
C) a chelate
D) a chelon
E) monodentate
A) bidentate
B) polydentate
C) a chelate
D) a chelon
E) monodentate

Unlock Deck
Unlock for access to all 124 flashcards in this deck.
Unlock Deck
k this deck
6
Consider a complex in which manganese (III) is bonded to six identical ligands. Which one of the following ligands will result in the smallest value of O?
A) Cl-
B) H2O
C) F-
D) NH3
E) CN-
A) Cl-
B) H2O
C) F-
D) NH3
E) CN-

Unlock Deck
Unlock for access to all 124 flashcards in this deck.
Unlock Deck
k this deck
7
What form of hemoglobin is purplish- red?
A) oxyhemoglobin
B) deoxyhemoglobin
A) oxyhemoglobin
B) deoxyhemoglobin

Unlock Deck
Unlock for access to all 124 flashcards in this deck.
Unlock Deck
k this deck
8
What is the most common geometry found in four- coordinate complexes?
A) icosahedral
B) trigonal bipyramidal
C) square planar
D) octahedral
E) tetrahedral
A) icosahedral
B) trigonal bipyramidal
C) square planar
D) octahedral
E) tetrahedral

Unlock Deck
Unlock for access to all 124 flashcards in this deck.
Unlock Deck
k this deck
9
Which one of the following geometries does not exhibit geometrical isomerism?
A) trigonal bipyramidal
B) tetrahedral
C) octahedral
D) square planar
E) linear
A) trigonal bipyramidal
B) tetrahedral
C) octahedral
D) square planar
E) linear

Unlock Deck
Unlock for access to all 124 flashcards in this deck.
Unlock Deck
k this deck
10
What are the donor atoms in ferrichrome and how many of them are in one molecule?
A) Cr, 5
B) Fe, 4
C) N, 4
D) O, 6
E) S, 6
A) Cr, 5
B) Fe, 4
C) N, 4
D) O, 6
E) S, 6

Unlock Deck
Unlock for access to all 124 flashcards in this deck.
Unlock Deck
k this deck
11
Which one of the following is a strong- field ligand?
A) H2O
B) NH3
C) CN-
D) Cl-
E) F-
A) H2O
B) NH3
C) CN-
D) Cl-
E) F-

Unlock Deck
Unlock for access to all 124 flashcards in this deck.
Unlock Deck
k this deck
12
What metal is complexed in chlorophyll?
A) manganese
B) vanadium
C) magnesium
D) chromium
E) iron
A) manganese
B) vanadium
C) magnesium
D) chromium
E) iron

Unlock Deck
Unlock for access to all 124 flashcards in this deck.
Unlock Deck
k this deck
13
Which one of the following coordination compounds would be paramagnetic?
A) [Cd(H2O)6]SO4
B) Na[CoCl6] (low spin)
C) [Cu(H2O)4]Br
D) [Zn(NH3)4]Cl2
E) K[FeCl4] (low spin)
A) [Cd(H2O)6]SO4
B) Na[CoCl6] (low spin)
C) [Cu(H2O)4]Br
D) [Zn(NH3)4]Cl2
E) K[FeCl4] (low spin)

Unlock Deck
Unlock for access to all 124 flashcards in this deck.
Unlock Deck
k this deck
14
Which of the following can form both high- and low- spin octahedral complexes?
A) Zn2+
B) Cu+
C) Cr3+
D) Cr2+
E) All of the above can form either high- or low- spin complexes.
A) Zn2+
B) Cu+
C) Cr3+
D) Cr2+
E) All of the above can form either high- or low- spin complexes.

Unlock Deck
Unlock for access to all 124 flashcards in this deck.
Unlock Deck
k this deck
15
Which of the following will display optical isomerism?
A) octahedral Co(H2NC2H4NH2)33+
B) octahedral Co(NH3)63+
C) octahedral Co(NH3)5Cl2+
D) square- planar Pt(H2NC2H4NH2)22+
E) square- planar Rh(CO)2Cl2-
A) octahedral Co(H2NC2H4NH2)33+
B) octahedral Co(NH3)63+
C) octahedral Co(NH3)5Cl2+
D) square- planar Pt(H2NC2H4NH2)22+
E) square- planar Rh(CO)2Cl2-

Unlock Deck
Unlock for access to all 124 flashcards in this deck.
Unlock Deck
k this deck
16
How many bonds can ethylenediamine form to a metal ion?
A) 6
B) 4
C) 1
D) 3
E) 2
A) 6
B) 4
C) 1
D) 3
E) 2

Unlock Deck
Unlock for access to all 124 flashcards in this deck.
Unlock Deck
k this deck
17
Based on the crystal- field strengths F- < CH2CN < NH3 < NO2- < CN- , which Co(III) complex is most likely high- spin?
A) [Co(CN)6]3-
B) [Co(NO2)6]3-
C) [Co(NH3)6]3+
D) [CoF6]3-
E) [Co(CH3CN)6]3+
A) [Co(CN)6]3-
B) [Co(NO2)6]3-
C) [Co(NH3)6]3+
D) [CoF6]3-
E) [Co(CH3CN)6]3+

Unlock Deck
Unlock for access to all 124 flashcards in this deck.
Unlock Deck
k this deck
18
Based on entropy considerations alone, which homogeneous aqueous equilibrium would be expected to lie to the right?
A) AgI2- + 2Br-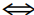 AgBr2- + 2I-
AgBr2- + 2I-
B) Fe(NH3)62+ + C20H10N42- 11ec835b_aed0_b9fc_b7a5_cf026f9e50f9_TB34225555_11Fe(NH3)2(C20H10N4) + 4NH3
C) CoCl42+ + 6H2O 11ec835b_aed0_b9fc_b7a5_cf026f9e50f9_TB34225555_11Co(H2O)62+ + 4Cl-
D) Ni(H2NC2H4NH2)32+ + 6NH3 11ec835b_aed0_b9fc_b7a5_cf026f9e50f9_TB34225555_11Ni(NH3)62+ + 3H2NC2H4NH2
E) Cu(NH3)42+ + 6H2O 11ec835b_aed0_b9fc_b7a5_cf026f9e50f9_TB34225555_11Cu( H2O)62+ + 4NH3
A) AgI2- + 2Br-
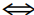 AgBr2- + 2I-
AgBr2- + 2I-B) Fe(NH3)62+ + C20H10N42- 11ec835b_aed0_b9fc_b7a5_cf026f9e50f9_TB34225555_11Fe(NH3)2(C20H10N4) + 4NH3
C) CoCl42+ + 6H2O 11ec835b_aed0_b9fc_b7a5_cf026f9e50f9_TB34225555_11Co(H2O)62+ + 4Cl-
D) Ni(H2NC2H4NH2)32+ + 6NH3 11ec835b_aed0_b9fc_b7a5_cf026f9e50f9_TB34225555_11Ni(NH3)62+ + 3H2NC2H4NH2
E) Cu(NH3)42+ + 6H2O 11ec835b_aed0_b9fc_b7a5_cf026f9e50f9_TB34225555_11Cu( H2O)62+ + 4NH3

Unlock Deck
Unlock for access to all 124 flashcards in this deck.
Unlock Deck
k this deck
19
Linkage isomerism would most likely occur when which of the following ligands is present?
A) Cl-
B) PF3
C) H2O
D) NH3
E) NCS-
A) Cl-
B) PF3
C) H2O
D) NH3
E) NCS-

Unlock Deck
Unlock for access to all 124 flashcards in this deck.
Unlock Deck
k this deck
20
What are the donor atoms in a porphine molecule?
A) F
B) O
C) S
D) N
E) Br
A) F
B) O
C) S
D) N
E) Br

Unlock Deck
Unlock for access to all 124 flashcards in this deck.
Unlock Deck
k this deck
21
Which complex below has 2 unpaired electrons?
A) square- planar [Ni(CN)4]2-
B) tetrahedral [CoCl4]2-
C) octahedral [Ni(NH3)6]2+
D) low- spin octahedral [Fe(CN)6]3-
E) tetrahedral [FeI4]2-
A) square- planar [Ni(CN)4]2-
B) tetrahedral [CoCl4]2-
C) octahedral [Ni(NH3)6]2+
D) low- spin octahedral [Fe(CN)6]3-
E) tetrahedral [FeI4]2-

Unlock Deck
Unlock for access to all 124 flashcards in this deck.
Unlock Deck
k this deck
22
Complexes containing metals with d10 electron configurations are typically _.
A) blue
B) colorless
C) yellow
D) violet
E) green
A) blue
B) colorless
C) yellow
D) violet
E) green

Unlock Deck
Unlock for access to all 124 flashcards in this deck.
Unlock Deck
k this deck
23
The chelate effect is best attributed to considerations of which type?
A) entropy
B) hydrogen bonding
C) enthalpy
D) resonance
E) hydration
A) entropy
B) hydrogen bonding
C) enthalpy
D) resonance
E) hydration

Unlock Deck
Unlock for access to all 124 flashcards in this deck.
Unlock Deck
k this deck
24
Which one of the following complexes can exhibit geometrical isomerism?
A) [Pt(NH3)2Cl2] (square planar)
B) [Zn(NH3)2Cl2] (tetrahedral)
C) [Cu(NH3)5Cl]2+ (octahedral)
D) [Cu(NH3)4]2+ (square planar)
E) All of the above can exhibit geometrical isomerism.
A) [Pt(NH3)2Cl2] (square planar)
B) [Zn(NH3)2Cl2] (tetrahedral)
C) [Cu(NH3)5Cl]2+ (octahedral)
D) [Cu(NH3)4]2+ (square planar)
E) All of the above can exhibit geometrical isomerism.

Unlock Deck
Unlock for access to all 124 flashcards in this deck.
Unlock Deck
k this deck
25
Which one of the following species is paramagnetic?
A) Ag+
B) Zn
C) Ca
D) Cu+
E) Cr3+
A) Ag+
B) Zn
C) Ca
D) Cu+
E) Cr3+

Unlock Deck
Unlock for access to all 124 flashcards in this deck.
Unlock Deck
k this deck
26
What is the purpose of adding EDTA to prepared foods?
A) to complex trace metal ions that catalyze decomposition reactions
B) to keep ions such as Ca2+ in solution so the foods look good
C) to complex iron(III) ions so they can catalyze protein decomposition on cooking
D) to aid in browning of the surface during cooking
E) to prevent dissolution of the container in the food when stored for long periods of time
A) to complex trace metal ions that catalyze decomposition reactions
B) to keep ions such as Ca2+ in solution so the foods look good
C) to complex iron(III) ions so they can catalyze protein decomposition on cooking
D) to aid in browning of the surface during cooking
E) to prevent dissolution of the container in the food when stored for long periods of time

Unlock Deck
Unlock for access to all 124 flashcards in this deck.
Unlock Deck
k this deck
27
Which of the following complexes has a coordination number of 6?
A) [Pt(NH3)2Cl2]
B) [Ag(NH3)2]+
C) [Cu(NH3)4]2+
D) [Co(en)2Cl2]+
E) None of these complexes has coordination number 6.
A) [Pt(NH3)2Cl2]
B) [Ag(NH3)2]+
C) [Cu(NH3)4]2+
D) [Co(en)2Cl2]+
E) None of these complexes has coordination number 6.

Unlock Deck
Unlock for access to all 124 flashcards in this deck.
Unlock Deck
k this deck
28
A racemic mixture is
A) a mixture of an optically active species with an optically inactive species.
B) a mixture of metal ions and ligands in equilibrium.
C) an equal mixture of both enantiomers of an optically active species.
D) a mixture of structural isomers.
E) an equal mixture of cis- and trans- isomers.
A) a mixture of an optically active species with an optically inactive species.
B) a mixture of metal ions and ligands in equilibrium.
C) an equal mixture of both enantiomers of an optically active species.
D) a mixture of structural isomers.
E) an equal mixture of cis- and trans- isomers.

Unlock Deck
Unlock for access to all 124 flashcards in this deck.
Unlock Deck
k this deck
29
Changes in the coordination sphere of a complex compound may lead to changes in .
A) chemical properties
B) color
C) physical properties
D) stability
E) all of the above
A) chemical properties
B) color
C) physical properties
D) stability
E) all of the above

Unlock Deck
Unlock for access to all 124 flashcards in this deck.
Unlock Deck
k this deck
30
Which one of the following substances has three unpaired d electrons?
A) [Ag(NH3)2]+
B) [Zn(NH3)4]2+
C) [Cr(CN)6]3-
D) [V(H2O)6]4+
E) [Cu(NH3)4]2+
A) [Ag(NH3)2]+
B) [Zn(NH3)4]2+
C) [Cr(CN)6]3-
D) [V(H2O)6]4+
E) [Cu(NH3)4]2+

Unlock Deck
Unlock for access to all 124 flashcards in this deck.
Unlock Deck
k this deck
31
Which one of the following ions cannot form both a high spin and a low spin octahedral complex ion?
A) Cr2+
B) Fe3+
C) Co2+
D) Cr3+
E) Mn3+
A) Cr2+
B) Fe3+
C) Co2+
D) Cr3+
E) Mn3+

Unlock Deck
Unlock for access to all 124 flashcards in this deck.
Unlock Deck
k this deck
32
How many ligands are there in the coordination sphere of [Co(en)2Cl2]+?
A) 4
B) 0
C) 1
D) 3
E) 6
A) 4
B) 0
C) 1
D) 3
E) 6

Unlock Deck
Unlock for access to all 124 flashcards in this deck.
Unlock Deck
k this deck
33
Does either or both cis- or trans- [Mn(en)2Br2] have optical isomers?
A) cis only
B) trans only
C) both cis and trans
D) neither cis nor trans
E) [Mn(en)2Br2] does not exhibit cis- trans- isomerism.
A) cis only
B) trans only
C) both cis and trans
D) neither cis nor trans
E) [Mn(en)2Br2] does not exhibit cis- trans- isomerism.

Unlock Deck
Unlock for access to all 124 flashcards in this deck.
Unlock Deck
k this deck
34
Which one of the following is the correct formula for potassium diaquatetrachloromolybdate (III)?
A) K3[Mo(H2O)2Cl4]
B) K2[Mo(H2O)2Cl4]
C) K[Mo(H2O)2Cl4]
D) K[Mo(H2O)2Cl2]Cl2
E) Mo[K(H2O)2]Cl4
A) K3[Mo(H2O)2Cl4]
B) K2[Mo(H2O)2Cl4]
C) K[Mo(H2O)2Cl4]
D) K[Mo(H2O)2Cl2]Cl2
E) Mo[K(H2O)2]Cl4

Unlock Deck
Unlock for access to all 124 flashcards in this deck.
Unlock Deck
k this deck
35
What are the respective central- metal oxidation state, coordination number, and overall charge on the complex ion in 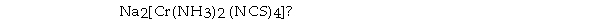
A) +1; 6; - 2
B) +2; 6; - 2
C) +2; 4; - 1
D) +3; 6; - 1
E) +3; 6; +1
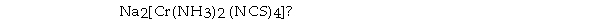
A) +1; 6; - 2
B) +2; 6; - 2
C) +2; 4; - 1
D) +3; 6; - 1
E) +3; 6; +1

Unlock Deck
Unlock for access to all 124 flashcards in this deck.
Unlock Deck
k this deck
36
Which of the following cannot form both high- and low- spin octahedral complexes?
A) Mn2+
B) Co3+
C) V2+
D) Cr2+
E) All of the above can form both high- and low- spin complexes.
A) Mn2+
B) Co3+
C) V2+
D) Cr2+
E) All of the above can form both high- and low- spin complexes.

Unlock Deck
Unlock for access to all 124 flashcards in this deck.
Unlock Deck
k this deck
37
Formation of a complex species of Mn+ metal ion with ligands often .
A) "masks" original chemical properties of both the Mn+ ion and the ligands
B) may cause changes in the ease with which Mn+ is reduced or oxidized
C) alters original physical properties of Mn+
D) reduces availability of the free Mn+ ions in solution
E) all of the above
A) "masks" original chemical properties of both the Mn+ ion and the ligands
B) may cause changes in the ease with which Mn+ is reduced or oxidized
C) alters original physical properties of Mn+
D) reduces availability of the free Mn+ ions in solution
E) all of the above

Unlock Deck
Unlock for access to all 124 flashcards in this deck.
Unlock Deck
k this deck
38
Based on the crystal- field strengths Cl- < F- < H2O < NH3 < H2NC2H4NH2, which octahedral Ti(III) complex below has its d- d electronic transition at shortest wavelength?
A) [TiF6]3-
B) [TiCl6]3-
C) [Ti(NH3)6]3+
D) [Ti(H2NC2H4NH2)3]3+
E) [Ti( H2O)6]3+
A) [TiF6]3-
B) [TiCl6]3-
C) [Ti(NH3)6]3+
D) [Ti(H2NC2H4NH2)3]3+
E) [Ti( H2O)6]3+

Unlock Deck
Unlock for access to all 124 flashcards in this deck.
Unlock Deck
k this deck
39
Which one of the following complex ions will be paramagnetic?
A) [Zn(H2O)4]2+
B) [Co(H2O)6]3+ (low spin)
C) [Fe(H2O)6]3+ (low spin)
D) [Fe(H2O)6]2+ (low spin)
E) [Zn(NH3)4]2+
A) [Zn(H2O)4]2+
B) [Co(H2O)6]3+ (low spin)
C) [Fe(H2O)6]3+ (low spin)
D) [Fe(H2O)6]2+ (low spin)
E) [Zn(NH3)4]2+

Unlock Deck
Unlock for access to all 124 flashcards in this deck.
Unlock Deck
k this deck
40
In the compound, CaNa[Fe(CN)6], what ligands are in the coordination sphere?
A) CN-
B) H2O
C) Na+
D) Ca2+
E) none of the above
A) CN-
B) H2O
C) Na+
D) Ca2+
E) none of the above

Unlock Deck
Unlock for access to all 124 flashcards in this deck.
Unlock Deck
k this deck
41
What is the coordination number of the iron atom in CaNa[Fe(CN)6]?

Unlock Deck
Unlock for access to all 124 flashcards in this deck.
Unlock Deck
k this deck
42
The most common coordination numbers are _ .

Unlock Deck
Unlock for access to all 124 flashcards in this deck.
Unlock Deck
k this deck
43
Based on electron configuration, which is most likely colorless?
A) [Ni(NH3)6]2+
B) [Cd(NH3)4]2+
C) [Cu(NH3)4]2+
D) [Co(NH3)6]2+
E) [Cr(NH3)5Cl]2+
A) [Ni(NH3)6]2+
B) [Cd(NH3)4]2+
C) [Cu(NH3)4]2+
D) [Co(NH3)6]2+
E) [Cr(NH3)5Cl]2+

Unlock Deck
Unlock for access to all 124 flashcards in this deck.
Unlock Deck
k this deck
44
Using the following abbreviated spectrochemical series, determine which complex ion is most likely to absorb light in the red region of the visible spectrum. ![<strong>Using the following abbreviated spectrochemical series, determine which complex ion is most likely to absorb light in the red region of the visible spectrum. </strong> A) [Cu(H<sub>2</sub>O)<sub>4</sub>]<sup>2</sup><sup>+</sup> B) [CuCl<sub>4</sub>]<sup>2</sup><sup>-</sup> C) [Cu(CN)<sub>4</sub>]<sup>2</sup><sup>-</sup> D) [Cu(NH<sub>3</sub>)<sub>4</sub>]<sup>2</sup><sup>+</sup> E) not enough information given to determine](https://storage.examlex.com/TB1819/11ead62c_1ef0_bd41_ac95_efb1e972b370_TB1819_00.jpg)
A) [Cu(H2O)4]2+
B) [CuCl4]2-
C) [Cu(CN)4]2-
D) [Cu(NH3)4]2+
E) not enough information given to determine
![<strong>Using the following abbreviated spectrochemical series, determine which complex ion is most likely to absorb light in the red region of the visible spectrum. </strong> A) [Cu(H<sub>2</sub>O)<sub>4</sub>]<sup>2</sup><sup>+</sup> B) [CuCl<sub>4</sub>]<sup>2</sup><sup>-</sup> C) [Cu(CN)<sub>4</sub>]<sup>2</sup><sup>-</sup> D) [Cu(NH<sub>3</sub>)<sub>4</sub>]<sup>2</sup><sup>+</sup> E) not enough information given to determine](https://storage.examlex.com/TB1819/11ead62c_1ef0_bd41_ac95_efb1e972b370_TB1819_00.jpg)
A) [Cu(H2O)4]2+
B) [CuCl4]2-
C) [Cu(CN)4]2-
D) [Cu(NH3)4]2+
E) not enough information given to determine

Unlock Deck
Unlock for access to all 124 flashcards in this deck.
Unlock Deck
k this deck
45
Which of the following is not a chelating agent?
A) oxalate anion
B) ethylenediamine
C) EDTA
D) chloride anion
E) porphine
A) oxalate anion
B) ethylenediamine
C) EDTA
D) chloride anion
E) porphine

Unlock Deck
Unlock for access to all 124 flashcards in this deck.
Unlock Deck
k this deck
46
Two compounds have the same formula and contain an SCN- ligand. In one compound the SCN- ligand is bonded to the metal atom via the N atom and in the other it is bonded via the S atom. These two compounds are examples of _ _ isomers.

Unlock Deck
Unlock for access to all 124 flashcards in this deck.
Unlock Deck
k this deck
47
Which one of the following complexes would most likely have tetrahedral geometry?
A) [Fe(CN)6]3
B) [Pt(NH3)2Cl2]
C) [Cr(NH3)6]3+
D) [Co(H2O)6]2+
E) [NiCl4]2-
A) [Fe(CN)6]3
B) [Pt(NH3)2Cl2]
C) [Cr(NH3)6]3+
D) [Co(H2O)6]2+
E) [NiCl4]2-

Unlock Deck
Unlock for access to all 124 flashcards in this deck.
Unlock Deck
k this deck
48
Complexes containing metals with which one of the following electron configurations are usually colorless?
A) d1
B) d10
C) d8
D) d2
E) d5
A) d1
B) d10
C) d8
D) d2
E) d5

Unlock Deck
Unlock for access to all 124 flashcards in this deck.
Unlock Deck
k this deck
49
Complexes containing metals with d0 electron configurations are typically colorless because
A) d electrons must be emitted by the complex in order for it to appear colored.
B) the empty d orbitals absorb all of the visible wavelengths.
C) there is no d electron that can be promoted via the absorption of visible light.
D) a complex must be charged to be colored.
E) there are no d electrons to form bonds to ligands.
A) d electrons must be emitted by the complex in order for it to appear colored.
B) the empty d orbitals absorb all of the visible wavelengths.
C) there is no d electron that can be promoted via the absorption of visible light.
D) a complex must be charged to be colored.
E) there are no d electrons to form bonds to ligands.

Unlock Deck
Unlock for access to all 124 flashcards in this deck.
Unlock Deck
k this deck
50
Coordination sphere isomers
A) have the same formula and coordination number.
B) have the same formula but different coordination numbers.
C) are the same as resonance structures.
D) have different formulas but the same coordination number.
E) have different formulas and different coordination numbers.
A) have the same formula and coordination number.
B) have the same formula but different coordination numbers.
C) are the same as resonance structures.
D) have different formulas but the same coordination number.
E) have different formulas and different coordination numbers.

Unlock Deck
Unlock for access to all 124 flashcards in this deck.
Unlock Deck
k this deck
51
List three of the seven transition metals required for human life.

Unlock Deck
Unlock for access to all 124 flashcards in this deck.
Unlock Deck
k this deck
52
The cis geometric isomer of what compound is a powerful anti cancer agent ?

Unlock Deck
Unlock for access to all 124 flashcards in this deck.
Unlock Deck
k this deck
53
Werner's theory of primary and secondary valences for transition metal complexes has given us the concepts of and .

Unlock Deck
Unlock for access to all 124 flashcards in this deck.
Unlock Deck
k this deck
54
Name Na[Ru(H2O)2(C2O4)2]

Unlock Deck
Unlock for access to all 124 flashcards in this deck.
Unlock Deck
k this deck
55
Non superimposable isomers are isomers.

Unlock Deck
Unlock for access to all 124 flashcards in this deck.
Unlock Deck
k this deck
56
The transport of iron into bacteria is facilitated by the formation of the complex
.
.

Unlock Deck
Unlock for access to all 124 flashcards in this deck.
Unlock Deck
k this deck
57
Which of the following is a polydentate ligand?
A) oxalate ion
B) water
C) hydroxide ion
D) ammonia
E) chloride ion
A) oxalate ion
B) water
C) hydroxide ion
D) ammonia
E) chloride ion

Unlock Deck
Unlock for access to all 124 flashcards in this deck.
Unlock Deck
k this deck
58
A compound that can occupy two coordination sites is a (an) .

Unlock Deck
Unlock for access to all 124 flashcards in this deck.
Unlock Deck
k this deck
59
What is the oxidation state of the iron atom in CaNa[Fe(CN)6]?

Unlock Deck
Unlock for access to all 124 flashcards in this deck.
Unlock Deck
k this deck
60
Transition metal ions with empty valence orbitals act as .

Unlock Deck
Unlock for access to all 124 flashcards in this deck.
Unlock Deck
k this deck
61
Trans- [Fe(H2O)2Cl4]2- must be .
A) trigonal bipyramidal
B) tetrahedral
C) square planar
D) octahedral
E) linear
A) trigonal bipyramidal
B) tetrahedral
C) square planar
D) octahedral
E) linear

Unlock Deck
Unlock for access to all 124 flashcards in this deck.
Unlock Deck
k this deck
62
What is the oxidation number of cobalt in [Co(NH3)4F2] ?
A) +1
B) +3
C) +6
D) +2
E) - 3
A) +1
B) +3
C) +6
D) +2
E) - 3

Unlock Deck
Unlock for access to all 124 flashcards in this deck.
Unlock Deck
k this deck
63
Define the chelate effect.

Unlock Deck
Unlock for access to all 124 flashcards in this deck.
Unlock Deck
k this deck
64
What is the coordination number of cobalt in [Co(NH3)5Cl](NO3)2 ?
A) 4
B) 2
C) 12
D) 6
E) 8
A) 4
B) 2
C) 12
D) 6
E) 8

Unlock Deck
Unlock for access to all 124 flashcards in this deck.
Unlock Deck
k this deck
65
A large difference in formation constant (Kf ) is a poly- versus monodentate ligand is called .

Unlock Deck
Unlock for access to all 124 flashcards in this deck.
Unlock Deck
k this deck
66
Six- coordinate complexes generally have geometry.

Unlock Deck
Unlock for access to all 124 flashcards in this deck.
Unlock Deck
k this deck
67
The "dentation " of a ligand is defined by _ _.
A) how many "dents" or "deceptions" there are in the coordination sphere of a complex species it forms
B) how many electron donor atoms it utilizes to form coordinate bonds to the central metal ion
C) how many metal ions it can sequester from solution
D) the total number of lone pairs of electrons it possesses
E) none of the above
A) how many "dents" or "deceptions" there are in the coordination sphere of a complex species it forms
B) how many electron donor atoms it utilizes to form coordinate bonds to the central metal ion
C) how many metal ions it can sequester from solution
D) the total number of lone pairs of electrons it possesses
E) none of the above

Unlock Deck
Unlock for access to all 124 flashcards in this deck.
Unlock Deck
k this deck
68
The coordination number of platinum in complexes is always _ .
A) 2
B) 4
C) 5
D) 3
E) 6
A) 2
B) 4
C) 5
D) 3
E) 6

Unlock Deck
Unlock for access to all 124 flashcards in this deck.
Unlock Deck
k this deck
69
How many d electrons are in the cobalt ion of K3[Co(CN)6] ?
A) 7
B) 5
C) 3
D) 4
E) 6
A) 7
B) 5
C) 3
D) 4
E) 6

Unlock Deck
Unlock for access to all 124 flashcards in this deck.
Unlock Deck
k this deck
70
The correct name for Na3[CoF6] is .
A) trisodium hexakisfluorocobalt(III)
B) sodium hexafluorocobaltate(IV)
C) trisodium hexakisfluorocobalt(II)
D) trisodium hexakisfluorocobalt(IV)
E) sodium hexafluorocobaltate(III)
A) trisodium hexakisfluorocobalt(III)
B) sodium hexafluorocobaltate(IV)
C) trisodium hexakisfluorocobalt(II)
D) trisodium hexakisfluorocobalt(IV)
E) sodium hexafluorocobaltate(III)

Unlock Deck
Unlock for access to all 124 flashcards in this deck.
Unlock Deck
k this deck
71
What is the oxidation state of chromium in [Cr(H2O)4Cl2]+ ?
A) 0
B) - 3
C) - 2
D) +3
E) +2
A) 0
B) - 3
C) - 2
D) +3
E) +2

Unlock Deck
Unlock for access to all 124 flashcards in this deck.
Unlock Deck
k this deck
72
How can high- spin and low- spin transition metal complexes be distinguished from each other?

Unlock Deck
Unlock for access to all 124 flashcards in this deck.
Unlock Deck
k this deck
73
Linkage isomerism can only occur .
A) with tetrahedral complexes
B) with ligands that have more than one possible donor atom
C) with cobalt complexes
D) with coordination number 6
E) in cis- isomers of octahedral complexes
A) with tetrahedral complexes
B) with ligands that have more than one possible donor atom
C) with cobalt complexes
D) with coordination number 6
E) in cis- isomers of octahedral complexes

Unlock Deck
Unlock for access to all 124 flashcards in this deck.
Unlock Deck
k this deck
74
The complex [Zn(NH3)2Cl2]2+ does not exhibit cis- trans- isomerism. The geometry of this complex must be .
A) octahedral
B) tetrahedral
C) trigonal bipyramidal
D) square planar
E) either tetrahedral or square planar
A) octahedral
B) tetrahedral
C) trigonal bipyramidal
D) square planar
E) either tetrahedral or square planar

Unlock Deck
Unlock for access to all 124 flashcards in this deck.
Unlock Deck
k this deck
75
What is the oxidation number of the central metal in [Mo(H2O)5NO3] Cl2

Unlock Deck
Unlock for access to all 124 flashcards in this deck.
Unlock Deck
k this deck
76
EDTA is - dantate ligand.
A) tetra
B) bi
C) tri
D) mono
E) hexa
A) tetra
B) bi
C) tri
D) mono
E) hexa

Unlock Deck
Unlock for access to all 124 flashcards in this deck.
Unlock Deck
k this deck
77
What transition metal is responsible for the color of topaz _?
A) manganese
B) iron
C) cobalt
D) gold
E) chromium
A) manganese
B) iron
C) cobalt
D) gold
E) chromium

Unlock Deck
Unlock for access to all 124 flashcards in this deck.
Unlock Deck
k this deck
78
The porphyrin compound that contrains Mg(II) is called .

Unlock Deck
Unlock for access to all 124 flashcards in this deck.
Unlock Deck
k this deck
79
What transition metal is responsible for the color of ruby ?
A) titanium
B) chromium
C) gold
D) manganese
E) cobalt
A) titanium
B) chromium
C) gold
D) manganese
E) cobalt

Unlock Deck
Unlock for access to all 124 flashcards in this deck.
Unlock Deck
k this deck
80
The coordination number for [Zn(H2O)3Cl]Cl is _ .
A) 5
B) 2
C) 1
D) 4
E) 6
A) 5
B) 2
C) 1
D) 4
E) 6

Unlock Deck
Unlock for access to all 124 flashcards in this deck.
Unlock Deck
k this deck


