Deck 22: Chemistry of the Nonmetals
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/176
Play
Full screen (f)
Deck 22: Chemistry of the Nonmetals
1
Consider the following xenon compounds:
(i) XeF2
(ii) XeF4
(iii) XeO4
(iv) XeOF4
(v) XeO3
Which of the compounds is(are) polar?
A) (i) only
B) (iii) and (iv)
C) (iv) and (v)
D) (ii) and (iii)
E) (iv) only
(i) XeF2
(ii) XeF4
(iii) XeO4
(iv) XeOF4
(v) XeO3
Which of the compounds is(are) polar?
A) (i) only
B) (iii) and (iv)
C) (iv) and (v)
D) (ii) and (iii)
E) (iv) only
(iv) and (v)
2
Of the following compounds, which is the most stable?
A) XeO3
B) XeF2
C) XeO2F2
D) XeF6
E) XeOF4
A) XeO3
B) XeF2
C) XeO2F2
D) XeF6
E) XeOF4
XeF6
3
Addition of B2O3 to soda- lime glass
A) results in a glass with a lower melting point.
B) results in opaque glass.
C) imparts a greater ability to withstand temperature change.
D) imparts a deep blue color.
E) results in a denser glass with a higher refractive index.
A) results in a glass with a lower melting point.
B) results in opaque glass.
C) imparts a greater ability to withstand temperature change.
D) imparts a deep blue color.
E) results in a denser glass with a higher refractive index.
imparts a greater ability to withstand temperature change.
4
Which one of the following is true concerning borax?
A) It is the hydrated sodium salt of tetraboric acid.
B) It is commonly used in cleaning products.
C) Its aqueous solutions are alkaline.
D) It is found in dry lake deposits in California.
E) All of the above are true.
A) It is the hydrated sodium salt of tetraboric acid.
B) It is commonly used in cleaning products.
C) Its aqueous solutions are alkaline.
D) It is found in dry lake deposits in California.
E) All of the above are true.

Unlock Deck
Unlock for access to all 176 flashcards in this deck.
Unlock Deck
k this deck
5
Which of the following statements is false?
A) Ozone decomposes to O2 and O.
B) Ozone is an allotrope of oxygen.
C) Ozone is a better reducing agent than O2 (g).
D) Ozone oxidizes all of the common metals except gold and platinum.
E) Ozone is produced by passing electricity through dry O2 (g).
A) Ozone decomposes to O2 and O.
B) Ozone is an allotrope of oxygen.
C) Ozone is a better reducing agent than O2 (g).
D) Ozone oxidizes all of the common metals except gold and platinum.
E) Ozone is produced by passing electricity through dry O2 (g).

Unlock Deck
Unlock for access to all 176 flashcards in this deck.
Unlock Deck
k this deck
6
How many oxygen atoms are bonded to each silicon atom in SiO2?
A) 1
B) 2
C) 3
D) 4
E) none
A) 1
B) 2
C) 3
D) 4
E) none

Unlock Deck
Unlock for access to all 176 flashcards in this deck.
Unlock Deck
k this deck
7
Nearly all commercial oxygen is obtained .
A) by thermal decomposition of potassium chlorate
B) by thermal cracking of petroleum
C) from air
D) by electrolysis of water
E) as a byproduct of the preparation of aluminum in the Hall process
A) by thermal decomposition of potassium chlorate
B) by thermal cracking of petroleum
C) from air
D) by electrolysis of water
E) as a byproduct of the preparation of aluminum in the Hall process

Unlock Deck
Unlock for access to all 176 flashcards in this deck.
Unlock Deck
k this deck
8
Which elemental halogen(s) can be used to prepare I2 from NaI?
A) Br2 only
B) F2 only
C) Cl2 only
D) both Cl2 and Br2, but not F2
E) F2, Cl2, and Br2
A) Br2 only
B) F2 only
C) Cl2 only
D) both Cl2 and Br2, but not F2
E) F2, Cl2, and Br2

Unlock Deck
Unlock for access to all 176 flashcards in this deck.
Unlock Deck
k this deck
9
Which of the following would produce the most strongly basic aqueous solution?
A) CO2
B) CO
C) HCO3-
D) CO32-
E) NaHCO3
A) CO2
B) CO
C) HCO3-
D) CO32-
E) NaHCO3

Unlock Deck
Unlock for access to all 176 flashcards in this deck.
Unlock Deck
k this deck
10
The oxidation numbers of sulfur in the sulfate ion, sulfite ion, sulfur trioxide, and hydrogen sulfide are _, , _,and ,respectively.
A) +6, +4, +6, - 2
B) +4, +6, +4, - 2
C) - 2, +6, - 2, 0
D) +4, - 2, +4, +6
E) +6, +2, +4, +6
A) +6, +4, +6, - 2
B) +4, +6, +4, - 2
C) - 2, +6, - 2, 0
D) +4, - 2, +4, +6
E) +6, +2, +4, +6

Unlock Deck
Unlock for access to all 176 flashcards in this deck.
Unlock Deck
k this deck
11
What is the major commercial source of elemental sulfur?
A) sulfate minerals
B) sulfide minerals
C) coal and petroleum
D) underground deposits of elemental sulfur
E) seawater
A) sulfate minerals
B) sulfide minerals
C) coal and petroleum
D) underground deposits of elemental sulfur
E) seawater

Unlock Deck
Unlock for access to all 176 flashcards in this deck.
Unlock Deck
k this deck
12
Which of the following equations correctly represents the combustion of hydrazine?
A) N2H4 (l) + 2O2 (g) → 2NO2 (g) + 2 H2 (g)
B) N2H4 (l) + O2 (g) → N2 (g) + 2 H2 (g) + O2 (g)
C) N2H4 (l) + O2 (g) → N2 (g) + 2 H2O (g)
D) N2H4 (l) + O2 (g) → NH3 (g) + HNO2 (g)
E) N2H4 (l) + O2 (g) → 2H2NO (g)
A) N2H4 (l) + 2O2 (g) → 2NO2 (g) + 2 H2 (g)
B) N2H4 (l) + O2 (g) → N2 (g) + 2 H2 (g) + O2 (g)
C) N2H4 (l) + O2 (g) → N2 (g) + 2 H2O (g)
D) N2H4 (l) + O2 (g) → NH3 (g) + HNO2 (g)
E) N2H4 (l) + O2 (g) → 2H2NO (g)

Unlock Deck
Unlock for access to all 176 flashcards in this deck.
Unlock Deck
k this deck
13
What is the function of the carbon fibers in a composite?
A) to provide ultraviolet protection
B) to provide resistance to oxidation
C) to provide a structure to help the epoxy resin solidify in the desired shape
D) to transmit loads evenly in all directions
E) to "spread out" the epoxy so that it remains more flexible
A) to provide ultraviolet protection
B) to provide resistance to oxidation
C) to provide a structure to help the epoxy resin solidify in the desired shape
D) to transmit loads evenly in all directions
E) to "spread out" the epoxy so that it remains more flexible

Unlock Deck
Unlock for access to all 176 flashcards in this deck.
Unlock Deck
k this deck
14
Silicones are
A) flat sheets of silicon atoms.
B) three- dimensional covalent networks of silicon atoms.
C) three- dimensional covalent networks of SiO4 tetrahedra.
D) flat sheets of silicon and hydrogen atoms.
E) chains of alternating silicon and oxygen atoms with attached organic groups.
A) flat sheets of silicon atoms.
B) three- dimensional covalent networks of silicon atoms.
C) three- dimensional covalent networks of SiO4 tetrahedra.
D) flat sheets of silicon and hydrogen atoms.
E) chains of alternating silicon and oxygen atoms with attached organic groups.

Unlock Deck
Unlock for access to all 176 flashcards in this deck.
Unlock Deck
k this deck
15
Which one of the following is false concerning pure hydrazine?
A) It is used as a rocket fuel.
B) It can be made by reaction of hypochlorite and ammonia.
C) It is an oily, colorless liquid.
D) It is a clear, red liquid that is highly viscous.
E) Hydrazine is quite poisonous.
A) It is used as a rocket fuel.
B) It can be made by reaction of hypochlorite and ammonia.
C) It is an oily, colorless liquid.
D) It is a clear, red liquid that is highly viscous.
E) Hydrazine is quite poisonous.

Unlock Deck
Unlock for access to all 176 flashcards in this deck.
Unlock Deck
k this deck
16
Which one of the following compounds is peroxide?
A) H2O
B) both Na2O2 and CsO2
C) Na2O2
D) CsO2
E) Li2O
A) H2O
B) both Na2O2 and CsO2
C) Na2O2
D) CsO2
E) Li2O

Unlock Deck
Unlock for access to all 176 flashcards in this deck.
Unlock Deck
k this deck
17
The least electronegative of the elements below is .
A) I
B) H
C) Br
D) F
E) Cl
A) I
B) H
C) Br
D) F
E) Cl

Unlock Deck
Unlock for access to all 176 flashcards in this deck.
Unlock Deck
k this deck
18
Carbon dioxide is produced
A) by fermentation of sugar during the production of ethanol.
B) by combustion of carbon- containing substances in an excess of oxygen.
C) when carbonates are heated.
D) by all of these processes.
E) in blast furnaces when metal oxides are reduced with CO.
A) by fermentation of sugar during the production of ethanol.
B) by combustion of carbon- containing substances in an excess of oxygen.
C) when carbonates are heated.
D) by all of these processes.
E) in blast furnaces when metal oxides are reduced with CO.

Unlock Deck
Unlock for access to all 176 flashcards in this deck.
Unlock Deck
k this deck
19
Which group 6A element is not commonly found in a positive oxidation state?
A) tellurium
B) oxygen
C) selenium
D) polonium
E) sulfur
A) tellurium
B) oxygen
C) selenium
D) polonium
E) sulfur

Unlock Deck
Unlock for access to all 176 flashcards in this deck.
Unlock Deck
k this deck
20
Interhalogen compounds _.
A) are very active fluorinating agents
B) that contain fluorine are powerful oxidizing agents
C) contain halogens in both positive and negative oxidation states
D) are exceedingly reactive
E) all of the above
A) are very active fluorinating agents
B) that contain fluorine are powerful oxidizing agents
C) contain halogens in both positive and negative oxidation states
D) are exceedingly reactive
E) all of the above

Unlock Deck
Unlock for access to all 176 flashcards in this deck.
Unlock Deck
k this deck
21
Which one of the following is false concerning tritium?
A) The atomic number of tritium is 1.
B) It has the same chemical properties as protium but reacts more slowly.
C) It is radioactive, emitting alpha particles with a half- life of 12.3 yr.
D) It can be produced by neutron bombardment of lithium- 6.
E) It is formed continuously in the upper atmosphere.
A) The atomic number of tritium is 1.
B) It has the same chemical properties as protium but reacts more slowly.
C) It is radioactive, emitting alpha particles with a half- life of 12.3 yr.
D) It can be produced by neutron bombardment of lithium- 6.
E) It is formed continuously in the upper atmosphere.

Unlock Deck
Unlock for access to all 176 flashcards in this deck.
Unlock Deck
k this deck
22
Which of the following is the nitride ion?
A) N-
B) N3-
C) N3-
D) NO2-
E) NO3-
A) N-
B) N3-
C) N3-
D) NO2-
E) NO3-

Unlock Deck
Unlock for access to all 176 flashcards in this deck.
Unlock Deck
k this deck
23
Which form of elemental sulfur is the most stable at room temperature?
A) triclinic
B) hexagonal
C) rhombic sulfur
D) tetraclinic
E) monoclinic
A) triclinic
B) hexagonal
C) rhombic sulfur
D) tetraclinic
E) monoclinic

Unlock Deck
Unlock for access to all 176 flashcards in this deck.
Unlock Deck
k this deck
24
The prefix "thio" denotes
A) a sulfur-sulfur double bond.
B) replacement of an oxygen atom by a sulfur atom.
C) an allotropic form of sulfur.
D) sulfur in a negative oxidation state.
E) a sulfur-oxygen double bond.
A) a sulfur-sulfur double bond.
B) replacement of an oxygen atom by a sulfur atom.
C) an allotropic form of sulfur.
D) sulfur in a negative oxidation state.
E) a sulfur-oxygen double bond.

Unlock Deck
Unlock for access to all 176 flashcards in this deck.
Unlock Deck
k this deck
25
Which of the following would produce the most strongly acidic aqueous solution?
A) CaCO3
B) CO32-
C) CO2
D) CO
E) HCO3-
A) CaCO3
B) CO32-
C) CO2
D) CO
E) HCO3-

Unlock Deck
Unlock for access to all 176 flashcards in this deck.
Unlock Deck
k this deck
26
In the reaction of phosphorus with chlorine to form a phosphorus chloride, whether PCl3 or PCl5 forms depends on
A) whether the chlorine used is molecular or atomic.
B) the amount of moisture present.
C) whether the reaction is carried out in the gas phase or in solution.
D) which allotropic form of phosphorus is used.
E) the amount of chlorine present.
A) whether the chlorine used is molecular or atomic.
B) the amount of moisture present.
C) whether the reaction is carried out in the gas phase or in solution.
D) which allotropic form of phosphorus is used.
E) the amount of chlorine present.

Unlock Deck
Unlock for access to all 176 flashcards in this deck.
Unlock Deck
k this deck
27
The reaction between nitrogen dioxide and water is a
A) neutralization
B) decomposition
C) combustion
D) disproportionation
E) replacement
A) neutralization
B) decomposition
C) combustion
D) disproportionation
E) replacement

Unlock Deck
Unlock for access to all 176 flashcards in this deck.
Unlock Deck
k this deck
28
Isotopes of hydrogen
A) have different atomic numbers and different mass numbers.
B) have different atomic numbers and the same mass number.
C) are exactly alike.
D) have the same atomic number and the same mass number.
E) have the same atomic number and different mass numbers.
A) have different atomic numbers and different mass numbers.
B) have different atomic numbers and the same mass number.
C) are exactly alike.
D) have the same atomic number and the same mass number.
E) have the same atomic number and different mass numbers.

Unlock Deck
Unlock for access to all 176 flashcards in this deck.
Unlock Deck
k this deck
29
Which compound would produce a basic aqueous solution?
A) HCl
B) CH3OH
C) H2S
D) MgH2
E) HI
A) HCl
B) CH3OH
C) H2S
D) MgH2
E) HI

Unlock Deck
Unlock for access to all 176 flashcards in this deck.
Unlock Deck
k this deck
30
What method is used to produce the most hydrogen gas in the United States?
A) reaction of metallic sodium with water
B) reaction of coke (carbon) with steam
C) reaction of zinc with acid
D) reaction of methane with steam
E) electrolysis of water
A) reaction of metallic sodium with water
B) reaction of coke (carbon) with steam
C) reaction of zinc with acid
D) reaction of methane with steam
E) electrolysis of water

Unlock Deck
Unlock for access to all 176 flashcards in this deck.
Unlock Deck
k this deck
31
Which of the following is not an allotropic form of carbon?
A) carbide
B) buckminsterfullerene
C) graphite
D) diamond
E) All of the above are allotropic forms of carbon.
A) carbide
B) buckminsterfullerene
C) graphite
D) diamond
E) All of the above are allotropic forms of carbon.

Unlock Deck
Unlock for access to all 176 flashcards in this deck.
Unlock Deck
k this deck
32
What is the F-Xe-F bond angle in XeF2?
A) 109°
B) 60°
C) 90°
D) 120°
E) 180°
A) 109°
B) 60°
C) 90°
D) 120°
E) 180°

Unlock Deck
Unlock for access to all 176 flashcards in this deck.
Unlock Deck
k this deck
33
Which equation correctly represents what happens when NO2 dissolves in water?
A) NO2 (g) + H2O (l) → H2O2 (aq) + NO (g)
B) 2NO2 (g) + H2O (l) → 2H+ (aq) + NO42- (aq) + NO (g)
C) NO2 (g) + H2O (l) → 2H+ (aq) + NO3- (aq)
D) 2NO2 (g) + 2H2O (l) → 2 HNO2 (aq) + O2 (g) + H2 (g)
E) 3NO2 (g) + H2O (l) → 2H+ (aq) + 2NO3- (aq) + NO (g)
A) NO2 (g) + H2O (l) → H2O2 (aq) + NO (g)
B) 2NO2 (g) + H2O (l) → 2H+ (aq) + NO42- (aq) + NO (g)
C) NO2 (g) + H2O (l) → 2H+ (aq) + NO3- (aq)
D) 2NO2 (g) + 2H2O (l) → 2 HNO2 (aq) + O2 (g) + H2 (g)
E) 3NO2 (g) + H2O (l) → 2H+ (aq) + 2NO3- (aq) + NO (g)

Unlock Deck
Unlock for access to all 176 flashcards in this deck.
Unlock Deck
k this deck
34
The arrangement of oxygen atoms around a silicon atom in SiO44- is
A) octahedral
B) trigonal pyramidal
C) linear
D) tetrahedral
E) square planar
A) octahedral
B) trigonal pyramidal
C) linear
D) tetrahedral
E) square planar

Unlock Deck
Unlock for access to all 176 flashcards in this deck.
Unlock Deck
k this deck
35
Which one of the following is sodium thiosulfate?
A) Na2S2O3
B) Na2S
C) Na2SO3
D) Na2S4O6
E) Na2SO4
A) Na2S2O3
B) Na2S
C) Na2SO3
D) Na2S4O6
E) Na2SO4

Unlock Deck
Unlock for access to all 176 flashcards in this deck.
Unlock Deck
k this deck
36
Of the following, which is most likely to form interstitial carbides?
A) boron and silicon
B) transition metals
C) active metals
D) alkali metals
E) alkaline earth metals
A) boron and silicon
B) transition metals
C) active metals
D) alkali metals
E) alkaline earth metals

Unlock Deck
Unlock for access to all 176 flashcards in this deck.
Unlock Deck
k this deck
37
Sodium borohydride, NaBH4, is a strong reducing agent because .
A) Na+ is easily reduced to Na (s).
B) hydrogen can be easily oxidized from - 1 oxidation state to +1
C) boron easily changes its oxidation number from +3 to - 3
D) boron is readily oxidized from - 3 oxidation state to +3
E) hydrogen is easily reduced from +1 oxidation state to 0
A) Na+ is easily reduced to Na (s).
B) hydrogen can be easily oxidized from - 1 oxidation state to +1
C) boron easily changes its oxidation number from +3 to - 3
D) boron is readily oxidized from - 3 oxidation state to +3
E) hydrogen is easily reduced from +1 oxidation state to 0

Unlock Deck
Unlock for access to all 176 flashcards in this deck.
Unlock Deck
k this deck
38
Which noble gas is known to form a variety of binary compounds?
A) He
B) Ne
C) Xe
D) Ar
E) Kr
A) He
B) Ne
C) Xe
D) Ar
E) Kr

Unlock Deck
Unlock for access to all 176 flashcards in this deck.
Unlock Deck
k this deck
39
The primary commercial use of nitric acid is .
A) in the manufacture of anti- depressant drugs
B) in the manufacture of explosives
C) in the manufacture of fertilizers
D) in the manufacture of plastics
E) in pool water maintenance
A) in the manufacture of anti- depressant drugs
B) in the manufacture of explosives
C) in the manufacture of fertilizers
D) in the manufacture of plastics
E) in pool water maintenance

Unlock Deck
Unlock for access to all 176 flashcards in this deck.
Unlock Deck
k this deck
40
A disproportionation reaction is one in which
A) an insoluble salt separates into ions.
B) aqueous ions combine to form an insoluble salt.
C) the ratio of combination of two elements in a compound changes.
D) a compound is separated into its constituent elements.
E) a single element is both oxidized and reduced.
A) an insoluble salt separates into ions.
B) aqueous ions combine to form an insoluble salt.
C) the ratio of combination of two elements in a compound changes.
D) a compound is separated into its constituent elements.
E) a single element is both oxidized and reduced.

Unlock Deck
Unlock for access to all 176 flashcards in this deck.
Unlock Deck
k this deck
41
A borane is a
A) compound containing only boron and hydrogen.
B) compound containing only boron and oxygen.
C) compound containing only boron and carbon.
D) three- dimensional covalent network of boron atoms.
E) compound containing only boron and aluminum.
A) compound containing only boron and hydrogen.
B) compound containing only boron and oxygen.
C) compound containing only boron and carbon.
D) three- dimensional covalent network of boron atoms.
E) compound containing only boron and aluminum.

Unlock Deck
Unlock for access to all 176 flashcards in this deck.
Unlock Deck
k this deck
42
Which compound would produce an acidic aqueous solution?
A) H2S
B) H2O
C) CaH2
D) KH
E) NH3
A) H2S
B) H2O
C) CaH2
D) KH
E) NH3

Unlock Deck
Unlock for access to all 176 flashcards in this deck.
Unlock Deck
k this deck
43
Soda- lime glass contains
A) SiO2, CO2, and citric acid
B) SiO2, CO2, Na2O
C) pure SiO2
D) SiO2 and aluminum
E) SiO2, CaO, and Na2O
A) SiO2, CO2, and citric acid
B) SiO2, CO2, Na2O
C) pure SiO2
D) SiO2 and aluminum
E) SiO2, CaO, and Na2O

Unlock Deck
Unlock for access to all 176 flashcards in this deck.
Unlock Deck
k this deck
44
What is the primary commercial use of hydrogen in the United States?
A) manufacture of methanol
B) as a rocket fuel, especially on the space shuttle
C) as an automobile fuel
D) hydrogenation of vegetable oils
E) manufacture of ammonia by the Haber process
A) manufacture of methanol
B) as a rocket fuel, especially on the space shuttle
C) as an automobile fuel
D) hydrogenation of vegetable oils
E) manufacture of ammonia by the Haber process

Unlock Deck
Unlock for access to all 176 flashcards in this deck.
Unlock Deck
k this deck
45
Which equation correctly represents the reaction between carbon dioxide and water?
A) CO2 (aq) + H2O (l)→ H2 (g) + CO (g) + O2 (g)
B) CO2 (aq) + 2H2O (l) → CH4 (g) + 2O2 (aq)
C) CO2 (aq) + H2O (l) → H2O2 (aq) + CO (g)
D) CO2 (aq) + H2O (l) → H2CO (aq) + O2 (g)
E) CO2 (aq) + H2O (l) → H2CO3 (aq)
A) CO2 (aq) + H2O (l)→ H2 (g) + CO (g) + O2 (g)
B) CO2 (aq) + 2H2O (l) → CH4 (g) + 2O2 (aq)
C) CO2 (aq) + H2O (l) → H2O2 (aq) + CO (g)
D) CO2 (aq) + H2O (l) → H2CO (aq) + O2 (g)
E) CO2 (aq) + H2O (l) → H2CO3 (aq)

Unlock Deck
Unlock for access to all 176 flashcards in this deck.
Unlock Deck
k this deck
46
The oxidation numbers of nitrogen in the nitride ion, hydrazine, ammonium cation, and nitrate ion are _, , _,and ,respectively.
A) +3, - 2, - 3, +5
B) - 3, +2, +1, +5
C) +3, - 2, +1, +3
D) - 3, - 2, - 3, +5
E) - 3, +2, - 3, +3
A) +3, - 2, - 3, +5
B) - 3, +2, +1, +5
C) +3, - 2, +1, +3
D) - 3, - 2, - 3, +5
E) - 3, +2, - 3, +3

Unlock Deck
Unlock for access to all 176 flashcards in this deck.
Unlock Deck
k this deck
47
What are the products of the reaction of PF3 (g) and water?
A) phosphoric acid and phosphorous acid
B) elemental phosphorus and fluorine gas
C) phosphoric acid and fluorine gas
D) phosphorous acid and hydrofluoric acid
E) elemental phosphorus and hydrofluoric acid
A) phosphoric acid and phosphorous acid
B) elemental phosphorus and fluorine gas
C) phosphoric acid and fluorine gas
D) phosphorous acid and hydrofluoric acid
E) elemental phosphorus and hydrofluoric acid

Unlock Deck
Unlock for access to all 176 flashcards in this deck.
Unlock Deck
k this deck
48
Boron can violate the octet rule in its compounds in that
A) it can have an expanded octet.
B) boron cannot violate the octet rule.
C) it can exist in a molecule with an odd number of electrons.
D) it can have fewer than eight valence electrons.
E) its compounds are all ionic.
A) it can have an expanded octet.
B) boron cannot violate the octet rule.
C) it can exist in a molecule with an odd number of electrons.
D) it can have fewer than eight valence electrons.
E) its compounds are all ionic.

Unlock Deck
Unlock for access to all 176 flashcards in this deck.
Unlock Deck
k this deck
49
Which of the following would produce a basic solution? 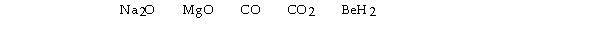
A) BeH2 only
B) CO, CO2, and BeH2
C) CO and CO2
D) Na2O, MgO, and BeH2
E) Na2O and MgO
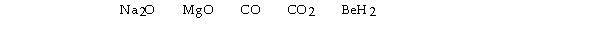
A) BeH2 only
B) CO, CO2, and BeH2
C) CO and CO2
D) Na2O, MgO, and BeH2
E) Na2O and MgO

Unlock Deck
Unlock for access to all 176 flashcards in this deck.
Unlock Deck
k this deck
50
The oxidation state of oxygen in OF2 is
A) +2
B) - 1
C) - 2
D) +1
E) 0
A) +2
B) - 1
C) - 2
D) +1
E) 0

Unlock Deck
Unlock for access to all 176 flashcards in this deck.
Unlock Deck
k this deck
51
Which element in group 6A is not found in compounds with an expanded valence shell?
A) selenium
B) oxygen
C) sulfur
D) polonium
E) tellurium
A) selenium
B) oxygen
C) sulfur
D) polonium
E) tellurium

Unlock Deck
Unlock for access to all 176 flashcards in this deck.
Unlock Deck
k this deck
52
Of the following, which is an ionic hydride?
A) BaH2
B) CaH2
C) LiH
D) SrH2
E) all of the above
A) BaH2
B) CaH2
C) LiH
D) SrH2
E) all of the above

Unlock Deck
Unlock for access to all 176 flashcards in this deck.
Unlock Deck
k this deck
53
The pentahydrated salt of sodium thiosulfate, Na2S2O3 · 5H2O, is used in photographic developing to convert _.
A) insoluble AgBr to insoluble Ag2S2O3
B) insoluble AgBr to soluble Na3[Ag(S2O3)2]
C) insoluble AgBr to soluble Ag2S2O3
D) soluble AgBr to insoluble Ag2S2O3
E) insoluble AgBr to insoluble Na3[Ag(S2O3)2]
A) insoluble AgBr to insoluble Ag2S2O3
B) insoluble AgBr to soluble Na3[Ag(S2O3)2]
C) insoluble AgBr to soluble Ag2S2O3
D) soluble AgBr to insoluble Ag2S2O3
E) insoluble AgBr to insoluble Na3[Ag(S2O3)2]

Unlock Deck
Unlock for access to all 176 flashcards in this deck.
Unlock Deck
k this deck
54
In a group of nonmetals, which element(s) is (are) most likely to be form stable n bonds?
A) the top element
B) the second element
C) the bottom element
D) the middle element
E) None of them, nonmetals do not do this.
A) the top element
B) the second element
C) the bottom element
D) the middle element
E) None of them, nonmetals do not do this.

Unlock Deck
Unlock for access to all 176 flashcards in this deck.
Unlock Deck
k this deck
55
The most stable allotrope of oxygen is .
A) H2O
B) HClO
C) O2
D) O3
E) O
A) H2O
B) HClO
C) O2
D) O3
E) O

Unlock Deck
Unlock for access to all 176 flashcards in this deck.
Unlock Deck
k this deck
56
Chlorine can have a positive oxidation state
A) if it combines with hydrogen.
B) if it combines with bromine or iodine.
C) if it combines with oxygen or fluorine.
D) if it combines with an alkali metal.
E) in its elemental form.
A) if it combines with hydrogen.
B) if it combines with bromine or iodine.
C) if it combines with oxygen or fluorine.
D) if it combines with an alkali metal.
E) in its elemental form.

Unlock Deck
Unlock for access to all 176 flashcards in this deck.
Unlock Deck
k this deck
57
Replacement of CaO by PbO in soda- lime glass results in
A) denser glass with a high refractive index.
B) a softer glass with a lower melting point.
C) glass with a deep blue color.
D) opaque glass.
E) a harder glass with a higher melting point.
A) denser glass with a high refractive index.
B) a softer glass with a lower melting point.
C) glass with a deep blue color.
D) opaque glass.
E) a harder glass with a higher melting point.

Unlock Deck
Unlock for access to all 176 flashcards in this deck.
Unlock Deck
k this deck
58
Boric acid condenses to form tetraboric acid according to the equation
A) 2H3BO3 (s) → HB2O2 (s) + 4H2O (g).
B) 4H3BO3 (s) → HB4O8 (s) + 4H2O (g).
C) 4H3BO3 (s) → H2B4O7 (s) + 5H2O (g).
D) 4H3BO3 (s) → 2H2B2O7 (s) + 3H2O (g).
E) 2H3BO3 (s) → H2B4O7 (s) + 3H2O (g).
A) 2H3BO3 (s) → HB2O2 (s) + 4H2O (g).
B) 4H3BO3 (s) → HB4O8 (s) + 4H2O (g).
C) 4H3BO3 (s) → H2B4O7 (s) + 5H2O (g).
D) 4H3BO3 (s) → 2H2B2O7 (s) + 3H2O (g).
E) 2H3BO3 (s) → H2B4O7 (s) + 3H2O (g).

Unlock Deck
Unlock for access to all 176 flashcards in this deck.
Unlock Deck
k this deck
59
Replacement of Na2O by K2O in soda- lime glass results in
A) a harder glass with a higher melting point.
B) glass with a deep blue color.
C) opaque glass.
D) denser glass with a high refractive index.
E) a softer glass with a lower melting point.
A) a harder glass with a higher melting point.
B) glass with a deep blue color.
C) opaque glass.
D) denser glass with a high refractive index.
E) a softer glass with a lower melting point.

Unlock Deck
Unlock for access to all 176 flashcards in this deck.
Unlock Deck
k this deck
60
Metal oxides are typically while nonmetal oxides are typically _.
A) amphoteric, acidic
B) basic, acidic
C) amphoteric, basic
D) basic, amphoteric
E) acidic, basic
A) amphoteric, acidic
B) basic, acidic
C) amphoteric, basic
D) basic, amphoteric
E) acidic, basic

Unlock Deck
Unlock for access to all 176 flashcards in this deck.
Unlock Deck
k this deck
61
What are the products of the reaction of PCl3 (g) and water?
A) elemental phosphorus and hydrochloric acid
B) elemental phosphorus and chlorine gas
C) phosphoric acid and chlorine gas
D) phosphoric acid and hydrochloric acid
E) phosphoric acid and phosphorous acid
A) elemental phosphorus and hydrochloric acid
B) elemental phosphorus and chlorine gas
C) phosphoric acid and chlorine gas
D) phosphoric acid and hydrochloric acid
E) phosphoric acid and phosphorous acid

Unlock Deck
Unlock for access to all 176 flashcards in this deck.
Unlock Deck
k this deck
62
How are the oxygen- containing compounds of xenon made?
A) by direct combination of the elements
B) by thermal decomposition of the xenon hydroxide
C) by reaction of the corresponding xenon fluoride with water
D) by reaction of xenon with peroxide
E) Xenon is inert and does not form compounds with oxygen.
A) by direct combination of the elements
B) by thermal decomposition of the xenon hydroxide
C) by reaction of the corresponding xenon fluoride with water
D) by reaction of xenon with peroxide
E) Xenon is inert and does not form compounds with oxygen.

Unlock Deck
Unlock for access to all 176 flashcards in this deck.
Unlock Deck
k this deck
63
The careful, thermal decomposition of solid ammonium nitrate will yield .
A) N2O
B) N2O3
C) N2O5
D) NO2
E) NO
A) N2O
B) N2O3
C) N2O5
D) NO2
E) NO

Unlock Deck
Unlock for access to all 176 flashcards in this deck.
Unlock Deck
k this deck
64
Which pair of formula/name is incorrect?
A) N2O4 / dinitrogen trioxide
B) NO / nitric oxide
C) N2O / nitrous oxide
D) N2O5 / dinitrogen pentoxide
E) NO2 / nitrogen dioxide
A) N2O4 / dinitrogen trioxide
B) NO / nitric oxide
C) N2O / nitrous oxide
D) N2O5 / dinitrogen pentoxide
E) NO2 / nitrogen dioxide

Unlock Deck
Unlock for access to all 176 flashcards in this deck.
Unlock Deck
k this deck
65
The interhalogen compound ICl3 can form but BrCl3 cannot form. This is because
A) bromine is not electronegative enough to react with chlorine.
B) iodine can have a negative oxidation state but bromine cannot.
C) bromine is too electronegative to react with chlorine.
D) iodine is large enough to accommodate three chlorine atoms around itself
E) iodine can have a positive oxidation state but bromine cannot.
A) bromine is not electronegative enough to react with chlorine.
B) iodine can have a negative oxidation state but bromine cannot.
C) bromine is too electronegative to react with chlorine.
D) iodine is large enough to accommodate three chlorine atoms around itself
E) iodine can have a positive oxidation state but bromine cannot.

Unlock Deck
Unlock for access to all 176 flashcards in this deck.
Unlock Deck
k this deck
66
Which of the following would produce an acidic solution? 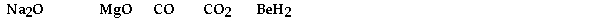
A) CO2 only
B) BeH2 only
C) CO, CO2, and BeH2
D) Na2O, MgO, and BeH2
E) Na2O and MgO
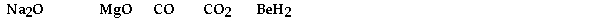
A) CO2 only
B) BeH2 only
C) CO, CO2, and BeH2
D) Na2O, MgO, and BeH2
E) Na2O and MgO

Unlock Deck
Unlock for access to all 176 flashcards in this deck.
Unlock Deck
k this deck
67
In the following chemical equation  the products (when the equation is balanced) are
the products (when the equation is balanced) are
A) 3NaO + PH6
B) 3NaOH + PH3
C) 3NaH + POH3
D) NaOH + 3PH
E) H2PO3 + 3NaH
 the products (when the equation is balanced) are
the products (when the equation is balanced) areA) 3NaO + PH6
B) 3NaOH + PH3
C) 3NaH + POH3
D) NaOH + 3PH
E) H2PO3 + 3NaH

Unlock Deck
Unlock for access to all 176 flashcards in this deck.
Unlock Deck
k this deck
68
B2O3is the anhydride of
A) diborane
B) borax
C) boric acid
D) borous acid
E) tetraboric acid
A) diborane
B) borax
C) boric acid
D) borous acid
E) tetraboric acid

Unlock Deck
Unlock for access to all 176 flashcards in this deck.
Unlock Deck
k this deck
69
Which equation correctly represents the reaction between elemental fluorine and sodium iodide?
A) F + NaI → I + NaF
B) F- + NaI → I- + NaF
C) F2 + NaI → NaF2 + I-
D) F + NaI → 1/2I2 + NaF
E) F2 + 2NaI → I2 + 2NaF
A) F + NaI → I + NaF
B) F- + NaI → I- + NaF
C) F2 + NaI → NaF2 + I-
D) F + NaI → 1/2I2 + NaF
E) F2 + 2NaI → I2 + 2NaF

Unlock Deck
Unlock for access to all 176 flashcards in this deck.
Unlock Deck
k this deck
70
Write the correctly balanced equation for the reaction between elemental fluorine and sodium iodide.

Unlock Deck
Unlock for access to all 176 flashcards in this deck.
Unlock Deck
k this deck
71
Which of the following approaches will produce PF3 reliably?
A) 2PCl3 (l) + (excess) F2 (g)→ 2PF3 (g) + 3Cl2 (g) + (excess) F2 (g)
B) PCl3 (l) + AsF3 (l) → PF3 (g) + AsCl3 (l)
C) P (s) + (excess) F2 (g) → PF3 (g) + (excess) F2 (g)
D) PF5 (g) → PF3 (g) + F2 (g)
E) none of these approaches will produce PF3
A) 2PCl3 (l) + (excess) F2 (g)→ 2PF3 (g) + 3Cl2 (g) + (excess) F2 (g)
B) PCl3 (l) + AsF3 (l) → PF3 (g) + AsCl3 (l)
C) P (s) + (excess) F2 (g) → PF3 (g) + (excess) F2 (g)
D) PF5 (g) → PF3 (g) + F2 (g)
E) none of these approaches will produce PF3

Unlock Deck
Unlock for access to all 176 flashcards in this deck.
Unlock Deck
k this deck
72
The oxidation state of fluorine in its compounds is
A) negative unless it combines with oxygen.
B) negative unless it combines with another halogen.
C) positive unless it combines with another halogen.
D) always negative.
E) negative unless it combines with an active metal.
A) negative unless it combines with oxygen.
B) negative unless it combines with another halogen.
C) positive unless it combines with another halogen.
D) always negative.
E) negative unless it combines with an active metal.

Unlock Deck
Unlock for access to all 176 flashcards in this deck.
Unlock Deck
k this deck
73
A carbonyl compound contains
A) a carbon- oxygen double bond
B) a carbon- carbon triple bond
C) a carbon- oxygen triple bond
D) a carbon- carbon double bond
E) a carbon atom with a lone pair of electrons
A) a carbon- oxygen double bond
B) a carbon- carbon triple bond
C) a carbon- oxygen triple bond
D) a carbon- carbon double bond
E) a carbon atom with a lone pair of electrons

Unlock Deck
Unlock for access to all 176 flashcards in this deck.
Unlock Deck
k this deck
74
The heavier noble gases are more reactive than the lighter ones because
A) the lighter noble gases have complete octets.
B) the heavier noble gases are more abundant.
C) the lighter noble gases exist as diatomic molecules.
D) the heavier noble gases have low ionization energies relative to the lighter ones.
E) the heavier noble gases have greater electron affinities.
A) the lighter noble gases have complete octets.
B) the heavier noble gases are more abundant.
C) the lighter noble gases exist as diatomic molecules.
D) the heavier noble gases have low ionization energies relative to the lighter ones.
E) the heavier noble gases have greater electron affinities.

Unlock Deck
Unlock for access to all 176 flashcards in this deck.
Unlock Deck
k this deck
75
Which one of the following is false concerning buckminsterfullerene?
A) It consists of individual molecules like C60 and C70.
B) It is made up of molecules that resemble soccer balls.
C) It is made up of Cl2molecules.
D) It is the most recently discovered crystalline allotrope of carbon.
E) It is a molecular form of carbon.
A) It consists of individual molecules like C60 and C70.
B) It is made up of molecules that resemble soccer balls.
C) It is made up of Cl2molecules.
D) It is the most recently discovered crystalline allotrope of carbon.
E) It is a molecular form of carbon.

Unlock Deck
Unlock for access to all 176 flashcards in this deck.
Unlock Deck
k this deck
76
The oxidation state of oxygen in O2F2 is
A) +2
B) 0
C) - 1
D) +1
E) - 2
A) +2
B) 0
C) - 1
D) +1
E) - 2

Unlock Deck
Unlock for access to all 176 flashcards in this deck.
Unlock Deck
k this deck
77
The molecular shape of the SF6 molecule is .
A) trigonal pyramidal
B) octahedral
C) tetrahedral
D) trigonal bipyramidal
E) T- shaped
A) trigonal pyramidal
B) octahedral
C) tetrahedral
D) trigonal bipyramidal
E) T- shaped

Unlock Deck
Unlock for access to all 176 flashcards in this deck.
Unlock Deck
k this deck
78
Additives can be used in soda- lime glass to alter its .
A) hardness
B) ability to withstand temperature change
C) color
D) melting point
E) any of the above
A) hardness
B) ability to withstand temperature change
C) color
D) melting point
E) any of the above

Unlock Deck
Unlock for access to all 176 flashcards in this deck.
Unlock Deck
k this deck
79
Which of the following equations correctly represents the reaction of B2H6 with oxygen?
A) B2H6 (g) + 2O2 (g) → B2O2 (s) + 3H2 + O2 (g)
B) B2H6 (g) + O2 (g) → H2B2O2 (s) + 2H2 (g)
C) B2H6 (g) + O2 (g) → B2O2 (s) + 3H2 (g)
D) B2H6 (g) + 2O2 (g) → B2H2 (s) + 2H2O2 (aq)
E) B2H6 (g) + 3O2 (g) → B2O3 (s) + 3H2O (g)
A) B2H6 (g) + 2O2 (g) → B2O2 (s) + 3H2 + O2 (g)
B) B2H6 (g) + O2 (g) → H2B2O2 (s) + 2H2 (g)
C) B2H6 (g) + O2 (g) → B2O2 (s) + 3H2 (g)
D) B2H6 (g) + 2O2 (g) → B2H2 (s) + 2H2O2 (aq)
E) B2H6 (g) + 3O2 (g) → B2O3 (s) + 3H2O (g)

Unlock Deck
Unlock for access to all 176 flashcards in this deck.
Unlock Deck
k this deck
80
Silicones can be oils or rubber- like materials depending on
A) the oxidation state of silicon in the chain.
B) the silicon- to- oxygen ratio.
C) the percentage of sulfur in the chain.
D) the length of the chain and degree of cross- linking.
E) the percentage of carbon in the chain.
A) the oxidation state of silicon in the chain.
B) the silicon- to- oxygen ratio.
C) the percentage of sulfur in the chain.
D) the length of the chain and degree of cross- linking.
E) the percentage of carbon in the chain.

Unlock Deck
Unlock for access to all 176 flashcards in this deck.
Unlock Deck
k this deck


