Deck 8: The Structure of Atoms and Periodic Trends
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/82
Play
Full screen (f)
Deck 8: The Structure of Atoms and Periodic Trends
1
The procedure by which electrons are assigned to (or built up into) orbitals is known as the ____ principle.
A) Aufbau
B) Bohr
C) Planck
D) Hund
E) Pauli
A) Aufbau
B) Bohr
C) Planck
D) Hund
E) Pauli
Aufbau
2
Which of the following statements concerning the Pauli exclusion principle is/are CORRECT?

Unlock Deck
Unlock for access to all 82 flashcards in this deck.
Unlock Deck
k this deck
3
The small, but important, energy differences between 3s, 3p, and 3d electrons is a consequence of
A) the number of electrons they can hold
B) their principal quantum number
C) the Heisenberg uncertainty principle
D) thier effective nuclear charge
E) Hund's rule
A) the number of electrons they can hold
B) their principal quantum number
C) the Heisenberg uncertainty principle
D) thier effective nuclear charge
E) Hund's rule
thier effective nuclear charge
4
What is the maximum number of electrons that can occupy the n = 3 shell?
A) 2
B) 8
C) 18
D) 32
E) 50
A) 2
B) 8
C) 18
D) 32
E) 50

Unlock Deck
Unlock for access to all 82 flashcards in this deck.
Unlock Deck
k this deck
5
How many electrons can be described by the quantum numbers n= 3 and l= 2?
A) 14
B) 6
C) 2
D) 18
E) 10
A) 14
B) 6
C) 2
D) 18
E) 10

Unlock Deck
Unlock for access to all 82 flashcards in this deck.
Unlock Deck
k this deck
6
Which of the following statements is true concerning the electron configuration [Xe]6p2?
A) This configuration cannot be the ground-state electron configuration for a Ba atom because it violates the Pauli exclusion principle.
B) This configuration cannot be the ground-state electron configuration for a Ba atom because it violates Hund's rule.
C) This configuration is the ground-state electron configuration for a Ba atom.
D) This configuration cannot be the ground-state electron configuration for a Ba atom because it violates the Heisenberg uncertainty principle.
E) This configuration cannot be the ground-state electron configuration for a Ba atom because it violates the Aufbau principle.
A) This configuration cannot be the ground-state electron configuration for a Ba atom because it violates the Pauli exclusion principle.
B) This configuration cannot be the ground-state electron configuration for a Ba atom because it violates Hund's rule.
C) This configuration is the ground-state electron configuration for a Ba atom.
D) This configuration cannot be the ground-state electron configuration for a Ba atom because it violates the Heisenberg uncertainty principle.
E) This configuration cannot be the ground-state electron configuration for a Ba atom because it violates the Aufbau principle.

Unlock Deck
Unlock for access to all 82 flashcards in this deck.
Unlock Deck
k this deck
7
What is the maximum number of electrons that can occupy one s orbital?
A) 1
B) 2
C) 6
D) 10
E) 14
A) 1
B) 2
C) 6
D) 10
E) 14

Unlock Deck
Unlock for access to all 82 flashcards in this deck.
Unlock Deck
k this deck
8
Which of the following orbital occupancy designations is incorrect?
A) 2s2
B) 3d6
C) 1s2
D) 4p3
E) 3d12
A) 2s2
B) 3d6
C) 1s2
D) 4p3
E) 3d12

Unlock Deck
Unlock for access to all 82 flashcards in this deck.
Unlock Deck
k this deck
10
Which of the following statements is true?
A) Outer electrons efficiently shield one another from nuclear charge.
B) Core electrons effectively shield outer electrons from nuclear charge.
C) Valence electrons are the most difficult of all electrons to remove.
D) Core electrons have the lowest ionization energies of all electrons.
E) Valence electrons in the outermost shell of all elements have the highest ionization energy.
A) Outer electrons efficiently shield one another from nuclear charge.
B) Core electrons effectively shield outer electrons from nuclear charge.
C) Valence electrons are the most difficult of all electrons to remove.
D) Core electrons have the lowest ionization energies of all electrons.
E) Valence electrons in the outermost shell of all elements have the highest ionization energy.

Unlock Deck
Unlock for access to all 82 flashcards in this deck.
Unlock Deck
k this deck
11
Which of the following elements is found in the d-block of the periodic table?
A) Ir
B) Tb
C) Li
D) Cl
E) None of these
A) Ir
B) Tb
C) Li
D) Cl
E) None of these

Unlock Deck
Unlock for access to all 82 flashcards in this deck.
Unlock Deck
k this deck
12
According to the Aufbau principle, which of the following subshells is typically filled next after the 4s subshell?
A) 3d
B) 4s
C) 3p
D) 2p
E) 2s
A) 3d
B) 4s
C) 3p
D) 2p
E) 2s

Unlock Deck
Unlock for access to all 82 flashcards in this deck.
Unlock Deck
k this deck
13
Which of the following sets of quantum numbers is allowed?
A) n = 2, = 1, = +1/2, ms = -1/2
B) n = 3, = 2, = +1, ms = +1
C) n = 4, = 1, = 0, ms = -1/2
D) n = 4, = 3, = -1, ms = 0
E) n = 5, = 2, = +2, ms = -1
A) n = 2, = 1, = +1/2, ms = -1/2
B) n = 3, = 2, = +1, ms = +1
C) n = 4, = 1, = 0, ms = -1/2
D) n = 4, = 3, = -1, ms = 0
E) n = 5, = 2, = +2, ms = -1

Unlock Deck
Unlock for access to all 82 flashcards in this deck.
Unlock Deck
k this deck
14
An element that has the same ground state valence-shell electron configuration as thallium is
A) gallium.
B) carbon.
C) krypton.
D) cesium.
E) magnesium.
A) gallium.
B) carbon.
C) krypton.
D) cesium.
E) magnesium.

Unlock Deck
Unlock for access to all 82 flashcards in this deck.
Unlock Deck
k this deck
15
How many unpaired electrons are found in the ground state electron configuration of barium (ba)?
A) 0
B) 1
C) 2
D) 3
E) 5
A) 0
B) 1
C) 2
D) 3
E) 5

Unlock Deck
Unlock for access to all 82 flashcards in this deck.
Unlock Deck
k this deck
17
How many electrons can be described by the following quantum numbers: n = 3, = 2, = 1?
A) 1
B) 2
C) 6
D) 10
E) 18
A) 1
B) 2
C) 6
D) 10
E) 18

Unlock Deck
Unlock for access to all 82 flashcards in this deck.
Unlock Deck
k this deck
18
How many electrons can be described by the following quantum numbers: n = 4, = 2, = 2, ms = -1/2?
A) 1
B) 2
C) 6
D) 10
E) 18
A) 1
B) 2
C) 6
D) 10
E) 18

Unlock Deck
Unlock for access to all 82 flashcards in this deck.
Unlock Deck
k this deck
19
Which of the following statements concerning the Pauli exclusion principle is/are CORRECT?
1. If two electrons occupy the same orbital they must have opposite spins.
2. No two electrons in an atom can have the same four quantum numbers.
3. Electrons with opposing spins are attracted to each other.
A) 1 only
B) 2 only
C) 3 only
D) 1 and 2
E) 1, 2, and 3
1. If two electrons occupy the same orbital they must have opposite spins.
2. No two electrons in an atom can have the same four quantum numbers.
3. Electrons with opposing spins are attracted to each other.
A) 1 only
B) 2 only
C) 3 only
D) 1 and 2
E) 1, 2, and 3

Unlock Deck
Unlock for access to all 82 flashcards in this deck.
Unlock Deck
k this deck
20
How many valence electrons does an arsenic atom have?
A) 5
B) 8
C) 7
D) 2
E) 33
A) 5
B) 8
C) 7
D) 2
E) 33

Unlock Deck
Unlock for access to all 82 flashcards in this deck.
Unlock Deck
k this deck
21
Which of the following elements in its 1+ ionic state has the ground state electron configuration [Kr]4d10?
A) Ru
B) Au
C) Ag
D) In
E) Cd
A) Ru
B) Au
C) Ag
D) In
E) Cd

Unlock Deck
Unlock for access to all 82 flashcards in this deck.
Unlock Deck
k this deck
22
The complete electron configuration of tin is _____.
A) 1s22s22p63s23p64s23d104p65s24d105p2
B) 1s22s22p63s23p64s24p65s24d105d105p2
C) 1s22s22p63s23p64s23d104d104p2
D) 1s22s22p63s23p64s23d104p65s24d105d105p2
E) None of these
A) 1s22s22p63s23p64s23d104p65s24d105p2
B) 1s22s22p63s23p64s24p65s24d105d105p2
C) 1s22s22p63s23p64s23d104d104p2
D) 1s22s22p63s23p64s23d104p65s24d105d105p2
E) None of these

Unlock Deck
Unlock for access to all 82 flashcards in this deck.
Unlock Deck
k this deck
23
Which ground-state electron configuration is incorrect?
A) Br: [Ar]3d104s24p5
B) K: [Ar]4s1
C) Ni: [Ar]3d5
D) Mg: 1s22s22p63s2
E) Co: [Ar]3d74s2
A) Br: [Ar]3d104s24p5
B) K: [Ar]4s1
C) Ni: [Ar]3d5
D) Mg: 1s22s22p63s2
E) Co: [Ar]3d74s2

Unlock Deck
Unlock for access to all 82 flashcards in this deck.
Unlock Deck
k this deck
24
Which of the following electron configurations corresponds to the ground state of an atom of a transition element?
A) 1s22s22p1
B) 1s22s22p63s23p63d104s24p3
C) 1s22s22p63s23p63d14s2
D) 1s22s22p63s23p64s1
E) 1s22s22p63s23p4
A) 1s22s22p1
B) 1s22s22p63s23p63d104s24p3
C) 1s22s22p63s23p63d14s2
D) 1s22s22p63s23p64s1
E) 1s22s22p63s23p4

Unlock Deck
Unlock for access to all 82 flashcards in this deck.
Unlock Deck
k this deck
25
Which of the following atoms is diamagnetic in its ground state?
A) mercury (Hg)
B) tin (Sn)
C) rhenium (Re)
D) berkelium (Bk)
E) phosphorus (P)
A) mercury (Hg)
B) tin (Sn)
C) rhenium (Re)
D) berkelium (Bk)
E) phosphorus (P)

Unlock Deck
Unlock for access to all 82 flashcards in this deck.
Unlock Deck
k this deck
26
Which of the following orbital diagrams represents a diamagnetic atom? 1s 2s 2p
A)
B)
C)
D)
E)
A)

B)

C)

D)

E)


Unlock Deck
Unlock for access to all 82 flashcards in this deck.
Unlock Deck
k this deck
27
Which atom has the ground state electronic configuration 1s22s22p63s23p64s23d3?
A) Ga
B) V
C) As
D) Nb
E) none
A) Ga
B) V
C) As
D) Nb
E) none

Unlock Deck
Unlock for access to all 82 flashcards in this deck.
Unlock Deck
k this deck
28
Which element has the following ground state electron configuration? 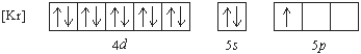
A) In
B) Y
C) Nb
D) Tl
E) Ga

A) In
B) Y
C) Nb
D) Tl
E) Ga

Unlock Deck
Unlock for access to all 82 flashcards in this deck.
Unlock Deck
k this deck
29
What is the ground-state electron configuration of sulfur (S)?
A) [Ne]3s33p3
B) [Ar]3s23p4
C) [Ar]3p6
D) [Ne]3s23p4
E) [Ar]3p6..
A) [Ne]3s33p3
B) [Ar]3s23p4
C) [Ar]3p6
D) [Ne]3s23p4
E) [Ar]3p6..

Unlock Deck
Unlock for access to all 82 flashcards in this deck.
Unlock Deck
k this deck
30
If the ground state electron configuration of an element is [Ar]3d104s24p5, what is the typical charge on the monatomic anion of the element?
A) 4+
B) 2+
C) 1-
D) 2-
E) 3-
A) 4+
B) 2+
C) 1-
D) 2-
E) 3-

Unlock Deck
Unlock for access to all 82 flashcards in this deck.
Unlock Deck
k this deck
31
Which of the following orbital diagrams represents a paramagnetic atom? 1s 2s 2p
1)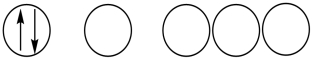 2.
2. 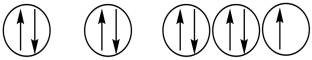 3.
3. 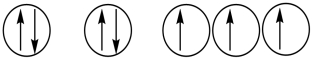
A) 1 only
B) 2 only
C) 3 only
D) 1 and 2
E) 2 and 3
1)
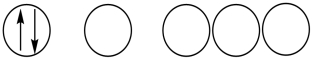 2.
2.  3.
3. 
A) 1 only
B) 2 only
C) 3 only
D) 1 and 2
E) 2 and 3

Unlock Deck
Unlock for access to all 82 flashcards in this deck.
Unlock Deck
k this deck
32
For which of the following atoms is the 2+ ion diamagnetic in the ground state?
A) Ni
B) Fe
C) Zn
D) Mn
E) Cu
A) Ni
B) Fe
C) Zn
D) Mn
E) Cu

Unlock Deck
Unlock for access to all 82 flashcards in this deck.
Unlock Deck
k this deck
33
Which of the following elements has the ground state electron configuration [Ar]3d104s1?
A) Cu
B) Zn
C) Ge
D) Ag
E) Cd
A) Cu
B) Zn
C) Ge
D) Ag
E) Cd

Unlock Deck
Unlock for access to all 82 flashcards in this deck.
Unlock Deck
k this deck
34
What is a possible set of quantum numbers for an unpaired electron in the orbital box diagram below? 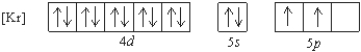
A) n = 1, = 1, = -1, ms = +1/2
B) n = 4, = 2, = -1, ms = -1/2
C) n = 5, = 2, = -2, ms = +1/2
D) n = 5, = 0, = 0, ms = -1/2
E) n = 5, = 1, = -1, ms = +1/2

A) n = 1, = 1, = -1, ms = +1/2
B) n = 4, = 2, = -1, ms = -1/2
C) n = 5, = 2, = -2, ms = +1/2
D) n = 5, = 0, = 0, ms = -1/2
E) n = 5, = 1, = -1, ms = +1/2

Unlock Deck
Unlock for access to all 82 flashcards in this deck.
Unlock Deck
k this deck
35
What noble gas core precedes the valence shell ground state electron configuration for potassium (K)?
A) [Ar]
B) [Rn]
C) [Kr]
D) [Ne]
E) [Xe]
A) [Ar]
B) [Rn]
C) [Kr]
D) [Ne]
E) [Xe]

Unlock Deck
Unlock for access to all 82 flashcards in this deck.
Unlock Deck
k this deck
36
Hund's rule states that the most stable arrangement of electrons (for a ground state electron configuration)
A) has a filled valence shell of electrons.
B) has three electrons per orbital, each with identical spins.
C) has values greater than or equal to +1.
D) has the maximum number of unpaired electrons, all with the same spin.
E) has two electrons per orbital, each with opposing spins.
A) has a filled valence shell of electrons.
B) has three electrons per orbital, each with identical spins.
C) has values greater than or equal to +1.
D) has the maximum number of unpaired electrons, all with the same spin.
E) has two electrons per orbital, each with opposing spins.

Unlock Deck
Unlock for access to all 82 flashcards in this deck.
Unlock Deck
k this deck
37
Which is the correct valence shell orbital box notation for the ground state electron configuration of Fe?
A)
B)
C)
D)
E)
A)

B)

C)

D)

E)


Unlock Deck
Unlock for access to all 82 flashcards in this deck.
Unlock Deck
k this deck
38
Which element has the following ground state electron configuration? 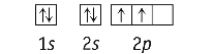
A) Be
B) O
C) Li
D) Si
E) N

A) Be
B) O
C) Li
D) Si
E) N

Unlock Deck
Unlock for access to all 82 flashcards in this deck.
Unlock Deck
k this deck
39
All of the following ground-state electron configurations are correct except
A) V: [Ar]4s24d3
B) K: [Ar]4s1
C) Sb: [Kr]4d105s25p3
D) Cr: [Ar]3d54s1
E) Te: [Kr]4d105s25p4
A) V: [Ar]4s24d3
B) K: [Ar]4s1
C) Sb: [Kr]4d105s25p3
D) Cr: [Ar]3d54s1
E) Te: [Kr]4d105s25p4

Unlock Deck
Unlock for access to all 82 flashcards in this deck.
Unlock Deck
k this deck
40
Which element has the following ground state electron configuration? 3d 4s
[Ar]![<strong>Which element has the following ground state electron configuration? 3d 4s [Ar] </strong> A) Sc B) Ni C) Co D) Fe E) V](https://storage.examlex.com/TB7130/11ead6f3_24ad_ac95_9e2d_2f8c64539ecc_TB7130_11.jpg)
A) Sc
B) Ni
C) Co
D) Fe
E) V
[Ar]
![<strong>Which element has the following ground state electron configuration? 3d 4s [Ar] </strong> A) Sc B) Ni C) Co D) Fe E) V](https://storage.examlex.com/TB7130/11ead6f3_24ad_ac95_9e2d_2f8c64539ecc_TB7130_11.jpg)
A) Sc
B) Ni
C) Co
D) Fe
E) V

Unlock Deck
Unlock for access to all 82 flashcards in this deck.
Unlock Deck
k this deck
41
Place the following atoms in order of increasing atomic radii: Se, Sb, Br, and Te.
A) Br < Se < Te < Sb
B) Se < Br < Sb < Te
C) Se < Br < Te < As
D) Sb < Te < Se < Br
E) Te < Sb < Se < Br
A) Br < Se < Te < Sb
B) Se < Br < Sb < Te
C) Se < Br < Te < As
D) Sb < Te < Se < Br
E) Te < Sb < Se < Br

Unlock Deck
Unlock for access to all 82 flashcards in this deck.
Unlock Deck
k this deck
42
Which of the following equations corresponds to the second ionization of magnesium?
A) Mg(g) → Mg+(g) + e-..
B) Mg(g) → Mg+(g) + e-.
C) Mg(g) → Mg+(g) + e-
D) Mg(g) → Mg2+(g) + 2e-
E) Mg(g) + e- → Mg-(g)
A) Mg(g) → Mg+(g) + e-..
B) Mg(g) → Mg+(g) + e-.
C) Mg(g) → Mg+(g) + e-
D) Mg(g) → Mg2+(g) + 2e-
E) Mg(g) + e- → Mg-(g)

Unlock Deck
Unlock for access to all 82 flashcards in this deck.
Unlock Deck
k this deck
43
The change in energy for which of the following processes corresponds to the first ionization energy of beryllium?
A) Be(g) → Be+(g) + e-
B) Be(s) → Be+(s) + e-
C) Be(s) → Be+(g) + e-
D) Be(g) → Be2+(g) + 2e-
E) Be(s) + e- → Be-(s)
A) Be(g) → Be+(g) + e-
B) Be(s) → Be+(s) + e-
C) Be(s) → Be+(g) + e-
D) Be(g) → Be2+(g) + 2e-
E) Be(s) + e- → Be-(s)

Unlock Deck
Unlock for access to all 82 flashcards in this deck.
Unlock Deck
k this deck
44
Which of the following atoms of elements has the largest atomic radius?
A) Ga
B) In
C) Al
D) Tl
E) B
A) Ga
B) In
C) Al
D) Tl
E) B

Unlock Deck
Unlock for access to all 82 flashcards in this deck.
Unlock Deck
k this deck
45
An atom of which of the following elements has the smallest ionization energy?
A) At
B) Bi
C) Pb
D) Cs
E) Po
A) At
B) Bi
C) Pb
D) Cs
E) Po

Unlock Deck
Unlock for access to all 82 flashcards in this deck.
Unlock Deck
k this deck
46
Rank the following atoms in order decreasing atomic radii: Be, Be, Be, B.
A) B > Be > Be > Be
B) Be > B > Be > Be
C) Be > Be > B > Be
D) Be > Be > Be > B
E) B > Be > Be > Be..
A) B > Be > Be > Be
B) Be > B > Be > Be
C) Be > Be > B > Be
D) Be > Be > Be > B
E) B > Be > Be > Be..

Unlock Deck
Unlock for access to all 82 flashcards in this deck.
Unlock Deck
k this deck
47
What 2+ ion has the following ground state electron configuration? 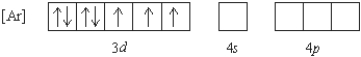
A) Mn2+
B) Co2+
C) Ni2+
D) Cu2+
E) Ge2+

A) Mn2+
B) Co2+
C) Ni2+
D) Cu2+
E) Ge2+

Unlock Deck
Unlock for access to all 82 flashcards in this deck.
Unlock Deck
k this deck
48
Which of the following ions has the given ground state electron configuration? 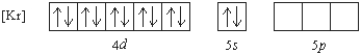
A) Cd2+
B) Sr2+
C) Zn2+
D) Sn2+
E) None of these

A) Cd2+
B) Sr2+
C) Zn2+
D) Sn2+
E) None of these

Unlock Deck
Unlock for access to all 82 flashcards in this deck.
Unlock Deck
k this deck
49
Which of the following has the same (total) number of electrons as Ar?
A) Na+
B) Ca2+
C) Ga3+
D) O2-
E) none
A) Na+
B) Ca2+
C) Ga3+
D) O2-
E) none

Unlock Deck
Unlock for access to all 82 flashcards in this deck.
Unlock Deck
k this deck
50
What is the ground state electron configuration for Sn2+?
A) [Kr]4d105s2
B) [Kr]4d105p2
C) [Kr]5s2
D) [Kr]4d105s25p2
E) [Kr]4d105s25p4
A) [Kr]4d105s2
B) [Kr]4d105p2
C) [Kr]5s2
D) [Kr]4d105s25p2
E) [Kr]4d105s25p4

Unlock Deck
Unlock for access to all 82 flashcards in this deck.
Unlock Deck
k this deck
51
Which of the following statements is true of atomic radii?
A) They decrease down a group and remain constant across a period.
B) They decrease down a group and increase across a period.
C) They increase down a group and increase across a period.
D) They increase down a group and remain constant across a period.
E) They increase down a group and decrease across a period.
A) They decrease down a group and remain constant across a period.
B) They decrease down a group and increase across a period.
C) They increase down a group and increase across a period.
D) They increase down a group and remain constant across a period.
E) They increase down a group and decrease across a period.

Unlock Deck
Unlock for access to all 82 flashcards in this deck.
Unlock Deck
k this deck
52
Arrange F, Cl, and Br in order of their increasing first ionization energies.
A) F < Cl < Br
B) Cl < F < Br
C) Cl < Br < F
D) Br < F < Cl
E) Br < Cl < F
A) F < Cl < Br
B) Cl < F < Br
C) Cl < Br < F
D) Br < F < Cl
E) Br < Cl < F

Unlock Deck
Unlock for access to all 82 flashcards in this deck.
Unlock Deck
k this deck
53
For which one of the following elements is the second ionization energy over ten times larger than its first ionization energy?
A) B
B) N
C) Li
D) Ne
E) Cu
A) B
B) N
C) Li
D) Ne
E) Cu

Unlock Deck
Unlock for access to all 82 flashcards in this deck.
Unlock Deck
k this deck
54
The ground-state electron configuration of a Ni2+ ion is 1s22s22p63s23p63d8 . Therefore, Ni2+ is
A) paramagnetic with two unpaired electrons.
B) diamagnetic.
C) paramagnetic with one unpaired electron.
D) paramagnetic with four unpaired electrons.
E) paramagnetic with five unpaired electrons.
A) paramagnetic with two unpaired electrons.
B) diamagnetic.
C) paramagnetic with one unpaired electron.
D) paramagnetic with four unpaired electrons.
E) paramagnetic with five unpaired electrons.

Unlock Deck
Unlock for access to all 82 flashcards in this deck.
Unlock Deck
k this deck
55
What is the ground-state electron configuration of the chloride ion?
A)
B)
C)
D)
E)
A)
B)
C)
D)
E)

Unlock Deck
Unlock for access to all 82 flashcards in this deck.
Unlock Deck
k this deck
56
An atom of which of the following elements has the smallest atomic radius?
A) F
B) Rb
C) Ca
D) Ge
E) P
A) F
B) Rb
C) Ca
D) Ge
E) P

Unlock Deck
Unlock for access to all 82 flashcards in this deck.
Unlock Deck
k this deck
57
What is the ground state electron configuration for Cr3+?
A) [Ar]
B) [Ar]3d74s2
C) [Ar]3d14s2
D) [Ar]3d24s1
E) [Ar]3d3
A) [Ar]
B) [Ar]3d74s2
C) [Ar]3d14s2
D) [Ar]3d24s1
E) [Ar]3d3

Unlock Deck
Unlock for access to all 82 flashcards in this deck.
Unlock Deck
k this deck
58
Which of the given ions have the same ground state electron configuration: S2-, N3-, Mg2+, and Br-?
A) N3- and Mg2+
B) S2-, N3-, and Br-
C) S2- and Br-
D) Mg2+ and Br-
E) S2-, N3-, Mg2+, and Br-
A) N3- and Mg2+
B) S2-, N3-, and Br-
C) S2- and Br-
D) Mg2+ and Br-
E) S2-, N3-, Mg2+, and Br-

Unlock Deck
Unlock for access to all 82 flashcards in this deck.
Unlock Deck
k this deck
59
What 2- ion has the following ground state electron configuration? 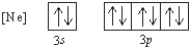
A) oxide ion
B) nitride ion
C) fluoride ion
D) sulfide ion
E) magnesium ion

A) oxide ion
B) nitride ion
C) fluoride ion
D) sulfide ion
E) magnesium ion

Unlock Deck
Unlock for access to all 82 flashcards in this deck.
Unlock Deck
k this deck
60
Which of the following cations has the same number of unpaired electrons as Fe2+?
A) Ni2+
B) Fe3+
C) Cr2+
D) Mn2+
E) Co2+
A) Ni2+
B) Fe3+
C) Cr2+
D) Mn2+
E) Co2+

Unlock Deck
Unlock for access to all 82 flashcards in this deck.
Unlock Deck
k this deck
61
Electron ________ is defined as the energy change for a process in which a gas phase atom acquires an electron.

Unlock Deck
Unlock for access to all 82 flashcards in this deck.
Unlock Deck
k this deck
62
Explain the difference between paramagnetic and ferromagnetic materials.

Unlock Deck
Unlock for access to all 82 flashcards in this deck.
Unlock Deck
k this deck
63
An atom of which of the following elements has the most negative electron affinity?
A) K
B) Sb
C) Cl
D) Br
E) O
A) K
B) Sb
C) Cl
D) Br
E) O

Unlock Deck
Unlock for access to all 82 flashcards in this deck.
Unlock Deck
k this deck
64
_____ have no affinity for electrons.
A) Transition metals
B) s-block elements
C) Main group nonmetals
D) Noble gases
E) Semiconductors
A) Transition metals
B) s-block elements
C) Main group nonmetals
D) Noble gases
E) Semiconductors

Unlock Deck
Unlock for access to all 82 flashcards in this deck.
Unlock Deck
k this deck
65
Place the following ions in order from smallest to largest ionic radii: K+, Na+, Mg2+, and Al3+.
A) Al3+ < Mg2+ < Na+ < K+
B) Na+ < Mg2+ < Al3+ < K+
C) K+ < Mg2+ < Na+ < Al3+
D) K+ < Al3+ < Mg2+ < Na+
E) Mg2+ < Al3+ < Na+ < K+
A) Al3+ < Mg2+ < Na+ < K+
B) Na+ < Mg2+ < Al3+ < K+
C) K+ < Mg2+ < Na+ < Al3+
D) K+ < Al3+ < Mg2+ < Na+
E) Mg2+ < Al3+ < Na+ < K+

Unlock Deck
Unlock for access to all 82 flashcards in this deck.
Unlock Deck
k this deck
66
A metal halide forms when potassium reacts with elemental chlorine. What is the most likely formula of this metal halide?
A) KCl
B) KCl2
C) K2Cl
D) KCl3
E) K3Cl2
A) KCl
B) KCl2
C) K2Cl
D) KCl3
E) K3Cl2

Unlock Deck
Unlock for access to all 82 flashcards in this deck.
Unlock Deck
k this deck
67
Which of the following elements would be expected to have chemical and physical properties most similar to Iodine (I)?
A) Fluorine (F)
B) Aluminum (Al)
C) Magnesium (Mg)
D) Rubidium (Rb)
E) Krypton (Kr)
A) Fluorine (F)
B) Aluminum (Al)
C) Magnesium (Mg)
D) Rubidium (Rb)
E) Krypton (Kr)

Unlock Deck
Unlock for access to all 82 flashcards in this deck.
Unlock Deck
k this deck
68
The change in energy for the following reaction is referred to as the ____ for boron. B(g) + e- → B-(g)
A) oxidation number
B) electron affinity
C) electronegativity energy
D) first ionization energy
E) second ionization energy
A) oxidation number
B) electron affinity
C) electronegativity energy
D) first ionization energy
E) second ionization energy

Unlock Deck
Unlock for access to all 82 flashcards in this deck.
Unlock Deck
k this deck
69
The f-block elements are also referred to as the ________ and actinides.

Unlock Deck
Unlock for access to all 82 flashcards in this deck.
Unlock Deck
k this deck
70
________ rule states that the most stable arrangement of electrons is that which contains the maximum number of unpaired electrons, all with the same spin direction.

Unlock Deck
Unlock for access to all 82 flashcards in this deck.
Unlock Deck
k this deck
71
What is the charge formed by alkaline earth metals when they react with nonmetals?
A) +1
B) −1
C) +2
D) −2
E) +3
A) +1
B) −1
C) +2
D) −2
E) +3

Unlock Deck
Unlock for access to all 82 flashcards in this deck.
Unlock Deck
k this deck
72
Rank the following ions in order of decreasing ionic radii: Al3+, Mg2+, Al3+, Al3+.
A) Al3+ > Al3+ > Mg2+ > Al3+
B) Al3+ > Al3+ > Mg2+ > Al3+..
C) Al3+> Mg2+ > Al3+ > Al3+
D) Al3+ > Mg2+ > Al3+ > Al3+..
E) Mg2+ > Al3+ > Al3+ > Al3+
A) Al3+ > Al3+ > Mg2+ > Al3+
B) Al3+ > Al3+ > Mg2+ > Al3+..
C) Al3+> Mg2+ > Al3+ > Al3+
D) Al3+ > Mg2+ > Al3+ > Al3+..
E) Mg2+ > Al3+ > Al3+ > Al3+

Unlock Deck
Unlock for access to all 82 flashcards in this deck.
Unlock Deck
k this deck
73
As one moves horizontally from left to right across a period, the effective ________ charge increases, resulting in decreasing atomic radii.

Unlock Deck
Unlock for access to all 82 flashcards in this deck.
Unlock Deck
k this deck
74
A metal phosphide forms when potassium reacts with elemental phosphorus. What is the most likely formula of this metal phosphide?
A) KP
B) K3P
C) K2P3
D) K3P2
E) KP3
A) KP
B) K3P
C) K2P3
D) K3P2
E) KP3

Unlock Deck
Unlock for access to all 82 flashcards in this deck.
Unlock Deck
k this deck
75
The element _____ has the electron configuration [Rn]5f127s2.

Unlock Deck
Unlock for access to all 82 flashcards in this deck.
Unlock Deck
k this deck
76
Which of the following species has the largest radius?
A) Cl-
B) P
C) K-
D) Br-
E) Ca2+
A) Cl-
B) P
C) K-
D) Br-
E) Ca2+

Unlock Deck
Unlock for access to all 82 flashcards in this deck.
Unlock Deck
k this deck
77
A metal oxide forms when potassium reacts with oxygen. What is the most likely formula of this metal oxide?
A) KO
B) K2O
C) K2O3
D) KO2
E) KO3
A) KO
B) K2O
C) K2O3
D) KO2
E) KO3

Unlock Deck
Unlock for access to all 82 flashcards in this deck.
Unlock Deck
k this deck
78
Which group of the periodic table of elements forms only 2+ ions?
A) group 1A
B) group 2A
C) group 1B
D) group 7A
E) group 8A
A) group 1A
B) group 2A
C) group 1B
D) group 7A
E) group 8A

Unlock Deck
Unlock for access to all 82 flashcards in this deck.
Unlock Deck
k this deck
79
Explain why the first ionization energy for oxygen is lower than that for nitrogen.

Unlock Deck
Unlock for access to all 82 flashcards in this deck.
Unlock Deck
k this deck
80
According to the general trend in electron affinities, which group (or family) of elements tends to form the most stable anions in the gas phase?
A) Noble gases
B) Halogens
C) Transition metals
D) Alkaline earth metals
E) Alkali metals
A) Noble gases
B) Halogens
C) Transition metals
D) Alkaline earth metals
E) Alkali metals

Unlock Deck
Unlock for access to all 82 flashcards in this deck.
Unlock Deck
k this deck
81
Which of the following groups is a part of the s-block elements?
A) Group 2A
B) Group 6A
C) Group 8A
D) Group 7A
E) Group 5A
A) Group 2A
B) Group 6A
C) Group 8A
D) Group 7A
E) Group 5A

Unlock Deck
Unlock for access to all 82 flashcards in this deck.
Unlock Deck
k this deck
82
In the context of magnetism, Cu2+ is _____.
A) diamagnetic
B) paramagnetic
C) ferromagnetic
D) ferrimagnetic
E) antiferromagnetic
A) diamagnetic
B) paramagnetic
C) ferromagnetic
D) ferrimagnetic
E) antiferromagnetic

Unlock Deck
Unlock for access to all 82 flashcards in this deck.
Unlock Deck
k this deck


