Deck 19: Enzymes
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/74
Play
Full screen (f)
Deck 19: Enzymes
1
The rate of an enzyme-catalyzed reaction initially increases with an increase in the substrate concentration,but eventually reaches a maximum value,even though the concentration of substrate continues to increase.Which of the following best explains why?
A)As substrate concentration increases,the substrates preferentially bind with each other instead of the active site of the enzyme,and no additional catalysis occurs.
B)As substrate concentration increases,the active sites of all the enzyme molecules become occupied with substrate molecules,and no additional binding can occur.
C)An increase in substrate concentration eventually increases the temperature of the reaction mixture,and the enzyme becomes denatured.
D)An increase in substrate concentration allows for greater competition between substrate and inhibitor binding,and enzyme activity ceases.
E)When substrate concentrations become too high,local variations of pH occur that cause the enzyme to become denatured.
A)As substrate concentration increases,the substrates preferentially bind with each other instead of the active site of the enzyme,and no additional catalysis occurs.
B)As substrate concentration increases,the active sites of all the enzyme molecules become occupied with substrate molecules,and no additional binding can occur.
C)An increase in substrate concentration eventually increases the temperature of the reaction mixture,and the enzyme becomes denatured.
D)An increase in substrate concentration allows for greater competition between substrate and inhibitor binding,and enzyme activity ceases.
E)When substrate concentrations become too high,local variations of pH occur that cause the enzyme to become denatured.
As substrate concentration increases,the active sites of all the enzyme molecules become occupied with substrate molecules,and no additional binding can occur.
2
The enzyme acetylcholinesterase catalyzes the hydrolysis of acetylcholine.Which of the following is a product of this hydrolysis reaction? 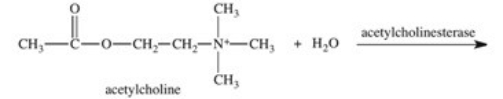
A)
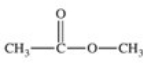
B)
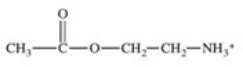
C)
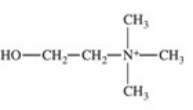
D)
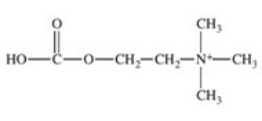
E)
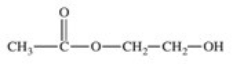

A)
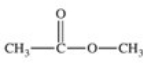
B)
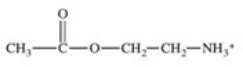
C)

D)

E)
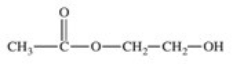

3
Trypsin is a protease enzyme that cleaves peptide bonds on the carbonyl side of basic amino acids.Which labeled bond(s)in the peptide below would be cleaved by trypsin? 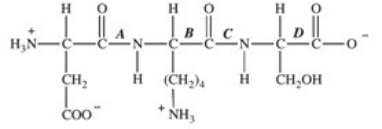
A)A
B)B
C)C
D)D
E)A,C,and D

A)A
B)B
C)C
D)D
E)A,C,and D
C
4
Acetylcholine is a neurotransmitter that transmits a signal from a nerve cell to a muscle cell,causing the muscle to contract.How is the neurotransmitter inactivated so that the muscle can relax?
A)It is denatured by the local pH and loses its active conformation.
B)It is hydrolyzed by the enzyme acetylcholinesterase.
C)It is taken up and stored by the muscle cells.
D)It is dissolved in the blood and is transported through the circulatory system.
E)All of the choices are correct.
A)It is denatured by the local pH and loses its active conformation.
B)It is hydrolyzed by the enzyme acetylcholinesterase.
C)It is taken up and stored by the muscle cells.
D)It is dissolved in the blood and is transported through the circulatory system.
E)All of the choices are correct.

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
5
Sucrase is an example of an enzyme that displays absolute specificity.What does the term "absolute specificity" indicate about an enzyme?
A)It catalyzes the reaction of only a single substrate.
B)It catalyzes the reaction of only one class of compounds.
C)It catalyzes one type of reaction for the same class of compounds.
D)It catalyzes the reaction of only a single enantiomer of a compound.
E)It catalyzes the reaction of only a single type of bond.
A)It catalyzes the reaction of only a single substrate.
B)It catalyzes the reaction of only one class of compounds.
C)It catalyzes one type of reaction for the same class of compounds.
D)It catalyzes the reaction of only a single enantiomer of a compound.
E)It catalyzes the reaction of only a single type of bond.

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
6
Proteolytic digestive enzymes are often produced in an inactive form.What is the name that refers to the inactive form of an enzyme?
A)isozyme
B)zwitterion
C)proenzyme
D)inhibitor
E)allele
A)isozyme
B)zwitterion
C)proenzyme
D)inhibitor
E)allele

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
7
Proteases,or proteolytic enzymes,are responsible for which of the following functions?
A)formation of the zwitterion form of a protein
B)hydrolysis of the ester bonds in dietary triglycerides
C)hydrolysis of the peptide bonds between amino acids in proteins
D)formation of the glycosidic linkages in disaccharides and polysaccharides
E)formation of bacterial cell walls
A)formation of the zwitterion form of a protein
B)hydrolysis of the ester bonds in dietary triglycerides
C)hydrolysis of the peptide bonds between amino acids in proteins
D)formation of the glycosidic linkages in disaccharides and polysaccharides
E)formation of bacterial cell walls

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
8
The protease enzyme chymotrypsin cleaves peptide bonds on the carbonyl side of aromatic amino acids.Which labeled bond in the peptide below would be cleaved by chymotrypsin? 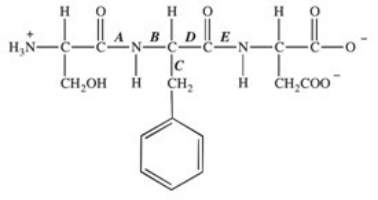
A)A
B)B
C)C
D)D
E)E

A)A
B)B
C)C
D)D
E)E

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
9
What is an allosteric enzyme?
A)an enzyme whose activity is regulated by the binding of a small effector molecule
B)the inactive form of an enzyme
C)an enzyme that has become denatured and can no longer catalyze reactions
D)an enzyme that is bound to an inhibitor molecule
E)an enzyme that catalyzes reactions in plants and animals,but not humans
A)an enzyme whose activity is regulated by the binding of a small effector molecule
B)the inactive form of an enzyme
C)an enzyme that has become denatured and can no longer catalyze reactions
D)an enzyme that is bound to an inhibitor molecule
E)an enzyme that catalyzes reactions in plants and animals,but not humans

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
10
Which of the following describes how an enzyme can affect the transition state in an enzyme-catalyzed reaction?
A)modifying the local pH by accepting or donating H+ ions
B)placing stress on a bond in the substrate,making it easier to break
C)bringing two reactants together in close proximity and in a suitable orientation for reaction
D)None of the choices are correct.
E)All of the choices are correct.
A)modifying the local pH by accepting or donating H+ ions
B)placing stress on a bond in the substrate,making it easier to break
C)bringing two reactants together in close proximity and in a suitable orientation for reaction
D)None of the choices are correct.
E)All of the choices are correct.

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
11
What term describes the pH at which an individual enzyme functions best?
A)isoelectric pH
B)pH optimum
C)physiological pH
D)neutral pH
E)zwitterion pH
A)isoelectric pH
B)pH optimum
C)physiological pH
D)neutral pH
E)zwitterion pH

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
12
The pancreatic enzymes trypsin,chymotrypsin,and elastase all hydrolyze peptide bonds on the carbonyl side of several different amino acids in a protein.What term best describes their enzyme specificity?
A)stereospecific
B)peptide
C)absolute
D)linkage
E)group
A)stereospecific
B)peptide
C)absolute
D)linkage
E)group

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
13
The diagram below shows the energy pathway of an uncatalyzed reaction.How does the energy diagram change when an enzyme is used in the same reaction? 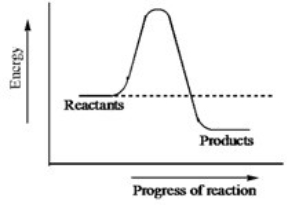
A)The energy of the products decreases.
B)The energy of the reactants increases.
C)The energy of the reactants decreases.
D)The activation energy decreases.
E)The energy of the reactants becomes equal to the energy of the products.

A)The energy of the products decreases.
B)The energy of the reactants increases.
C)The energy of the reactants decreases.
D)The activation energy decreases.
E)The energy of the reactants becomes equal to the energy of the products.

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
14
In feedback inhibition of an enzyme-catalyzed biosynthetic pathway,what frequently serves as the negative allosteric effector?
A)the starting material of the pathway
B)acetylcholine
C)oxygen
D)the final product of the pathway
E)temperature
A)the starting material of the pathway
B)acetylcholine
C)oxygen
D)the final product of the pathway
E)temperature

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
15
Which of the following statements concerning enzymes is FALSE?
A)Enzymes are the body's biological catalysts,increasing the rate of cellular reactions.
B)Enzymes are usually globular proteins with flexible three-dimensional shapes.
C)The reactant in an enzyme-catalyzed reaction is called a cofactor.
D)The active site of an enzyme is where substrate binding and catalysis occur.
E)Inhibitors are molecules that cause an enzyme to lose its activity.
A)Enzymes are the body's biological catalysts,increasing the rate of cellular reactions.
B)Enzymes are usually globular proteins with flexible three-dimensional shapes.
C)The reactant in an enzyme-catalyzed reaction is called a cofactor.
D)The active site of an enzyme is where substrate binding and catalysis occur.
E)Inhibitors are molecules that cause an enzyme to lose its activity.

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
16
About half of the 223 amino acids in the enzyme trypsin are hydrophobic.Where in the tertiary structure of this globular protein are these amino acids most likely to be found?
A)at the N-terminal end of the protein chain
B)at the C-terminal end of the protein chain
C)on the exterior surface of the folded protein
D)in the interior of the folded protein
E)in the active site of the enzyme
A)at the N-terminal end of the protein chain
B)at the C-terminal end of the protein chain
C)on the exterior surface of the folded protein
D)in the interior of the folded protein
E)in the active site of the enzyme

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
17
What three structurally similar pancreatic serine proteases function to cleave certain peptide bonds in proteins?
A)pepsinogen,zymogen,and acetylcholinesterase
B)pepsinogen,trypsin,and pepsin
C)tryptophan,trypsin,and chymotrypsin
D)trypsin,chymotrypsin,and elastase
E)choline,tryptophan,and proline
A)pepsinogen,zymogen,and acetylcholinesterase
B)pepsinogen,trypsin,and pepsin
C)tryptophan,trypsin,and chymotrypsin
D)trypsin,chymotrypsin,and elastase
E)choline,tryptophan,and proline

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
18
Elastase is a protease enzyme that cleaves peptide bonds on the carbonyl side of the amino acids glycine and alanine.What products result from treatment of a peptide with the primary structure shown below with elastase? Pro-Gly-Phe-Ala
A)the individual amino acids proline,glycine,phenylalanine,and alanine
B)Pro-Gly,phenylalanine,and alanine
C)proline,Gly-Phe,and alanine
D)Pro-Gly,and Phe-Ala
E)proline,glycine,and Phe-Ala
A)the individual amino acids proline,glycine,phenylalanine,and alanine
B)Pro-Gly,phenylalanine,and alanine
C)proline,Gly-Phe,and alanine
D)Pro-Gly,and Phe-Ala
E)proline,glycine,and Phe-Ala

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
19
Which of the following correctly describes pepsinogen?
A)It is the inactive form of the enzyme pepsin.
B)It is the cofactor for the enzyme pepsin.
C)It is the inhibitor of the enzyme pepsin.
D)It is an isoenzyme of pepsin.
E)It is the name of the reaction catalyzed by pepsin.
A)It is the inactive form of the enzyme pepsin.
B)It is the cofactor for the enzyme pepsin.
C)It is the inhibitor of the enzyme pepsin.
D)It is an isoenzyme of pepsin.
E)It is the name of the reaction catalyzed by pepsin.

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
20
Which of the following best describes the type of binding between an irreversible inhibitor and an enzyme?
A)weak
B)non-covalent
C)hydrogen bonding
D)hydrophobic interactions
E)very tight
A)weak
B)non-covalent
C)hydrogen bonding
D)hydrophobic interactions
E)very tight

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
21
Which of the following is a medical use of enzymes?
A)enzyme assays to diagnose damage or disease to a particular organ or tissues
B)injection of enzymes to counteract blood clots
C)enzyme replacement therapy for missing or malfunctioning enzymes
D)analytical reagents in the clinic laboratory
E)All of the choices are correct.
A)enzyme assays to diagnose damage or disease to a particular organ or tissues
B)injection of enzymes to counteract blood clots
C)enzyme replacement therapy for missing or malfunctioning enzymes
D)analytical reagents in the clinic laboratory
E)All of the choices are correct.

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
22
The graph shows how the rate of an enzyme-catalyzed reaction depends on the variable x.Which of the following quantities could x most likely represent? 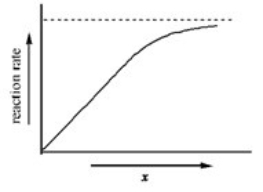
A)enzyme concentration
B)pH
C)energy
D)substrate concentration
E)progress of reaction

A)enzyme concentration
B)pH
C)energy
D)substrate concentration
E)progress of reaction

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
23
For enzyme-catalyzed reactions,what happens as the concentration of the substrate is increased?
A)The rate continues to increase as substrate concentration increases.
B)The rate increases until it reaches a maximum,constant value.
C)The rate increases until it reaches a maximum,then the rate decreases.
D)The rate decreases with increasing concentration of the substrate.
E)The rate decreases until it reaches a minimum,constant value.
A)The rate continues to increase as substrate concentration increases.
B)The rate increases until it reaches a maximum,constant value.
C)The rate increases until it reaches a maximum,then the rate decreases.
D)The rate decreases with increasing concentration of the substrate.
E)The rate decreases until it reaches a minimum,constant value.

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
24
An enzyme catalyzes the removal of an amino group from one substrate and its addition to another compound.To what class of enzymes does it belong?
A)kinase
B)hydrolase
C)transferase
D)oxidoreductase
E)lyase
A)kinase
B)hydrolase
C)transferase
D)oxidoreductase
E)lyase

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
25
What is the water-soluble vitamin from which NAD+ is made?
A)riboflavin (B2)
B)niacin (B3)
C)pyridoxine (B6)
D)cyanocobalamine (B12)
E)riboflavin (B2)and niacin (B3)are correct.
A)riboflavin (B2)
B)niacin (B3)
C)pyridoxine (B6)
D)cyanocobalamine (B12)
E)riboflavin (B2)and niacin (B3)are correct.

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
26
Which of the following is NOT a class of enzymes?
A)isomerases
B)transferases
C)carnases
D)lyases
E)ligases
A)isomerases
B)transferases
C)carnases
D)lyases
E)ligases

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
27
To which class of enzymes does an enzyme belong if it catalyzes the rearrangement of functional groups within a molecule?
A)oxidoreductases
B)lyases
C)ligases
D)isomerases
E)lyases and ligases are correct.
A)oxidoreductases
B)lyases
C)ligases
D)isomerases
E)lyases and ligases are correct.

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
28
Lactase is an enzyme that catalyzes the following reaction.Which statement describing this reaction is FALSE? 
A)Lactose is the substrate in this reaction.
B)Glucose is a product in this reaction.
C)Galactose is a cofactor in this reaction.
D)This reaction occurs in the active site of lactase.
E)Lactase is a hydrolase enzyme.

A)Lactose is the substrate in this reaction.
B)Glucose is a product in this reaction.
C)Galactose is a cofactor in this reaction.
D)This reaction occurs in the active site of lactase.
E)Lactase is a hydrolase enzyme.

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
29
What parameter of a reaction does an enzyme alter?
A)product structure
B)activation energy
C)pH
D)temperature
E)concentration
A)product structure
B)activation energy
C)pH
D)temperature
E)concentration

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
30
Which class of enzymes catalyzes reactions involving the formation of double bonds?
A)elastases
B)oxidoreductases
C)lyases
D)catalases
E)isomerases
A)elastases
B)oxidoreductases
C)lyases
D)catalases
E)isomerases

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
31
To which class of enzymes would you assign an enzyme that carries out the dehydrogenation of its substrate?
A)oxidoreductases
B)transferases
C)lyases
D)hydrolases
E)All of the choices are correct.
A)oxidoreductases
B)transferases
C)lyases
D)hydrolases
E)All of the choices are correct.

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
32
What type of specificity is displayed by an enzyme that is able to catalyze a reaction of D-glucose,but not L-glucose?
A)absolute specificity
B)stereospecificity
C)group specificity
D)optical specificity
E)sugar specificity
A)absolute specificity
B)stereospecificity
C)group specificity
D)optical specificity
E)sugar specificity

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
33
What class of enzymes catalyzes the following reaction? 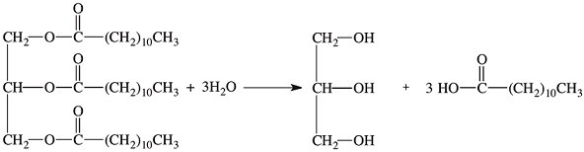
A)hydrogenase
B)catalase
C)isomerase
D)elastase
E)lipase

A)hydrogenase
B)catalase
C)isomerase
D)elastase
E)lipase

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
34
Carboxypetidase A is a highly folded,globular protein that functions as a protease enzyme.Which of the following is a likely assumption about the amino acid residues that exist on the exterior of this folded protein?
A)The majority of these amino acids have disulfide bonds between cysteine residues.
B)The majority of these amino acids are hydrophilic in nature.
C)The majority of these residues are glycine.
D)None of these residues is serine.
E)The majority of these residues are nonpolar in nature.
A)The majority of these amino acids have disulfide bonds between cysteine residues.
B)The majority of these amino acids are hydrophilic in nature.
C)The majority of these residues are glycine.
D)None of these residues is serine.
E)The majority of these residues are nonpolar in nature.

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
35
What is the effect of an enzyme on the equilibrium constant for the reaction it catalyzes?
A)It increases the equilibrium constant.
B)It decreases the equilibrium constant.
C)It may increase or decrease the equilibrium constant,depending upon the specific reaction.
D)It makes the equilibrium constant numerically equal to the reaction rate.
E)It has no effect on the equilibrium constant.
A)It increases the equilibrium constant.
B)It decreases the equilibrium constant.
C)It may increase or decrease the equilibrium constant,depending upon the specific reaction.
D)It makes the equilibrium constant numerically equal to the reaction rate.
E)It has no effect on the equilibrium constant.

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
36
Phenylketonuria (PKU)is an inherited disease caused by the inability to produce a properly working form of the enzyme phenylalanine hydroxylase.This enzyme catalyzes the conversion of the amino acid phenylalanine to the amino acid tyrosine by adding a hydroxyl group to the para position on the ring.Based on this information,what is the structure of tyrosine (Tyr)? 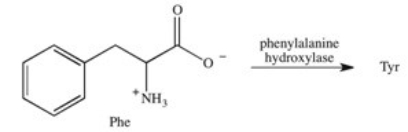
A)
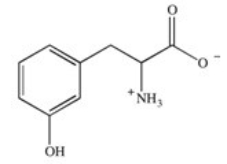
B)

C)
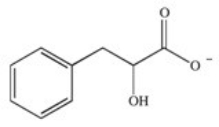
D)
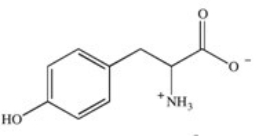
E)
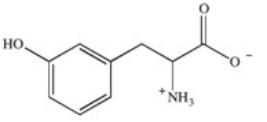

A)

B)

C)

D)

E)


Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
37
What portion of an enzyme is in direct contact with the substrate?
A)the inert site
B)the alpha site
C)the beta site
D)the delta site
E)the active site
A)the inert site
B)the alpha site
C)the beta site
D)the delta site
E)the active site

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
38
At extremes of pH,an enzyme will lose its biologically active conformation.What term describes the state of the enzyme under these conditions?
A)denatured
B)acidic
C)basic
D)buffered
E)noncovalent
A)denatured
B)acidic
C)basic
D)buffered
E)noncovalent

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
39
What is the classification of an enzyme that catalyzes the joining of two molecules?
A)kinase
B)ligase
C)oxidoreductase
D)transferase
E)hydrolase
A)kinase
B)ligase
C)oxidoreductase
D)transferase
E)hydrolase

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
40
What is the first step in an enzyme-catalyzed reaction?
A)catalysis of enzyme
B)dimerizing the substrate
C)ionization of enzyme
D)decrease of pH
E)formation of an enzyme-substrate complex
A)catalysis of enzyme
B)dimerizing the substrate
C)ionization of enzyme
D)decrease of pH
E)formation of an enzyme-substrate complex

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
41
What type of enzyme regulation occurs when the final product of a pathway shuts off the entire pathway for its own synthesis?
A)irreversible inhibition
B)competitive inhibition
C)feedback inhibition
D)poisoning
E)noncompetitive inhibition
A)irreversible inhibition
B)competitive inhibition
C)feedback inhibition
D)poisoning
E)noncompetitive inhibition

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
42
Which statement concerning an enzyme is true?
A)An enzyme increases the size of the equilibrium constant of the reaction it catalyzes.
B)An enzyme decreases the size of the equilibrium constant of the reaction it catalyzes.
C)An enzyme shifts the equilibrium so that more product is formed when equilibrium is reached.
D)An enzyme increases the rate of a chemical reaction by lowering the activation energy.
E)An enzyme slows down reactions that occur too rapidly to be effective in the body.
A)An enzyme increases the size of the equilibrium constant of the reaction it catalyzes.
B)An enzyme decreases the size of the equilibrium constant of the reaction it catalyzes.
C)An enzyme shifts the equilibrium so that more product is formed when equilibrium is reached.
D)An enzyme increases the rate of a chemical reaction by lowering the activation energy.
E)An enzyme slows down reactions that occur too rapidly to be effective in the body.

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
43
Many enzymes are regulated by having more than one binding site.What is the term used to describe these enzymes?
A)allosteric enzymes
B)lipases
C)proenzymes
D)lyases
E)isomerases
A)allosteric enzymes
B)lipases
C)proenzymes
D)lyases
E)isomerases

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
44
In 1956,Christian de Duve discovered organelles in the cytoplasm of certain cells that have hydrolytic enzymes that destroy bacteria and viruses.What are these organelles called?
A)lysosomes
B)mitochondria
C)endosomes
D)ribosomes
E)nucleosomes
A)lysosomes
B)mitochondria
C)endosomes
D)ribosomes
E)nucleosomes

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
45
Some enzymes require an additional nonprotein prosthetic group in order to function.What is the protein part of this combination called?
A)substrate
B)apoenzyme
C)effector
D)cofactor
E)holoenzyme
A)substrate
B)apoenzyme
C)effector
D)cofactor
E)holoenzyme

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
46
The arrival of a nerve impulse at the end plate of the nerve axon results in an influx of what ion?
A)calcium
B)sodium
C)potassium
D)bicarbonate
E)phosphate
A)calcium
B)sodium
C)potassium
D)bicarbonate
E)phosphate

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
47
The graph shows how the rate of an enzyme-catalyzed reaction depends on the variable x.Which of the following quantities could x most likely represent? 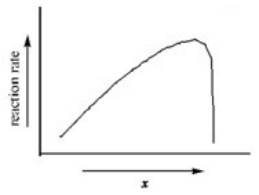
A)enzyme concentration
B)energy
C)temperature
D)substrate concentration
E)progress of reaction

A)enzyme concentration
B)energy
C)temperature
D)substrate concentration
E)progress of reaction

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
48
A holoenzyme is a functional enzyme composed of what two parts?
A)apoenzyme and cofactor
B)amino acid and NAD+
C)substrate and catalyst
D)transferase and ligase
E)substrate and inhibitor
A)apoenzyme and cofactor
B)amino acid and NAD+
C)substrate and catalyst
D)transferase and ligase
E)substrate and inhibitor

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
49
The enzyme hexokinase catalyzes the phosphorylation of glucose according to the reaction shown below.Glucosamine is a competitive inhibitor of hexokinase.Which of the following best describes the inhibition by glucosamine? 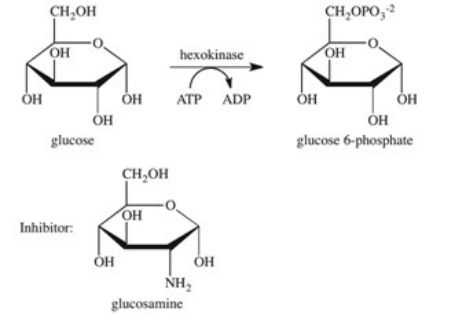
A)Glucosamine stabilizes the active site of the enzyme,preventing glucose-6-phosphate from being released.
B)Glucosamine reacts with glucose,preventing it from binding to the active site of the enzyme.
C)Glucosamine binds to the active site of the enzyme,preventing glucose from binding.
D)Glucosamine binds to the surface of the enzyme,causing a change in the shape of both the enzyme and the active site,preventing glucose from binding.
E)Glucosamine reacts with glucose-6-phosphate,preventing it from being released from the active site.

A)Glucosamine stabilizes the active site of the enzyme,preventing glucose-6-phosphate from being released.
B)Glucosamine reacts with glucose,preventing it from binding to the active site of the enzyme.
C)Glucosamine binds to the active site of the enzyme,preventing glucose from binding.
D)Glucosamine binds to the surface of the enzyme,causing a change in the shape of both the enzyme and the active site,preventing glucose from binding.
E)Glucosamine reacts with glucose-6-phosphate,preventing it from being released from the active site.

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
50
HIV protease inhibitors block the activity of enzymes responsible for which of the following?
A)viral replication
B)digestion
C)oxygen transport
D)antibiotic activity
E)pain production
A)viral replication
B)digestion
C)oxygen transport
D)antibiotic activity
E)pain production

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
51
What is the function of a lyase enzyme?
A)to catalyze redox reactions
B)to catalyze the transfer of a functional group from one molecule to another
C)to catalyze hydrolysis reactions
D)to catalyze the addition of a group to a double bond or the removal of a group to form a double bond
E)to catalyze the formation or cleavage of a C-C,C-S,C-O,or C-N bond
A)to catalyze redox reactions
B)to catalyze the transfer of a functional group from one molecule to another
C)to catalyze hydrolysis reactions
D)to catalyze the addition of a group to a double bond or the removal of a group to form a double bond
E)to catalyze the formation or cleavage of a C-C,C-S,C-O,or C-N bond

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
52
Most cellular enzymes work optimally at what pH?
A)2
B)5
C)7
D)9
E)12
A)2
B)5
C)7
D)9
E)12

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
53
A second binding site on an allosteric enzyme has the ability to bind to a molecule that alters the shape of the active site.What is this second site called?
A)structural analog binding site
B)effector binding site
C)feedback binding site
D)coenzyme binding site
E)cofactor binding site
A)structural analog binding site
B)effector binding site
C)feedback binding site
D)coenzyme binding site
E)cofactor binding site

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
54
Of the following types of molecular interactions,which is NOT likely to be involved in the binding of an enzyme to a substrate?
A)covalent linkages
B)hydrogen bonds
C)van der Waals attractions
D)polar-polar attractions
E)ionic attractions
A)covalent linkages
B)hydrogen bonds
C)van der Waals attractions
D)polar-polar attractions
E)ionic attractions

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
55
What term refers to an organic molecule that binds transiently to an enzyme and participates in the reaction by either accepting or donating a chemical group?
A)apoenzyme
B)cofactor
C)effector
D)coenzyme
E)analog
A)apoenzyme
B)cofactor
C)effector
D)coenzyme
E)analog

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
56
The penicillin class of antibiotics is inhibitors of an enzyme responsible for the formation of bacterial cell walls.The penicillin covalently binds to a serine residue in the active site of the enzyme,as depicted below.What type of bond is formed between the penicillin and the active site? 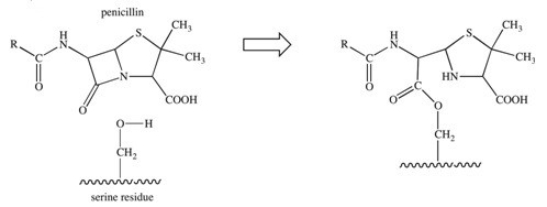
A)hydrogen bond
B)ionic bond
C)glycosidic linkage
D)ester bond
E)acetal bond

A)hydrogen bond
B)ionic bond
C)glycosidic linkage
D)ester bond
E)acetal bond

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
57
Some enzymes require an additional nonprotein prosthetic group in order to function.What is the nonprotein part of this combination called?
A)apoenzyme
B)effector
C)mineral
D)cofactor
E)vitamin
A)apoenzyme
B)effector
C)mineral
D)cofactor
E)vitamin

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
58
The enzyme fumarase is a lyase enzyme that catalyzes the hydration of fumarate to give malate.What is the structure of malate? 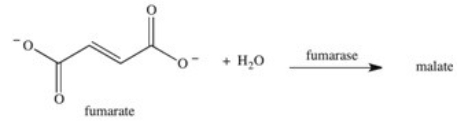
A)
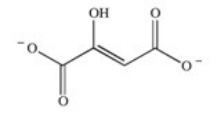
B)
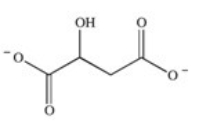
C)
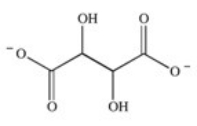
D)
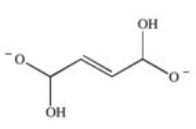
E)
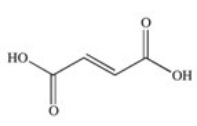
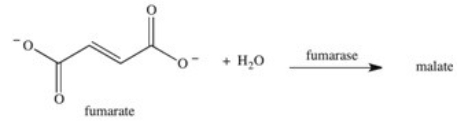
A)

B)

C)

D)

E)


Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
59
Which of the following is true for an enzyme catalyzed reaction?
A)The rate of the reaction will increase indefinitely with an increase in the substrate concentration.
B)The rate of the reaction will increase with an increase in the substrate concentration until it reaches a maximum rate.
C)The rate of the reaction will decrease as the substrate concentration increases.
D)The rate of the reaction is unaffected by substrate concentration.
E)The rate of the reaction decreases as the amount of enzyme available increases.
A)The rate of the reaction will increase indefinitely with an increase in the substrate concentration.
B)The rate of the reaction will increase with an increase in the substrate concentration until it reaches a maximum rate.
C)The rate of the reaction will decrease as the substrate concentration increases.
D)The rate of the reaction is unaffected by substrate concentration.
E)The rate of the reaction decreases as the amount of enzyme available increases.

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
60
What is the term for a molecule that is similar in structure and charge distribution to the substrate in an enzyme-catalyzed reaction?
A)effector
B)transition state
C)structural analog
D)irreversible inhibitor
E)noncompetitive inhibitor
A)effector
B)transition state
C)structural analog
D)irreversible inhibitor
E)noncompetitive inhibitor

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
61
The presence of an enzyme will alter the relative ratio of product to reactant for a biochemical reaction.

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
62
Chymotrypsin catalyzes the hydrolysis of dietary carbohydrates.

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
63
Irreversible inhibitors bind very tightly,and sometimes covalently,to enzymes.

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
64
The presence of an enzyme catalyst will affect the time taken for a reaction to reach equilibrium.

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
65
The vitamin biotin is a component of a coenzyme.

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
66
Which of the following describes the function of the enzyme cis-trans isomerase?
A)It breaks the double bond in an unsaturated fatty acid.
B)It converts pentose sugars to hexose sugars.
C)It converts between isomers on a disubstituted benzene ring.
D)It converts between the boat and chair conformations of a cyclohexane.
E)It breaks and re-forms a double bond to change the arrangement of the groups around a double bond.
A)It breaks the double bond in an unsaturated fatty acid.
B)It converts pentose sugars to hexose sugars.
C)It converts between isomers on a disubstituted benzene ring.
D)It converts between the boat and chair conformations of a cyclohexane.
E)It breaks and re-forms a double bond to change the arrangement of the groups around a double bond.

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
67
Binding of a negative effector converts an active site on an enzyme into the active configuration.

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
68
The rates of enzyme-catalyzed reactions increase with increasing pH.

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
69
Many enzymes are destroyed if the temperature rises significantly above 37ºC.

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
70
Which of the following will NOT destroy an enzyme by denaturation?
A)increase in temperature above 37°C (body temperature)
B)decrease in temperature below 37°C
C)significant increase in pH
D)significant decrease in pH
E)All of the choices will destroy an enzyme.
A)increase in temperature above 37°C (body temperature)
B)decrease in temperature below 37°C
C)significant increase in pH
D)significant decrease in pH
E)All of the choices will destroy an enzyme.

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
71
A proenzyme is an inactive form of an enzyme.

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
72
Arsenic is an example of an irreversible enzyme inhibitor.

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
73
Isomerases rearrange the functional groups within a molecule.

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
74
Hydrochloric acid in the stomach is partially responsible for activating pepsinogen.

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck


