Deck 10: An Introduction to Organic Chemistry
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/104
Play
Full screen (f)
Deck 10: An Introduction to Organic Chemistry
1
Which alkyl group is a secondary alkyl group?
A)
B)
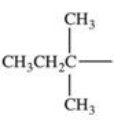
C)
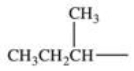
D)
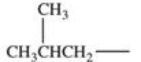
E)All of these are correct.
A)
B)

C)

D)
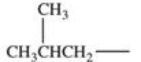
E)All of these are correct.

2
Which type of compound is NOT classified as an aliphatic hydrocarbon?
A)alkyne
B)aromatic
C)cycloalkane
D)alkene
E)alkane
A)alkyne
B)aromatic
C)cycloalkane
D)alkene
E)alkane
aromatic
3
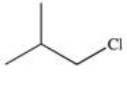
Which of the following is 1-chloro-3-methylbutane?
A)
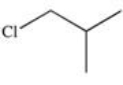
B)
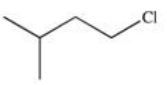
C)
D)
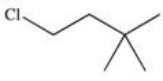
E)
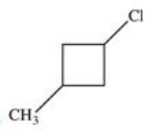
A)

B)

C)

D)

E)


4
Which statement concerning the two compounds shown below is FALSE? 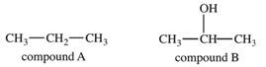
A)Compound A and compound B are both organic compounds.
B)Compound A and compound B are both hydrocarbons.
C)Compound B has stronger intermolecular forces than compound A.
D)Compound A is likely a gas at room temperature and compound B is likely a liquid at room temperature.
E)Compound B has a higher boiling point than compound A.
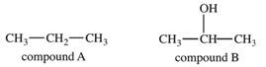
A)Compound A and compound B are both organic compounds.
B)Compound A and compound B are both hydrocarbons.
C)Compound B has stronger intermolecular forces than compound A.
D)Compound A is likely a gas at room temperature and compound B is likely a liquid at room temperature.
E)Compound B has a higher boiling point than compound A.

Unlock Deck
Unlock for access to all 104 flashcards in this deck.
Unlock Deck
k this deck
5
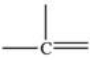
Which bonding pattern is NOT typical of carbon atoms in organic compounds?
A)
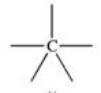
B)
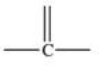
C)
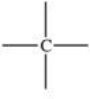
D)
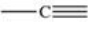
E)
A)

B)

C)

D)
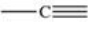
E)


Unlock Deck
Unlock for access to all 104 flashcards in this deck.
Unlock Deck
k this deck
6
The carbonyl group is a functional group found in many different types of compounds.What is the general structure of the carbonyl group?
A)
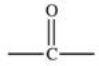
B)
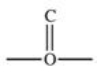
C)
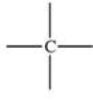
D)
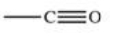
E)
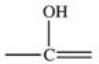
A)
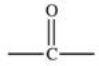
B)

C)

D)
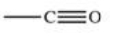
E)


Unlock Deck
Unlock for access to all 104 flashcards in this deck.
Unlock Deck
k this deck
7
Which of the following compounds would be named 3-ethyl-2-methylhexane?
A)
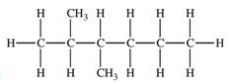
B)
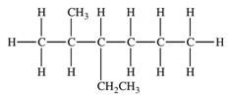
C)
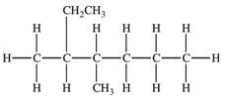
D)
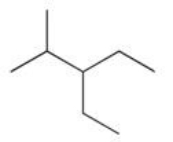
E)(CH3)2CHCH2CH2(CH2CH3)2
A)

B)

C)

D)

E)(CH3)2CHCH2CH2(CH2CH3)2

Unlock Deck
Unlock for access to all 104 flashcards in this deck.
Unlock Deck
k this deck
8
Benzene may be represented by the line formula below.What is the molecular formula of benzene? 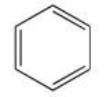
A)C12H6
B)C6H12
C)C6H6
D)C6H14
E)C6

A)C12H6
B)C6H12
C)C6H6
D)C6H14
E)C6

Unlock Deck
Unlock for access to all 104 flashcards in this deck.
Unlock Deck
k this deck
9
Which correctly describes the geometry and bond angles around the indicated carbon in the structure below? 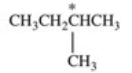
A)trigonal planar; 120°
B)tetrahedral,109.5°
C)trigonal pyramidal,109.5°
D)tetrahedral,90°
E)tetravalent,120°

A)trigonal planar; 120°
B)tetrahedral,109.5°
C)trigonal pyramidal,109.5°
D)tetrahedral,90°
E)tetravalent,120°

Unlock Deck
Unlock for access to all 104 flashcards in this deck.
Unlock Deck
k this deck
10
Which compound is classified as an ether?
A)
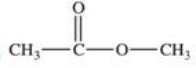
B)
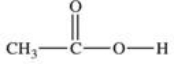
C)
D)
E)
A)
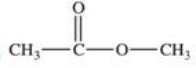
B)
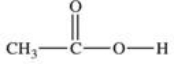
C)
D)
E)

Unlock Deck
Unlock for access to all 104 flashcards in this deck.
Unlock Deck
k this deck
11
The functional group shown below is found in what type of compound? 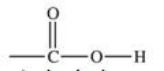
A)alcohols
B)ketones
C)carboxylic acids
D)esters
E)aldehydes
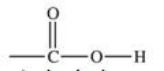
A)alcohols
B)ketones
C)carboxylic acids
D)esters
E)aldehydes

Unlock Deck
Unlock for access to all 104 flashcards in this deck.
Unlock Deck
k this deck
12
Which of the following best describes molecules related as conformational isomers?
A)same molecular formulas but different bonding patterns between atoms
B)different molecular formulas but the same connections between atoms
C)same molecular formulas,the same connections between atoms,but different spatial arrangement of atoms due to rotation around bonds
D)same molecular formulas but different cis-trans arrangement of atoms
E)different molecular formulas and different bonding patterns
A)same molecular formulas but different bonding patterns between atoms
B)different molecular formulas but the same connections between atoms
C)same molecular formulas,the same connections between atoms,but different spatial arrangement of atoms due to rotation around bonds
D)same molecular formulas but different cis-trans arrangement of atoms
E)different molecular formulas and different bonding patterns

Unlock Deck
Unlock for access to all 104 flashcards in this deck.
Unlock Deck
k this deck
13
Consider the compound 4-ethyl-2,2-dimethylhexane,shown below.What classification best describes the fourth carbon atom in this six-carbon parent chain? 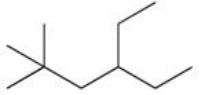
A)primary
B)secondary
C)tertiary
D)quaternary
E)It is impossible to determine without knowing the structural formula.

A)primary
B)secondary
C)tertiary
D)quaternary
E)It is impossible to determine without knowing the structural formula.

Unlock Deck
Unlock for access to all 104 flashcards in this deck.
Unlock Deck
k this deck
14
What is the classification of the carbon atom that is bonded to the -OH group in menthol? 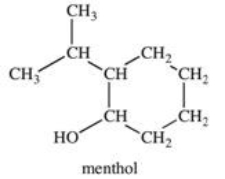
A)primary
B)secondary
C)tertiary
D)quaternary
E)pentary

A)primary
B)secondary
C)tertiary
D)quaternary
E)pentary

Unlock Deck
Unlock for access to all 104 flashcards in this deck.
Unlock Deck
k this deck
15
What is the name of the compound whose condensed formula is CH3(CH2)5CH3?
A)propane
B)pentane
C)hexane
D)heptane
E)1,5-dimethylpentane
A)propane
B)pentane
C)hexane
D)heptane
E)1,5-dimethylpentane

Unlock Deck
Unlock for access to all 104 flashcards in this deck.
Unlock Deck
k this deck
16
Which of the following is NOT a characteristic associated with undecane,an 11-carbon straight chain alkane?
A)hydrogen bonding
B)low water solubility
C)density less than water
D)flammable liquid
E)nonpolar
A)hydrogen bonding
B)low water solubility
C)density less than water
D)flammable liquid
E)nonpolar

Unlock Deck
Unlock for access to all 104 flashcards in this deck.
Unlock Deck
k this deck
17
An alkane used as a lubricant contains 25 carbon atoms.How many hydrogen atoms are present in its structure?
A)25
B)27
C)50
D)52
E)It is impossible to predict.
A)25
B)27
C)50
D)52
E)It is impossible to predict.

Unlock Deck
Unlock for access to all 104 flashcards in this deck.
Unlock Deck
k this deck
18
How does an alkyl group differ from its parent alkane?
A)An alkyl group has a larger structure than its parent alkane.
B)An alkyl group contains one more hydrogen atom than its parent alkane.
C)An alkyl group contains one less carbon atom than its parent alkane.
D)An alkyl group contains one less hydrogen atom than its parent alkane.
E)There is no difference between an alkyl group and its parent alkane.
A)An alkyl group has a larger structure than its parent alkane.
B)An alkyl group contains one more hydrogen atom than its parent alkane.
C)An alkyl group contains one less carbon atom than its parent alkane.
D)An alkyl group contains one less hydrogen atom than its parent alkane.
E)There is no difference between an alkyl group and its parent alkane.

Unlock Deck
Unlock for access to all 104 flashcards in this deck.
Unlock Deck
k this deck
19
Which of the following statements about organic compounds is FALSE?
A)Organic compounds are only synthesized by living systems.
B)Organic compounds are carbon-containing compounds.
C)Organic compounds contain covalent bonds.
D)The carbon atoms in organic compounds may be arranged in chains or rings.
E)Structural subunits called functional groups are responsible for the chemical behavior of organic compounds.
A)Organic compounds are only synthesized by living systems.
B)Organic compounds are carbon-containing compounds.
C)Organic compounds contain covalent bonds.
D)The carbon atoms in organic compounds may be arranged in chains or rings.
E)Structural subunits called functional groups are responsible for the chemical behavior of organic compounds.

Unlock Deck
Unlock for access to all 104 flashcards in this deck.
Unlock Deck
k this deck
20
The structure of an organic compound may be represented in several different ways.Which formula for an organic compound explicitly shows all of the atoms and bonds in a molecule?
A)molecular formula
B)condensed formula
C)structural formula
D)patterned formula
E)line formula
A)molecular formula
B)condensed formula
C)structural formula
D)patterned formula
E)line formula

Unlock Deck
Unlock for access to all 104 flashcards in this deck.
Unlock Deck
k this deck
21
The large number of organic compounds is due in part to the ability of these compounds to form constitutional isomers.Which of the following pairs of compounds are related as constitutional isomers?
A)

B)
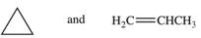
C)

D)

E)
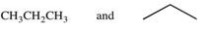
A)

B)
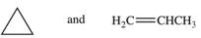
C)

D)

E)
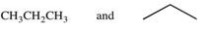

Unlock Deck
Unlock for access to all 104 flashcards in this deck.
Unlock Deck
k this deck
22
The name of an organic compound is 2-methyl-4-propyloctane.What information about the structure of this compound is FALSE?
A)It is a branched alkane.
B)There are eight carbons in the parent chain.
C)There is a one-carbon alkyl group on carbon 2 of the parent chain.
D)There is a three-carbon alkyl group on carbon 4 of the parent chain.
E)None of the above statements is false.
A)It is a branched alkane.
B)There are eight carbons in the parent chain.
C)There is a one-carbon alkyl group on carbon 2 of the parent chain.
D)There is a three-carbon alkyl group on carbon 4 of the parent chain.
E)None of the above statements is false.

Unlock Deck
Unlock for access to all 104 flashcards in this deck.
Unlock Deck
k this deck
23
What is the molecular formula of the simplest alkane capable of having constitutional isomers?
A)CH4
B)C2H6
C)C3H8
D)C4H10
E)C5H12
A)CH4
B)C2H6
C)C3H8
D)C4H10
E)C5H12

Unlock Deck
Unlock for access to all 104 flashcards in this deck.
Unlock Deck
k this deck
24
A cycloalkane molecule contains 8 carbon atoms.How many hydrogen atoms are present in the molecule?
A)4
B)8
C)14
D)16
E)18
A)4
B)8
C)14
D)16
E)18

Unlock Deck
Unlock for access to all 104 flashcards in this deck.
Unlock Deck
k this deck
25
The compound shown below can be made from the halogenation reaction of what compound? 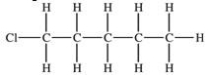
A)propane
B)hexane
C)pentane
D)benzene
E)1-methylbutane

A)propane
B)hexane
C)pentane
D)benzene
E)1-methylbutane

Unlock Deck
Unlock for access to all 104 flashcards in this deck.
Unlock Deck
k this deck
26
What is the proper name for the compound shown below? 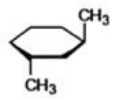
A)dimethylcyclohexane
B)trans-1,3-methylhexane
C)1-methyl-3-methyl-cis-cyclohexane
D)cis-1,3-dimethylcyclohexane
E)trans-1,3-dimethylcyclohexane

A)dimethylcyclohexane
B)trans-1,3-methylhexane
C)1-methyl-3-methyl-cis-cyclohexane
D)cis-1,3-dimethylcyclohexane
E)trans-1,3-dimethylcyclohexane

Unlock Deck
Unlock for access to all 104 flashcards in this deck.
Unlock Deck
k this deck
27
What class of organic compounds contains the structure shown below? 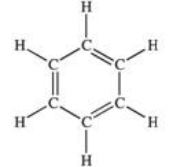
A)aliphatic
B)isotopic
C)alkene
D)alkyne
E)aromatic

A)aliphatic
B)isotopic
C)alkene
D)alkyne
E)aromatic

Unlock Deck
Unlock for access to all 104 flashcards in this deck.
Unlock Deck
k this deck
28
Traces of chloroform,CHCl3,were found in the trunk of a car of a Florida woman who was charged in the death of her child.Which of the following best represents the order of attachment of the atoms in chloroform?
A)
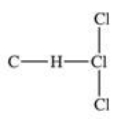
B)
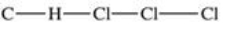
C)
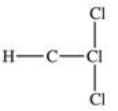
D)
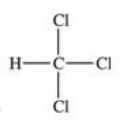
E)None of the choices are correct.
A)

B)
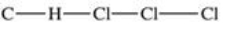
C)

D)

E)None of the choices are correct.

Unlock Deck
Unlock for access to all 104 flashcards in this deck.
Unlock Deck
k this deck
29
How does the molecular formula of a cycloalkane differ from that of the corresponding alkane?
A)The alkane has two fewer hydrogen atoms in its formula.
B)The cycloalkane has one fewer hydrogen atom in its formula.
C)The cycloalkane has one fewer carbon in its formula.
D)The cycloalkane has two fewer hydrogen atoms in its formula.
E)The alkane has two more carbon atoms in its formula.
A)The alkane has two fewer hydrogen atoms in its formula.
B)The cycloalkane has one fewer hydrogen atom in its formula.
C)The cycloalkane has one fewer carbon in its formula.
D)The cycloalkane has two fewer hydrogen atoms in its formula.
E)The alkane has two more carbon atoms in its formula.

Unlock Deck
Unlock for access to all 104 flashcards in this deck.
Unlock Deck
k this deck
30
What is the structure of trans-1,3-dibromocyclohexane?
A)
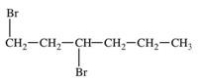
B)
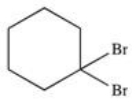
C)
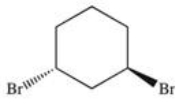
D)
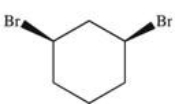
E)
A)

B)

C)

D)

E)


Unlock Deck
Unlock for access to all 104 flashcards in this deck.
Unlock Deck
k this deck
31
What type(s)of carbon-carbon bonds are never found in a saturated hydrocarbon?
A)single bonds
B)single and double bonds
C)double and triple bonds
D)single,double,and triple bonds
E)None of the choices are correct.
A)single bonds
B)single and double bonds
C)double and triple bonds
D)single,double,and triple bonds
E)None of the choices are correct.

Unlock Deck
Unlock for access to all 104 flashcards in this deck.
Unlock Deck
k this deck
32
Butanone (structure shown)may be classified as what type of compound? 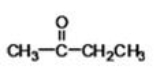
A)aldehyde
B)ketone
C)ether
D)carboxylic acid
E)ester

A)aldehyde
B)ketone
C)ether
D)carboxylic acid
E)ester

Unlock Deck
Unlock for access to all 104 flashcards in this deck.
Unlock Deck
k this deck
33
How many constitutional isomers are possible for the molecular formula C5H12?
A)one
B)three
C)four
D)five
E)six
A)one
B)three
C)four
D)five
E)six

Unlock Deck
Unlock for access to all 104 flashcards in this deck.
Unlock Deck
k this deck
34
How do the different conformations of an alkane molecule arise?
A)through C-C bond cleavage
B)by rotation about C-C single bonds
C)through oxidation of C-C bonds
D)by rearrangement of the order of attachment of atoms in the molecule
E)by combustion and halogenation reactions
A)through C-C bond cleavage
B)by rotation about C-C single bonds
C)through oxidation of C-C bonds
D)by rearrangement of the order of attachment of atoms in the molecule
E)by combustion and halogenation reactions

Unlock Deck
Unlock for access to all 104 flashcards in this deck.
Unlock Deck
k this deck
35
Which of the following statements concerning conformational isomers is FALSE?
A)Conformational isomers are different spatial arrangements of the same molecule,formed by rotation around single bonds.
B)Conformational isomers rapidly interconvert,and cannot be physically separated from each other.
C)The staggered and the eclipsed conformation are two types of conformational isomers of alkanes.
D)The staggered conformation is more stable than the eclipsed conformation,because in the staggered conformation electron repulsion is maximized.
E)The boat conformation and the chair conformation are two conformational isomers of cyclohexane; the chair conformation is the more stable of the two.
A)Conformational isomers are different spatial arrangements of the same molecule,formed by rotation around single bonds.
B)Conformational isomers rapidly interconvert,and cannot be physically separated from each other.
C)The staggered and the eclipsed conformation are two types of conformational isomers of alkanes.
D)The staggered conformation is more stable than the eclipsed conformation,because in the staggered conformation electron repulsion is maximized.
E)The boat conformation and the chair conformation are two conformational isomers of cyclohexane; the chair conformation is the more stable of the two.

Unlock Deck
Unlock for access to all 104 flashcards in this deck.
Unlock Deck
k this deck
36
The chair and boat conformations of cyclohexane are shown below.Which statement best explains why the chair conformation is more stable than the boat conformation? 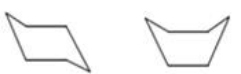
A)The hydrogen atoms in the chair conformation are all staggered,which minimizes the repulsion of the bonding electrons.
B)The carbon atoms in the chair conformation are all eclipsed,which provides for the ideal bond angle of 109.5°.
C)The carbon atoms in the chair conformation are closer together than in the boat conformation,which provides for stronger C-C bonds.
D)The hydrogen atoms in the chair conformation are closer together than in the boat conformation,which provides for stronger C-H bonds.
E)The C-C bonds in the chair conformation are all staggered,which maximizes the energy of the molecule.
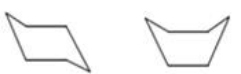
A)The hydrogen atoms in the chair conformation are all staggered,which minimizes the repulsion of the bonding electrons.
B)The carbon atoms in the chair conformation are all eclipsed,which provides for the ideal bond angle of 109.5°.
C)The carbon atoms in the chair conformation are closer together than in the boat conformation,which provides for stronger C-C bonds.
D)The hydrogen atoms in the chair conformation are closer together than in the boat conformation,which provides for stronger C-H bonds.
E)The C-C bonds in the chair conformation are all staggered,which maximizes the energy of the molecule.

Unlock Deck
Unlock for access to all 104 flashcards in this deck.
Unlock Deck
k this deck
37
What term is used to describe the various spatial arrangements of a molecule formed by rotation around C-C single bonds?
A)geometric isomers
B)conformations
C)constitutional isomers
D)enantiomers
E)structural isomers
A)geometric isomers
B)conformations
C)constitutional isomers
D)enantiomers
E)structural isomers

Unlock Deck
Unlock for access to all 104 flashcards in this deck.
Unlock Deck
k this deck
38
Which of the following properly describes constitutional isomers?
A)organic compounds with different molecular formulas
B)compounds with the same molecular formula,but a different order of attachment of atoms
C)compounds with the same molecular formula and order of attachment of atoms,but a different spatial arrangement of atoms
D)compounds with the same number of hydrogens,but a different number of carbons
E)compounds with the same number of carbons,but a different number of hydrogens
A)organic compounds with different molecular formulas
B)compounds with the same molecular formula,but a different order of attachment of atoms
C)compounds with the same molecular formula and order of attachment of atoms,but a different spatial arrangement of atoms
D)compounds with the same number of hydrogens,but a different number of carbons
E)compounds with the same number of carbons,but a different number of hydrogens

Unlock Deck
Unlock for access to all 104 flashcards in this deck.
Unlock Deck
k this deck
39
Eicosane is the name of the organic compound with the condensed formula CH3(CH2)18CH3.What does the prefix "eicos" specifically indicate about the structure of this compound?
A)It is an alkane.
B)It is a saturated hydrocarbon.
C)It contains a straight chain of carbons.
D)It is aliphatic.
E)It contains 20 carbons in a continuous chain.
A)It is an alkane.
B)It is a saturated hydrocarbon.
C)It contains a straight chain of carbons.
D)It is aliphatic.
E)It contains 20 carbons in a continuous chain.

Unlock Deck
Unlock for access to all 104 flashcards in this deck.
Unlock Deck
k this deck
40
What additional condition or reagent is necessary for methane to react with chlorine according to the reaction equation shown below? CH4 + Cl2 → CH3Cl + HCl
A)enzyme
B)water
C)light or high temperature
D)acid or base
E)complete darkness
A)enzyme
B)water
C)light or high temperature
D)acid or base
E)complete darkness

Unlock Deck
Unlock for access to all 104 flashcards in this deck.
Unlock Deck
k this deck
41
What is the IUPAC name of the following compound? 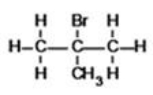
A)2-bromo-2-methylpropane
B)2-bromoisobutane
C)2,2-dibromomethylpropane
D)2-methyl-2-bromopropane
E)bromomethylbutane

A)2-bromo-2-methylpropane
B)2-bromoisobutane
C)2,2-dibromomethylpropane
D)2-methyl-2-bromopropane
E)bromomethylbutane

Unlock Deck
Unlock for access to all 104 flashcards in this deck.
Unlock Deck
k this deck
42
What is the correct classification for the compound shown below? 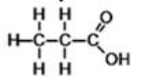
A)alcohol
B)aldehyde
C)carboxylic acid
D)ester
E)ketone

A)alcohol
B)aldehyde
C)carboxylic acid
D)ester
E)ketone

Unlock Deck
Unlock for access to all 104 flashcards in this deck.
Unlock Deck
k this deck
43
In 1828,Friedrich Wöhler carried out the first synthesis of an organic compound from inorganic substances.What compound did he accidentally make?
A)glucose
B)urea
C)ammonium cyanate
D)aspirin
E)formaldehyde
A)glucose
B)urea
C)ammonium cyanate
D)aspirin
E)formaldehyde

Unlock Deck
Unlock for access to all 104 flashcards in this deck.
Unlock Deck
k this deck
44
Which of the following is NOT a typical property of inorganic compounds?
A)high boiling point
B)flammable
C)water-soluble
D)rapid reactions
E)high melting point
A)high boiling point
B)flammable
C)water-soluble
D)rapid reactions
E)high melting point

Unlock Deck
Unlock for access to all 104 flashcards in this deck.
Unlock Deck
k this deck
45
The reaction shown below can be used to prepare alkyl halides.Which of the following best describes the type of reaction illustrated? 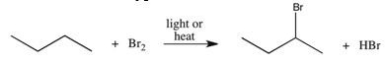
A)addition
B)substitution
C)elimination
D)combustion
E)condensation

A)addition
B)substitution
C)elimination
D)combustion
E)condensation

Unlock Deck
Unlock for access to all 104 flashcards in this deck.
Unlock Deck
k this deck
46
What is the balanced equation for the complete combustion of cyclooctane?
A)C8H18 + 12O2 → 8CO2 + 9H2O
B)C8H16 + O2 → CO2 + H2O
C)2C8H12 + 24O2 → 16CO2 + 12H2O
D)C8H16 + 12O2 → 8CO2 + 8H2O
E)C8H16 + 8O2 → 8CO + 8H2O
A)C8H18 + 12O2 → 8CO2 + 9H2O
B)C8H16 + O2 → CO2 + H2O
C)2C8H12 + 24O2 → 16CO2 + 12H2O
D)C8H16 + 12O2 → 8CO2 + 8H2O
E)C8H16 + 8O2 → 8CO + 8H2O

Unlock Deck
Unlock for access to all 104 flashcards in this deck.
Unlock Deck
k this deck
47
Which compound is NOT an isomer with the molecular formula C5H12?
A)

B)
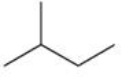
C)

D)
E)All of these are correct.
A)

B)

C)

D)

E)All of these are correct.

Unlock Deck
Unlock for access to all 104 flashcards in this deck.
Unlock Deck
k this deck
48
What functional groups are shown below? 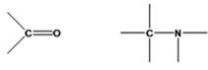
A)alkoxy,amino
B)carbonyl,aldehyde
C)hydroxyl,ammonia
D)carbonyl,amino
E)None of the choices are correct.
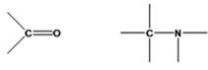
A)alkoxy,amino
B)carbonyl,aldehyde
C)hydroxyl,ammonia
D)carbonyl,amino
E)None of the choices are correct.

Unlock Deck
Unlock for access to all 104 flashcards in this deck.
Unlock Deck
k this deck
49
Functional groups are primarily responsible for the chemical properties of molecules in which they are found.Which of the following compounds have the same functional group,and would therefore have the same chemical properties? 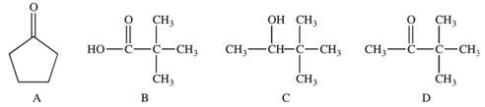
A)A and D
B)B and C
C)B and D
D)A,B,and D
E)B,C,and D

A)A and D
B)B and C
C)B and D
D)A,B,and D
E)B,C,and D

Unlock Deck
Unlock for access to all 104 flashcards in this deck.
Unlock Deck
k this deck
50
What is the IUPAC name of the following compound? 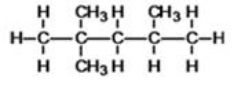
A)2,4,4-trimethylpentane
B)2,2,4-octane
C)2,2,4-trimethylpentane
D)trimethylpentane
E)None of the choices are correct.

A)2,4,4-trimethylpentane
B)2,2,4-octane
C)2,2,4-trimethylpentane
D)trimethylpentane
E)None of the choices are correct.

Unlock Deck
Unlock for access to all 104 flashcards in this deck.
Unlock Deck
k this deck
51
What is the source of most of the synthetic organic compounds that are used today?
A)methane
B)trees
C)petroleum
D)natural gas
E)coal
A)methane
B)trees
C)petroleum
D)natural gas
E)coal

Unlock Deck
Unlock for access to all 104 flashcards in this deck.
Unlock Deck
k this deck
52
What is the correct classification for the compound shown below? 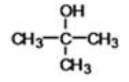
A)alcohol
B)ether
C)carboxylic acid
D)amine
E)ketone

A)alcohol
B)ether
C)carboxylic acid
D)amine
E)ketone

Unlock Deck
Unlock for access to all 104 flashcards in this deck.
Unlock Deck
k this deck
53
What is the molecular formula of butane?
A)C3H8
B)C4H8
C)C4H10
D)C2H6
E)None of the choices are correct.
A)C3H8
B)C4H8
C)C4H10
D)C2H6
E)None of the choices are correct.

Unlock Deck
Unlock for access to all 104 flashcards in this deck.
Unlock Deck
k this deck
54
Which of the following is the condensed formula of 3-methylheptane?
A)CH3CH2CH(CH3)CH2CH2CH2CH3
B)CH3CH2CH2(CH3)CH2CH2CH2CH3
C)CH3CH(CH3)CH2CH2CH2CH2CH3
D)CH3(CH3)CHCH2CH2CH2CH3
E)None of the choices are correct.
A)CH3CH2CH(CH3)CH2CH2CH2CH3
B)CH3CH2CH2(CH3)CH2CH2CH2CH3
C)CH3CH(CH3)CH2CH2CH2CH2CH3
D)CH3(CH3)CHCH2CH2CH2CH3
E)None of the choices are correct.

Unlock Deck
Unlock for access to all 104 flashcards in this deck.
Unlock Deck
k this deck
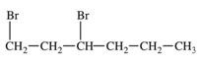
55
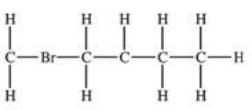
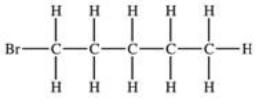
Which structural formula is represented by the condensed formula shown below? CH2BrCH2CH(CH3)2
A)
B)
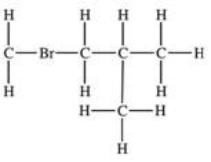
C)
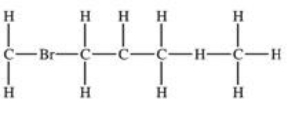
D)
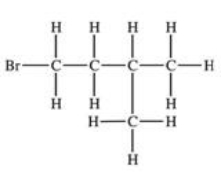
E)
A)

B)

C)

D)

E)


Unlock Deck
Unlock for access to all 104 flashcards in this deck.
Unlock Deck
k this deck
56
What is the correct classification for the compound shown below? 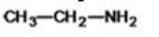
A)alcohol
B)ether
C)amide
D)amine
E)alkene
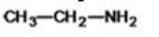
A)alcohol
B)ether
C)amide
D)amine
E)alkene

Unlock Deck
Unlock for access to all 104 flashcards in this deck.
Unlock Deck
k this deck
57
What is the IUPAC name of the compound below? 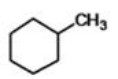
A)methylhexane
B)methylcyclohexane
C)toluene
D)hexylmethane
E)methylbenzene

A)methylhexane
B)methylcyclohexane
C)toluene
D)hexylmethane
E)methylbenzene

Unlock Deck
Unlock for access to all 104 flashcards in this deck.
Unlock Deck
k this deck
58
Which functional group is found in alcohols?
A)carboxyl
B)carboxy
C)amino
D)carbonyl
E)hydroxyl
A)carboxyl
B)carboxy
C)amino
D)carbonyl
E)hydroxyl

Unlock Deck
Unlock for access to all 104 flashcards in this deck.
Unlock Deck
k this deck
59
Which name describes a three-carbon alkane?
A)pentane
B)butane
C)propane
D)heptane
E)methane
A)pentane
B)butane
C)propane
D)heptane
E)methane

Unlock Deck
Unlock for access to all 104 flashcards in this deck.
Unlock Deck
k this deck
60
Methane reacts with oxygen in a combustion reaction to produce carbon dioxide and water.How many moles of water are produced by the complete combustion of one mole of methane?
A)1
B)2
C)3
D)4
E)5
A)1
B)2
C)3
D)4
E)5

Unlock Deck
Unlock for access to all 104 flashcards in this deck.
Unlock Deck
k this deck
61
Which compound is an isomer of pentane?
A)2-methylpentane
B)ethylcyclopropane
C)2,2-dimethylpropane
D)cyclopentane
E)methylcyclobutane
A)2-methylpentane
B)ethylcyclopropane
C)2,2-dimethylpropane
D)cyclopentane
E)methylcyclobutane

Unlock Deck
Unlock for access to all 104 flashcards in this deck.
Unlock Deck
k this deck
62
Molecules that have the same molecular formula,but have different structures are known as which of the following?
A)allotropes
B)isotopes
C)conformers
D)isomers
E)alkanes
A)allotropes
B)isotopes
C)conformers
D)isomers
E)alkanes

Unlock Deck
Unlock for access to all 104 flashcards in this deck.
Unlock Deck
k this deck
63
What is the IUPAC name of the following compound? 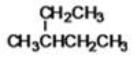
A)2-ethylbutane
B)2-methyl-2-ethylpropane
C)3-methylpentane
D)trimethylpropane
E)2-methyl-diethylmethane
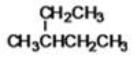
A)2-ethylbutane
B)2-methyl-2-ethylpropane
C)3-methylpentane
D)trimethylpropane
E)2-methyl-diethylmethane

Unlock Deck
Unlock for access to all 104 flashcards in this deck.
Unlock Deck
k this deck
64
Which of the following is a possible product of the halogenation reaction shown below? 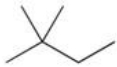
A)
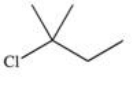
B)
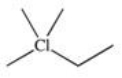
C)
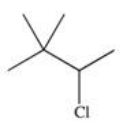
D)
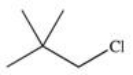
E)All of the compounds are possible products.

A)

B)

C)

D)

E)All of the compounds are possible products.

Unlock Deck
Unlock for access to all 104 flashcards in this deck.
Unlock Deck
k this deck
65
What is the IUPAC name of the following compound? 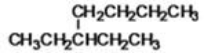
A)3-ethylpentane
B)3-methylheptane
C)1,4-dimethylpentane
D)3-ethylheptane
E)2-methyl-diethylmethane
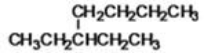
A)3-ethylpentane
B)3-methylheptane
C)1,4-dimethylpentane
D)3-ethylheptane
E)2-methyl-diethylmethane

Unlock Deck
Unlock for access to all 104 flashcards in this deck.
Unlock Deck
k this deck
66
Which of the following molecular formulas is not that of an alkane or cycloalkane?
A)C6H6
B)C6H12
C)C5H12
D)C3H6
E)C3H8
A)C6H6
B)C6H12
C)C5H12
D)C3H6
E)C3H8

Unlock Deck
Unlock for access to all 104 flashcards in this deck.
Unlock Deck
k this deck
67
Which equation properly represents the complete combustion of propane,C3H8?
A)C3H8 + 7O2 → 3CO2 + 8H2O
B)C3H8 + 5O2 → 3CO2 + 4H2O
C)C3H8 + 10O → 3CO2 + 4H2O
D)C3H8 + 7O → 3CO + 4H2O
E)2C3H8 + 7O2 → 6CO + 8H2O
A)C3H8 + 7O2 → 3CO2 + 8H2O
B)C3H8 + 5O2 → 3CO2 + 4H2O
C)C3H8 + 10O → 3CO2 + 4H2O
D)C3H8 + 7O → 3CO + 4H2O
E)2C3H8 + 7O2 → 6CO + 8H2O

Unlock Deck
Unlock for access to all 104 flashcards in this deck.
Unlock Deck
k this deck
68
Which compound is an isomer of 2-methylpropane?
A)2-chloropropane
B)2,2-dimethylpropane
C)propane
D)cyclobutane
E)butane
A)2-chloropropane
B)2,2-dimethylpropane
C)propane
D)cyclobutane
E)butane

Unlock Deck
Unlock for access to all 104 flashcards in this deck.
Unlock Deck
k this deck
69
Which is the correct order of melting points for the following alkanes,from highest temperature to lowest temperature?
A)nonane > hexane > methane > ethane
B)hexane > nonane > ethane > methane
C)nonane > hexane > ethane > methane
D)nonane > ethane > hexane > methane
E)methane > ethane > hexane > nonane
A)nonane > hexane > methane > ethane
B)hexane > nonane > ethane > methane
C)nonane > hexane > ethane > methane
D)nonane > ethane > hexane > methane
E)methane > ethane > hexane > nonane

Unlock Deck
Unlock for access to all 104 flashcards in this deck.
Unlock Deck
k this deck
70
What is the IUPAC name of the following compound? 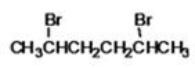
A)dibromopentane
B)2,4-dibromopentane
C)2,4-dibromohexane
D)2,5-dibromohexane
E)1-bromo-2,5-methylbromopentane
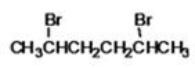
A)dibromopentane
B)2,4-dibromopentane
C)2,4-dibromohexane
D)2,5-dibromohexane
E)1-bromo-2,5-methylbromopentane

Unlock Deck
Unlock for access to all 104 flashcards in this deck.
Unlock Deck
k this deck
71
Which of the following compounds are related as geometric isomers? 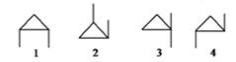
A)1 and 2
B)1 and 3
C)1 and 4
D)2 and 3
E)All are related as geometric isomers.

A)1 and 2
B)1 and 3
C)1 and 4
D)2 and 3
E)All are related as geometric isomers.

Unlock Deck
Unlock for access to all 104 flashcards in this deck.
Unlock Deck
k this deck
72
What word best describes the geometry (shape)around a carbon atom that has four single bonds to neighboring atoms?
A)tetrahedral
B)planar
C)regular
D)linear
E)unsaturated
A)tetrahedral
B)planar
C)regular
D)linear
E)unsaturated

Unlock Deck
Unlock for access to all 104 flashcards in this deck.
Unlock Deck
k this deck
73
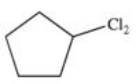
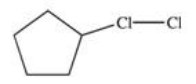
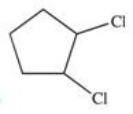
Which of the following is the monohalogenated product that results when cyclopentane undergoes chlorination according to the reaction below? 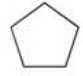
A)
B)
C)
D)
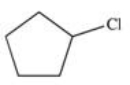
E)
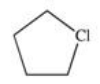

A)

B)

C)

D)

E)


Unlock Deck
Unlock for access to all 104 flashcards in this deck.
Unlock Deck
k this deck
74
What is the IUPAC name of the following compound? 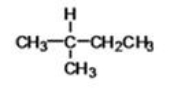
A)2,2-dimethylpropane
B)2-methylbutane
C)dimethylbutane
D)2-hydro-2-methylbutane
E)isopropylethane

A)2,2-dimethylpropane
B)2-methylbutane
C)dimethylbutane
D)2-hydro-2-methylbutane
E)isopropylethane

Unlock Deck
Unlock for access to all 104 flashcards in this deck.
Unlock Deck
k this deck
75
What is the IUPAC name of the following compound? 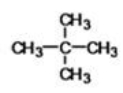
A)2,2-dimethylpropane
B)2-methylbutane
C)dimethylbutane
D)tetramethylethane
E)2,2,2-trimethylmethane

A)2,2-dimethylpropane
B)2-methylbutane
C)dimethylbutane
D)tetramethylethane
E)2,2,2-trimethylmethane

Unlock Deck
Unlock for access to all 104 flashcards in this deck.
Unlock Deck
k this deck
76
Which compound is an isomer of 1,1-dibromoethane?
A)Br2CHCH3
B)CH2BrCH2Br
C)CH3CHBr2
D)CHBr2CHBr2
E)CH3BrCH3Br
A)Br2CHCH3
B)CH2BrCH2Br
C)CH3CHBr2
D)CHBr2CHBr2
E)CH3BrCH3Br

Unlock Deck
Unlock for access to all 104 flashcards in this deck.
Unlock Deck
k this deck
77
What is the name of the alkyl group represented by the structure below? 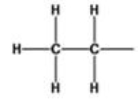
A)acetyl
B)dimethyl
C)propyl
D)ethanyl
E)ethyl

A)acetyl
B)dimethyl
C)propyl
D)ethanyl
E)ethyl

Unlock Deck
Unlock for access to all 104 flashcards in this deck.
Unlock Deck
k this deck
78
Which compound is an isomer of butane?
A)cyclobutane
B)CH3CH(CH3)2
C)benzene
D)CH3CHBrCH2CH3
E)2,2-dimethylpropane
A)cyclobutane
B)CH3CH(CH3)2
C)benzene
D)CH3CHBrCH2CH3
E)2,2-dimethylpropane

Unlock Deck
Unlock for access to all 104 flashcards in this deck.
Unlock Deck
k this deck
79
What is the IUPAC name of the following compound? 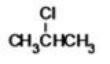
A)2-chloromethane
B)2-chloropropane
C)1-methyl-1-chloroethane
D)1,1-chloromethylethane
E)2-chloroethane

A)2-chloromethane
B)2-chloropropane
C)1-methyl-1-chloroethane
D)1,1-chloromethylethane
E)2-chloroethane

Unlock Deck
Unlock for access to all 104 flashcards in this deck.
Unlock Deck
k this deck
80
Of the structures shown,which are related as constitutional isomers? 
A)1 and 3
B)2 and 3
C)3 and 4
D)Both 1 and 3 and 2 and 3 are correct.
E)1 and 3,2 and 3,and 3 and 4 are all related.

A)1 and 3
B)2 and 3
C)3 and 4
D)Both 1 and 3 and 2 and 3 are correct.
E)1 and 3,2 and 3,and 3 and 4 are all related.

Unlock Deck
Unlock for access to all 104 flashcards in this deck.
Unlock Deck
k this deck


