Deck 13: Aldehydes and Ketones
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/55
Play
Full screen (f)
Deck 13: Aldehydes and Ketones
1
Propylene glycol, shown below, is a nontoxic diol used as a solvent for pharmaceutical drugs, and as a moisturizing agent for foods.When ingested, both alcohol functional groups are oxidized by enzymes in the liver to produce pyruvic acid, a compound with the formula C3H4O3.What is the structure of pyruvic acid? 
A)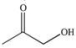
B)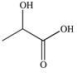
C)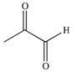
D)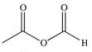
E)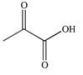

A)

B)

C)

D)

E)


2
What is the common name of the following compound? 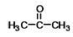
A)propanone
B)propanal
C)2-propyl ketone
D)1-propyl ketone
E)acetone
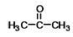
A)propanone
B)propanal
C)2-propyl ketone
D)1-propyl ketone
E)acetone
acetone
3
Which statement best describes the structural difference between aldehydes and ketones?
A)Aldehydes contain a hydroxyl group; ketones contain a carbonyl group.
B)Aldehydes contain a carbonyl group on an end carbon of the carbon chain; ketones contain a carbonyl group on a carbon within the carbon chain.
C)Ketones are cyclic compounds; aldehydes are acyclic compounds.
D)The carbonyl group of a ketone is bonded to an oxygen atom; the carbonyl group of an aldehyde is bonded to a hydrogen atom.
E)The carbonyl group of an aldehyde is bonded to two carbon atoms; the carbonyl group of a ketone is bonded to at least one hydrogen atom.
A)Aldehydes contain a hydroxyl group; ketones contain a carbonyl group.
B)Aldehydes contain a carbonyl group on an end carbon of the carbon chain; ketones contain a carbonyl group on a carbon within the carbon chain.
C)Ketones are cyclic compounds; aldehydes are acyclic compounds.
D)The carbonyl group of a ketone is bonded to an oxygen atom; the carbonyl group of an aldehyde is bonded to a hydrogen atom.
E)The carbonyl group of an aldehyde is bonded to two carbon atoms; the carbonyl group of a ketone is bonded to at least one hydrogen atom.
Aldehydes contain a carbonyl group on an end carbon of the carbon chain; ketones contain a carbonyl group on a carbon within the carbon chain.
4
What is the common name of the simplest aldehyde?
A)acetal
B)methal
C)acetaldehyde
D)formaldehyde
E)formal
A)acetal
B)methal
C)acetaldehyde
D)formaldehyde
E)formal

Unlock Deck
Unlock for access to all 55 flashcards in this deck.
Unlock Deck
k this deck
5
What is the structure of ethyl isopropyl ketone?
A)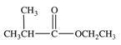
B)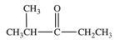
C)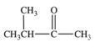
D)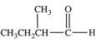
E)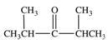
A)

B)
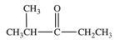
C)
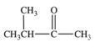
D)

E)
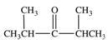

Unlock Deck
Unlock for access to all 55 flashcards in this deck.
Unlock Deck
k this deck
6
What is the name of the aqueous solution of formaldehyde that is available commercially as a tissue preservative?
A)acetone
B)formalin
C)acetaldehyde
D)glycerol
E)enol
A)acetone
B)formalin
C)acetaldehyde
D)glycerol
E)enol

Unlock Deck
Unlock for access to all 55 flashcards in this deck.
Unlock Deck
k this deck
7
The presence of a carbonyl group has which effect on the physical properties of a compound?
A)The carbonyl group decreases the polarity of the compound.
B)The carbonyl group increases the boiling point of the compound.
C)The carbonyl group decreases the water solubility of the compound.
D)The carbonyl group increases the ability of the compound to form ionic bonds.
E)The carbonyl group decreases the melting point of the compound.
A)The carbonyl group decreases the polarity of the compound.
B)The carbonyl group increases the boiling point of the compound.
C)The carbonyl group decreases the water solubility of the compound.
D)The carbonyl group increases the ability of the compound to form ionic bonds.
E)The carbonyl group decreases the melting point of the compound.

Unlock Deck
Unlock for access to all 55 flashcards in this deck.
Unlock Deck
k this deck
8
An unknown compound is highly water soluble, forms a silver mirror in the Tollens' test, and forms a red precipitate in the Benedict's test.Which of the following compounds is consistent with this data?
A)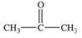
B)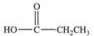
C)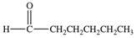
D)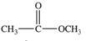
E)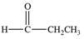
A)
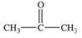
B)
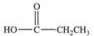
C)
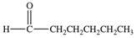
D)
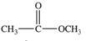
E)
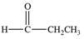

Unlock Deck
Unlock for access to all 55 flashcards in this deck.
Unlock Deck
k this deck
9
Which of the following compounds would be expected to react similarly in a chemical reaction? 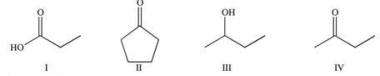
A)I and III
B)II and IV
C)I and IV
D)I, II, and IV
E)I, III, and IV

A)I and III
B)II and IV
C)I and IV
D)I, II, and IV
E)I, III, and IV

Unlock Deck
Unlock for access to all 55 flashcards in this deck.
Unlock Deck
k this deck
10
What is the proper IUPAC name of the three carbon aldehyde?
A)propanal
B)butanal
C)acetal
D)propanone
E)propanol
A)propanal
B)butanal
C)acetal
D)propanone
E)propanol

Unlock Deck
Unlock for access to all 55 flashcards in this deck.
Unlock Deck
k this deck
11
Nandrolone is an anabolic steroid sometimes taken by athletes to build muscle mass.Which of the following correctly describes the organic families to which nandrolone belongs? 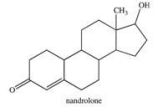
A)ketone, alkene, alcohol
B)aldehyde, alkene, phenol
C)ketone, alkene, phenol
D)aldehyde, aromatic, alcohol
E)keto, enol, aromatic

A)ketone, alkene, alcohol
B)aldehyde, alkene, phenol
C)ketone, alkene, phenol
D)aldehyde, aromatic, alcohol
E)keto, enol, aromatic

Unlock Deck
Unlock for access to all 55 flashcards in this deck.
Unlock Deck
k this deck
12
Methanol is toxic if ingested.The toxicity is due to the aldehyde produced when methanol is oxidized in the liver.Which of the following is the aldehyde formed by the oxidation of methanol?
A)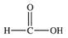
B)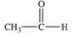
C)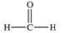
D)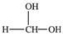
E)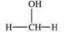
A)
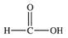
B)
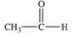
C)
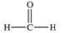
D)

E)


Unlock Deck
Unlock for access to all 55 flashcards in this deck.
Unlock Deck
k this deck
13
What compound is produced when 2-methyl-3-pentanol is oxidized?
A)2-methyl-3-pentanone
B)2-methyl-3-pentanal
C)2-methyl-3-pentene
D)2-methylpentone
E)It does not undergo oxidation.
A)2-methyl-3-pentanone
B)2-methyl-3-pentanal
C)2-methyl-3-pentene
D)2-methylpentone
E)It does not undergo oxidation.

Unlock Deck
Unlock for access to all 55 flashcards in this deck.
Unlock Deck
k this deck
14
Where in the body is ethanol oxidized to produce ethanal?
A)stomach
B)liver
C)small intestine
D)large intestine
E)Both small intestine and large intestine are correct.
A)stomach
B)liver
C)small intestine
D)large intestine
E)Both small intestine and large intestine are correct.

Unlock Deck
Unlock for access to all 55 flashcards in this deck.
Unlock Deck
k this deck
15
What is the IUPAC name of the aldehyde produced when ethanol is oxidized? 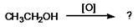
A)acetal
B)acetone
C)ethanal
D)ethanone
E)None of the choices are correct.
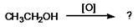
A)acetal
B)acetone
C)ethanal
D)ethanone
E)None of the choices are correct.

Unlock Deck
Unlock for access to all 55 flashcards in this deck.
Unlock Deck
k this deck
16
What is the structure of the compound produced by the oxidation of ethanal?
A)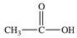
B)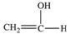
C)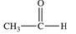
D)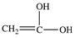
E)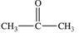
A)
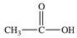
B)

C)
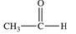
D)

E)
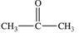

Unlock Deck
Unlock for access to all 55 flashcards in this deck.
Unlock Deck
k this deck
17
What class of compounds is formed by oxidation of aldehydes?
A)enols
B)carboxylic acids
C)ketones
D)acetals
E)Aldehydes do not undergo oxidation.
A)enols
B)carboxylic acids
C)ketones
D)acetals
E)Aldehydes do not undergo oxidation.

Unlock Deck
Unlock for access to all 55 flashcards in this deck.
Unlock Deck
k this deck
18
Which of the statements concerning the compound shown below is FALSE? 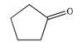
A)Its name is cyclopentanone.
B)Its molecular formula is C5H8O.
C)Molecules of this compound are held together in a collection by dipole-dipole attractions.
D)It may be formed by the oxidation of cyclopentanol.
E)It can be oxidized to produce a carboxylic acid.

A)Its name is cyclopentanone.
B)Its molecular formula is C5H8O.
C)Molecules of this compound are held together in a collection by dipole-dipole attractions.
D)It may be formed by the oxidation of cyclopentanol.
E)It can be oxidized to produce a carboxylic acid.

Unlock Deck
Unlock for access to all 55 flashcards in this deck.
Unlock Deck
k this deck
19
What is the general formula of a ketone?
A)R-O-R
B)R-O-H
C)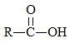
D)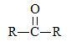
E)None of the choices are correct.
A)R-O-R
B)R-O-H
C)
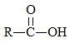
D)
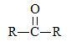
E)None of the choices are correct.

Unlock Deck
Unlock for access to all 55 flashcards in this deck.
Unlock Deck
k this deck
20
The compound decanal contributes to the odor and flavor of an orange.Which of the following statements about decanal is FALSE?
A)It is an aldehyde.
B)It contains a saturated chain of ten carbons.
C)It contains a carbonyl group on the first carbon of the carbon chain.
D)It can form intermolecular hydrogen bonds with other molecules of decanal.
E)It is insoluble in water.
A)It is an aldehyde.
B)It contains a saturated chain of ten carbons.
C)It contains a carbonyl group on the first carbon of the carbon chain.
D)It can form intermolecular hydrogen bonds with other molecules of decanal.
E)It is insoluble in water.

Unlock Deck
Unlock for access to all 55 flashcards in this deck.
Unlock Deck
k this deck
21
What simple ketone is commonly used as a solvent?
A)acetal
B)formalin
C)acetone
D)benzanal
E)butanone
A)acetal
B)formalin
C)acetone
D)benzanal
E)butanone

Unlock Deck
Unlock for access to all 55 flashcards in this deck.
Unlock Deck
k this deck
22
What type of compound is produced by the oxidation of an aldehyde?
A)carboxylic acid
B)alcohol
C)alkane
D)hemiacetal
E)acetone
A)carboxylic acid
B)alcohol
C)alkane
D)hemiacetal
E)acetone

Unlock Deck
Unlock for access to all 55 flashcards in this deck.
Unlock Deck
k this deck
23
What is the name of the organic product formed when the compound, CH3CH2CH2CHO, is reduced by addition of H2?
A)butane
B)1-butanol
C)1-butanone
D)butanal
E)butanoic acid
A)butane
B)1-butanol
C)1-butanone
D)butanal
E)butanoic acid

Unlock Deck
Unlock for access to all 55 flashcards in this deck.
Unlock Deck
k this deck
24
What kind of compound is produced by the reaction of an aldehyde with an alcohol in the presence of a trace of acid?
A)ether
B)ketone
C)carboxylic acid
D)hemiacetal
E)alkyne
A)ether
B)ketone
C)carboxylic acid
D)hemiacetal
E)alkyne

Unlock Deck
Unlock for access to all 55 flashcards in this deck.
Unlock Deck
k this deck
25
What is the IUPAC name of the compound shown? 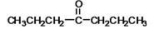
A)4-octanone
B)1-propyl butanal
C)-heptanone
D)dipropyl ether
E)4-heptanal
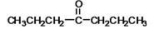
A)4-octanone
B)1-propyl butanal
C)-heptanone
D)dipropyl ether
E)4-heptanal

Unlock Deck
Unlock for access to all 55 flashcards in this deck.
Unlock Deck
k this deck
26
What is the name of the organic product formed when the compound shown is oxidized? 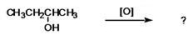
A)2-butanol
B)butanone
C)butanal
D)butyl ketone
E)methyl ethyl ether
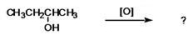
A)2-butanol
B)butanone
C)butanal
D)butyl ketone
E)methyl ethyl ether

Unlock Deck
Unlock for access to all 55 flashcards in this deck.
Unlock Deck
k this deck
27
What is the IUPAC name of the compound shown? 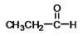
A)formaldehyde
B)acetone
C)propanone
D)propanal
E)ethanal
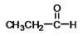
A)formaldehyde
B)acetone
C)propanone
D)propanal
E)ethanal

Unlock Deck
Unlock for access to all 55 flashcards in this deck.
Unlock Deck
k this deck
28
Which of the following does NOT represent an oxidation reaction?
A)ethanal → ethanol
B)benzaldehyde → benzoic acid
C)2-propanol → propanone
D)1-propanol → propanal
E)All of the reactions are oxidation reactions.
A)ethanal → ethanol
B)benzaldehyde → benzoic acid
C)2-propanol → propanone
D)1-propanol → propanal
E)All of the reactions are oxidation reactions.

Unlock Deck
Unlock for access to all 55 flashcards in this deck.
Unlock Deck
k this deck
29
What type of compound is formed in the following reaction? 
A)acetal
B)aldehyde
C)hemiacetal
D)enol
E)ketone

A)acetal
B)aldehyde
C)hemiacetal
D)enol
E)ketone

Unlock Deck
Unlock for access to all 55 flashcards in this deck.
Unlock Deck
k this deck
30
The compound butanone does not need a number to specify the location of the carbonyl group on the parent carbon chain.Which of the following best explains why?
A)The C=O is assumed to be on C-1 of the parent chain if it is not specified.
B)The C=O can only be on C-2 of the parent chain in this compound for it to be a ketone.
C)The C=O is on an end carbon in this compound, and therefore does not need to be specified.
D)The location of a C=O does not need to be specified for a cyclic ketone.
E)The location of the C=O in this compound is not significant to the name.
A)The C=O is assumed to be on C-1 of the parent chain if it is not specified.
B)The C=O can only be on C-2 of the parent chain in this compound for it to be a ketone.
C)The C=O is on an end carbon in this compound, and therefore does not need to be specified.
D)The location of a C=O does not need to be specified for a cyclic ketone.
E)The location of the C=O in this compound is not significant to the name.

Unlock Deck
Unlock for access to all 55 flashcards in this deck.
Unlock Deck
k this deck
31
Which of the following best describes the structure of an enol?
A)contains a triple bond and a carbonyl group
B)contains only single bonds and a hydroxyl group
C)contains a double bond and a hydroxyl group
D)contains a double bond and a carbonyl group
E)contains only single bonds and a carbonyl group
A)contains a triple bond and a carbonyl group
B)contains only single bonds and a hydroxyl group
C)contains a double bond and a hydroxyl group
D)contains a double bond and a carbonyl group
E)contains only single bonds and a carbonyl group

Unlock Deck
Unlock for access to all 55 flashcards in this deck.
Unlock Deck
k this deck
32
Which structure/name combination is incorrect? 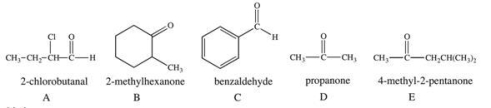
A)A
B)B
C)C
D)D
E)E

A)A
B)B
C)C
D)D
E)E

Unlock Deck
Unlock for access to all 55 flashcards in this deck.
Unlock Deck
k this deck
33
What is the IUPAC name for the product of the reaction shown? 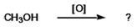
A)acetone
B)methanal
C)acetic acid
D)acetal
E)propanone
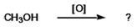
A)acetone
B)methanal
C)acetic acid
D)acetal
E)propanone

Unlock Deck
Unlock for access to all 55 flashcards in this deck.
Unlock Deck
k this deck
34
Which of the following is a hemiacetal?
A)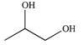
B)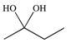
C)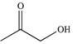
D)
E)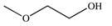
A)

B)

C)

D)

E)


Unlock Deck
Unlock for access to all 55 flashcards in this deck.
Unlock Deck
k this deck
35
Which of the following will give a silver mirror when treated with Tollens' reagent?
A)2-pentanone
B)propanone
C)hexanal
D)1-pentanol
E)2-pentanol
A)2-pentanone
B)propanone
C)hexanal
D)1-pentanol
E)2-pentanol

Unlock Deck
Unlock for access to all 55 flashcards in this deck.
Unlock Deck
k this deck
36
What alcohol undergoes oxidation to produce acetic acid?
A)methanol
B)ethanol
C)propanol
D)isopropyl alcohol
E)acetone
A)methanol
B)ethanol
C)propanol
D)isopropyl alcohol
E)acetone

Unlock Deck
Unlock for access to all 55 flashcards in this deck.
Unlock Deck
k this deck
37
What class of compounds is represented by the general formula RCHO?
A)ketones
B)carboxylic acids
C)alkenes
D)alcohols
E)aldehydes
A)ketones
B)carboxylic acids
C)alkenes
D)alcohols
E)aldehydes

Unlock Deck
Unlock for access to all 55 flashcards in this deck.
Unlock Deck
k this deck
38
Acetone is the simplest ketone.Not only is it used industrially as a solvent, but it is also produced by the body as a product of lipid metabolism.What is the structure of acetone?
A)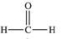
B)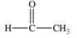
C)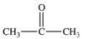
D)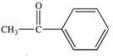
E)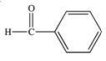
A)
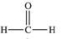
B)
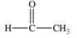
C)
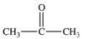
D)

E)


Unlock Deck
Unlock for access to all 55 flashcards in this deck.
Unlock Deck
k this deck
39
What is the IUPAC name of the compound produced in the liver that is responsible for the symptoms of a hangover?
A)acetone
B)acetic acid
C)ethanol
D)ethanal
E)2-propanol
A)acetone
B)acetic acid
C)ethanol
D)ethanal
E)2-propanol

Unlock Deck
Unlock for access to all 55 flashcards in this deck.
Unlock Deck
k this deck
40
What is the IUPAC name of the compound shown? 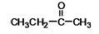
A)2-methylpropanone
B)2-butanal
C)2-butanyl
D)butanone
E)2-methyl-2-propanone
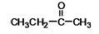
A)2-methylpropanone
B)2-butanal
C)2-butanyl
D)butanone
E)2-methyl-2-propanone

Unlock Deck
Unlock for access to all 55 flashcards in this deck.
Unlock Deck
k this deck
41
Benedict's test can be used to distinguish between an aldehyde and a ketone.

Unlock Deck
Unlock for access to all 55 flashcards in this deck.
Unlock Deck
k this deck
42
Aldehydes are easily oxidized to carboxylic acids.

Unlock Deck
Unlock for access to all 55 flashcards in this deck.
Unlock Deck
k this deck
43
An acetal contains two ether functional groups.

Unlock Deck
Unlock for access to all 55 flashcards in this deck.
Unlock Deck
k this deck
44
A hemiacetal contains two ether functional groups.

Unlock Deck
Unlock for access to all 55 flashcards in this deck.
Unlock Deck
k this deck
45
In any pair of keto-enol tautomers, the keto form is generally more stable than the enol form.

Unlock Deck
Unlock for access to all 55 flashcards in this deck.
Unlock Deck
k this deck
46
The most oxidized organic form of a primary alcohol is a ketone.

Unlock Deck
Unlock for access to all 55 flashcards in this deck.
Unlock Deck
k this deck
47
Small (low molecular weight) aldehyde molecules are generally more water-soluble than large ones.

Unlock Deck
Unlock for access to all 55 flashcards in this deck.
Unlock Deck
k this deck
48
A ketone will react with Tollens' reagent to produce a silver mirror, but an aldehyde will not.

Unlock Deck
Unlock for access to all 55 flashcards in this deck.
Unlock Deck
k this deck
49
Formaldehyde is used to kill viruses without damaging their genetic information.

Unlock Deck
Unlock for access to all 55 flashcards in this deck.
Unlock Deck
k this deck
50
The compound shown below is the enol form of butanal.



Unlock Deck
Unlock for access to all 55 flashcards in this deck.
Unlock Deck
k this deck
51
When a hemiacetal reacts with an alcohol, an acetal is formed.This is how disaccharides and polysaccharides are formed.What is the structure of the acetal formed in the following reaction? 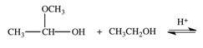
A)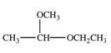
B)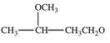
C)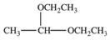
D)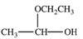
E)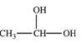

A)

B)
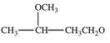
C)
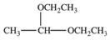
D)

E)


Unlock Deck
Unlock for access to all 55 flashcards in this deck.
Unlock Deck
k this deck
52
Pure formaldehyde is a liquid at room temperature.

Unlock Deck
Unlock for access to all 55 flashcards in this deck.
Unlock Deck
k this deck
53
Which of the following pairs of reactant molecules can react in the presence of a trace of acid to give a hemiacetal molecule?
A)a ketone and an aldehyde
B)an aldehyde and an alkene
C)an aldehyde and an alcohol
D)two ketones
E)two aldehydes
A)a ketone and an aldehyde
B)an aldehyde and an alkene
C)an aldehyde and an alcohol
D)two ketones
E)two aldehydes

Unlock Deck
Unlock for access to all 55 flashcards in this deck.
Unlock Deck
k this deck
54
A ketone can be reduced to a primary alcohol.

Unlock Deck
Unlock for access to all 55 flashcards in this deck.
Unlock Deck
k this deck
55
The oxidation of isopropyl alcohol produces propanone.

Unlock Deck
Unlock for access to all 55 flashcards in this deck.
Unlock Deck
k this deck


