Deck 6: Solutions
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/67
Play
Full screen (f)
Deck 6: Solutions
1
If 5.20 g of HCl is added to enough distilled water to form 3.00 L of solution, what is the molarity of the solution? [Molecular weight: HCl, 36.46 amu]
A)0.0475 M
B)0.143 M
C)0.428 M
D)1.73 M
E)2.34 M
A)0.0475 M
B)0.143 M
C)0.428 M
D)1.73 M
E)2.34 M
0.0475 M
2
Na3PO4 dissolves in water to produce an electrolytic solution.What is the osmolarity of a 2.0 × 10-3 M Na3PO4 solution?
A)1.0 × 10−3 osmolar
B)2.0 × 10−3 osmolar
C)4.0 × 10−3 osmolar
D)5.0 × 10−3 osmolar
E)8.0 × 10−3 osmolar
A)1.0 × 10−3 osmolar
B)2.0 × 10−3 osmolar
C)4.0 × 10−3 osmolar
D)5.0 × 10−3 osmolar
E)8.0 × 10−3 osmolar
8.0 × 10−3 osmolar
3
What is the molarity of a solution if 300.0 mL of it contains 16.8 g of KNO3? [Formula weight: KNO3, 101.11 amu]
A)5.53 × 10−4 M
B)0.056 M
C)0.166 M
D)0.554 M
E)5.66 M
A)5.53 × 10−4 M
B)0.056 M
C)0.166 M
D)0.554 M
E)5.66 M
0.554 M
4
What type of membrane allows solvent molecules to pass through but does not allow solute molecules to pass through?
A)transport
B)colloidal
C)transparent
D)semipermeable
E)filtering
A)transport
B)colloidal
C)transparent
D)semipermeable
E)filtering

Unlock Deck
Unlock for access to all 67 flashcards in this deck.
Unlock Deck
k this deck
5
Vitamin D is essential for the normal development of teeth and bones.A tablet of Vitamin D is tested for its solubility in water and benzene.The results are shown in the table below.What is the most appropriate interpretation of the solubility results? 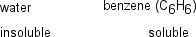












A)Vitamin D is likely a nonpolar substance.
B)Vitamin D is likely a polar substance.
C)Vitamin D is likely a bipolar substance.
D)Vitamin D is likely an ionic compound.
E)It is impossible to say anything about the polarity of Vitamin D without knowing its structure.
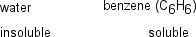












A)Vitamin D is likely a nonpolar substance.
B)Vitamin D is likely a polar substance.
C)Vitamin D is likely a bipolar substance.
D)Vitamin D is likely an ionic compound.
E)It is impossible to say anything about the polarity of Vitamin D without knowing its structure.

Unlock Deck
Unlock for access to all 67 flashcards in this deck.
Unlock Deck
k this deck
6
Enough water is added to 50.0 mL of a 0.660 M NaOH solution in order to bring the total volume to 450.0 mL.What is the molarity of this diluted solution?
A)0.0330 M
B)0.0733 M
C)0.594 M
D)5.94 M
E)0.660 M; the molarity remains unchanged if only solvent is added.
A)0.0330 M
B)0.0733 M
C)0.594 M
D)5.94 M
E)0.660 M; the molarity remains unchanged if only solvent is added.

Unlock Deck
Unlock for access to all 67 flashcards in this deck.
Unlock Deck
k this deck
7
What happens when a hypotonic solution is separated from a hypertonic solution by an osmotic membrane?
A)Water molecules move from the hypotonic solution to the hypertonic solution.
B)Solute molecules move from the hypertonic solution to the hypotonic solution.
C)Water molecules move from the hypertonic solution to the hypotonic solution.
D)Solute molecules move from the hypotonic solution to the hypertonic solution.
E)No net movement of solvent or solute molecules occurs, because the osmotic pressure on both sides is equal.
A)Water molecules move from the hypotonic solution to the hypertonic solution.
B)Solute molecules move from the hypertonic solution to the hypotonic solution.
C)Water molecules move from the hypertonic solution to the hypotonic solution.
D)Solute molecules move from the hypotonic solution to the hypertonic solution.
E)No net movement of solvent or solute molecules occurs, because the osmotic pressure on both sides is equal.

Unlock Deck
Unlock for access to all 67 flashcards in this deck.
Unlock Deck
k this deck
8
Which statement concerning solution concentration is FALSE?
A)An unsaturated solution contains less than the maximum amount of solute that can be dissolved in the solvent.
B)A saturated solution contains more than 100 g of dissolved solute.
C)The concentration of a saturated solution is equal to the solubility of the solute.
D)A solution can be made less concentrated by adding additional solvent.
E)The molarity of a solution is the number of moles of solute present in one liter of the solution.
A)An unsaturated solution contains less than the maximum amount of solute that can be dissolved in the solvent.
B)A saturated solution contains more than 100 g of dissolved solute.
C)The concentration of a saturated solution is equal to the solubility of the solute.
D)A solution can be made less concentrated by adding additional solvent.
E)The molarity of a solution is the number of moles of solute present in one liter of the solution.

Unlock Deck
Unlock for access to all 67 flashcards in this deck.
Unlock Deck
k this deck
9
Which of the following correctly describes what happens when red blood cells are placed in a hypertonic solution?
A)There will be a net movement of water into the red blood cells, the cells will swell and burst.
B)There will be a net movement of water out of the red blood cells, and the cells will shrink and collapse.
C)There will be a net movement of water from the hypertonic solution to the red blood cells, and the cells will shrink and burst.
D)There will be no net movement of water because red blood cells do not have a semipermeable membrane.
E)There will be no net movement of water because the red blood cells and the hypertonic solution have the same osmolarity.
A)There will be a net movement of water into the red blood cells, the cells will swell and burst.
B)There will be a net movement of water out of the red blood cells, and the cells will shrink and collapse.
C)There will be a net movement of water from the hypertonic solution to the red blood cells, and the cells will shrink and burst.
D)There will be no net movement of water because red blood cells do not have a semipermeable membrane.
E)There will be no net movement of water because the red blood cells and the hypertonic solution have the same osmolarity.

Unlock Deck
Unlock for access to all 67 flashcards in this deck.
Unlock Deck
k this deck
10
What mass of glucose is contained in 500.0 mL of a 5.00% (m/V) solution?
A)5.00 g
B)25.0 g
C)250 g
D)50.0 g
E)55.0 g
A)5.00 g
B)25.0 g
C)250 g
D)50.0 g
E)55.0 g

Unlock Deck
Unlock for access to all 67 flashcards in this deck.
Unlock Deck
k this deck
11
Ringer's solution is used in the treatment of burns and wounds.It is prepared by dissolving 8.6 g of NaCl, 0.30 g of KCl, and 0.33 g of CaCl2 in water, and diluting to a volume of 1.0 L.Which of the following is an accurate description of Ringer's solution?
A)NaCl is the solvent in this solution.
B)NaCl, KCl, and CaCl2 are all solvents in this solution.
C)Ringer's solution is a solid solution.
D)Ringer's solution is a heterogeneous mixture.
E)Ringer's solution is an aqueous solution.
A)NaCl is the solvent in this solution.
B)NaCl, KCl, and CaCl2 are all solvents in this solution.
C)Ringer's solution is a solid solution.
D)Ringer's solution is a heterogeneous mixture.
E)Ringer's solution is an aqueous solution.

Unlock Deck
Unlock for access to all 67 flashcards in this deck.
Unlock Deck
k this deck
12
Which of the following properly describes a colligative property of a solution?
A)a solution property that depends on the identity of the solute particles present
B)a solution property that depends on the electrical charges of the solute particles present
C)a solution property that depends on the concentration of solute particles present
D)a solution property that depends on the pressure of the solute particles present
E)a solution property that depends on the concentration, identity, and pressure of the solute particles present
A)a solution property that depends on the identity of the solute particles present
B)a solution property that depends on the electrical charges of the solute particles present
C)a solution property that depends on the concentration of solute particles present
D)a solution property that depends on the pressure of the solute particles present
E)a solution property that depends on the concentration, identity, and pressure of the solute particles present

Unlock Deck
Unlock for access to all 67 flashcards in this deck.
Unlock Deck
k this deck
13
A typical blood serum concentration of 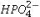 is 2 meq/L.What is the concentration of this ion expressed in mol/L?
is 2 meq/L.What is the concentration of this ion expressed in mol/L?
A)0.001 mol/L
B)1 mol/L
C)0.002 mol/L
D)0.0003 mol/L
E)0.004 mol/L
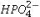 is 2 meq/L.What is the concentration of this ion expressed in mol/L?
is 2 meq/L.What is the concentration of this ion expressed in mol/L?A)0.001 mol/L
B)1 mol/L
C)0.002 mol/L
D)0.0003 mol/L
E)0.004 mol/L

Unlock Deck
Unlock for access to all 67 flashcards in this deck.
Unlock Deck
k this deck
14
A student mixes 4.0 g of KNO3 with 5.0 mL of water at room temperature, and observes that most, but not all, of the KNO3 has dissolved.Which statement concerning this solution is TRUE?
A)The solution is considered supersaturated, since no additional KNO3 will dissolve.
B)The solution is considered unsaturated, since not all of the KNO3 dissolved.
C)The solubility of KNO3 in water has been exceeded.
D)KNO3 is a water insoluble ionic compound.
E)In order to get the additional KNO3 to dissolve, the student can add more solute.
A)The solution is considered supersaturated, since no additional KNO3 will dissolve.
B)The solution is considered unsaturated, since not all of the KNO3 dissolved.
C)The solubility of KNO3 in water has been exceeded.
D)KNO3 is a water insoluble ionic compound.
E)In order to get the additional KNO3 to dissolve, the student can add more solute.

Unlock Deck
Unlock for access to all 67 flashcards in this deck.
Unlock Deck
k this deck
15
The solubility of a solute is best described as which of the following?
A)the ability of the solute to dissolve in water
B)the ability of the solute to dissociate into ions when dissolved in water
C)the mass of solute necessary to dissolve in 1 L of water
D)the maximum amount of solute that can be dissolved in a specified amount of solvent
E)the moles of solute that can dissolve in 1 L of solution
A)the ability of the solute to dissolve in water
B)the ability of the solute to dissociate into ions when dissolved in water
C)the mass of solute necessary to dissolve in 1 L of water
D)the maximum amount of solute that can be dissolved in a specified amount of solvent
E)the moles of solute that can dissolve in 1 L of solution

Unlock Deck
Unlock for access to all 67 flashcards in this deck.
Unlock Deck
k this deck
16
What is the osmotic pressure of a 6.0 × 10-2 M NaCl solution at 20°C (293 K)? [R=0.0821 L•atm/mol•K]
A)2.9 atm
B)1.4 atm
C)27 atm
D)0.20 atm
E)0.099 atm
A)2.9 atm
B)1.4 atm
C)27 atm
D)0.20 atm
E)0.099 atm

Unlock Deck
Unlock for access to all 67 flashcards in this deck.
Unlock Deck
k this deck
17
What type of liquid displays the Tyndall effect?
A)homogeneous mixture
B)heterogeneous mixture
C)solution
D)colloidal suspension
E)pure substance
A)homogeneous mixture
B)heterogeneous mixture
C)solution
D)colloidal suspension
E)pure substance

Unlock Deck
Unlock for access to all 67 flashcards in this deck.
Unlock Deck
k this deck
18
A solution contains the label 0.2 M KNO3.What is the correct interpretation of this concentration?
A)There are 0.2 moles of KNO3 in 100 mL of water.
B)There are 0.2 g of KNO3 in 100 mL of solution.
C)There are 0.2 molecules of KNO3 in 1 L of solution.
D)There are 0.2 g of KNO3 in 1 L of water.
E)There are 0.2 moles of KNO3 in 1 L of solution.
A)There are 0.2 moles of KNO3 in 100 mL of water.
B)There are 0.2 g of KNO3 in 100 mL of solution.
C)There are 0.2 molecules of KNO3 in 1 L of solution.
D)There are 0.2 g of KNO3 in 1 L of water.
E)There are 0.2 moles of KNO3 in 1 L of solution.

Unlock Deck
Unlock for access to all 67 flashcards in this deck.
Unlock Deck
k this deck
19
What term is used to describe a solution of lower osmolarity compared to one with a higher osmolarity?
A)hyposmotic
B)hypertonic
C)isotonic
D)isosmotic
E)hypotonic
A)hyposmotic
B)hypertonic
C)isotonic
D)isosmotic
E)hypotonic

Unlock Deck
Unlock for access to all 67 flashcards in this deck.
Unlock Deck
k this deck
20
A solution contains 1.65 g of NaF in a total volume of 150.0 mL.What is its concentration expressed as % (m/V)?
A)0.011%
B)1.10%
C)11.0%
D)110%
E)1110%
A)0.011%
B)1.10%
C)11.0%
D)110%
E)1110%

Unlock Deck
Unlock for access to all 67 flashcards in this deck.
Unlock Deck
k this deck
21
Which diagram best represents the composition of an aqueous calcium chloride (CaCl2) solution? (water molecules are not shown) 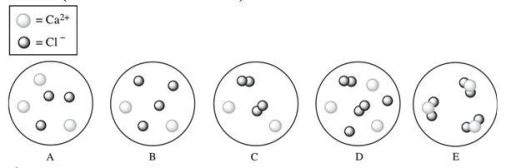
A)A
B)B
C)C
D)D
E)E

A)A
B)B
C)C
D)D
E)E

Unlock Deck
Unlock for access to all 67 flashcards in this deck.
Unlock Deck
k this deck
22
Calculate the mass in grams of NaCl that is present in 500.0 mL of a 0.900% (m/V) solution.
A)50.0 g
B)0.500 g
C)5.00 g
D)45.0 g
E)4.50 g
A)50.0 g
B)0.500 g
C)5.00 g
D)45.0 g
E)4.50 g

Unlock Deck
Unlock for access to all 67 flashcards in this deck.
Unlock Deck
k this deck
23
The solubility of gases in liquids is highest at
A)low temperature and low pressure.
B)low temperature and high pressure.
C)high temperature and low pressure.
D)high temperature and high pressure.
E)high pressure; temperature is immaterial.
A)low temperature and low pressure.
B)low temperature and high pressure.
C)high temperature and low pressure.
D)high temperature and high pressure.
E)high pressure; temperature is immaterial.

Unlock Deck
Unlock for access to all 67 flashcards in this deck.
Unlock Deck
k this deck
24
What term describes a solution that is in equilibrium with undissolved solute?
A)precipitating
B)aqueous
C)saturated
D)unsaturated
E)supersaturated
A)precipitating
B)aqueous
C)saturated
D)unsaturated
E)supersaturated

Unlock Deck
Unlock for access to all 67 flashcards in this deck.
Unlock Deck
k this deck
25
Assuming that air is a solution containing four molecules of N2 for every one molecule O2, what is the concentration of O2 in this solution, expressed as % (m/m)? [Formula weights: N2, 28.02 amu; O2, 32.00 amu]
A)22% (m/m)
B)47% (m/m)
C)53% (m/m)
D)78% (m/m)
E)114% (m/m)
A)22% (m/m)
B)47% (m/m)
C)53% (m/m)
D)78% (m/m)
E)114% (m/m)

Unlock Deck
Unlock for access to all 67 flashcards in this deck.
Unlock Deck
k this deck
26
What volume of a 0.1250 M KCl solution contains 2.330 g of KCl? [Formula weight: KCl, 74.55 amu]
A)0.2913 L
B)26.95 mL
C)25.00 mL
D)1.500 L
E)250.0 mL
A)0.2913 L
B)26.95 mL
C)25.00 mL
D)1.500 L
E)250.0 mL

Unlock Deck
Unlock for access to all 67 flashcards in this deck.
Unlock Deck
k this deck
27
If the concentration of Mg2+ in solution is 3.0 × 10-3 M, what is its concentration expressed in meq/L?
A)6.0 meq/L
B)3.0 meq/L
C)1.5 meq/L
D)6.0 × 10-6 meq/L
E)1.5 × 10-6 meq/L
A)6.0 meq/L
B)3.0 meq/L
C)1.5 meq/L
D)6.0 × 10-6 meq/L
E)1.5 × 10-6 meq/L

Unlock Deck
Unlock for access to all 67 flashcards in this deck.
Unlock Deck
k this deck
28
How many moles of KCl are present in 50.0 mL of a 0.552 M solution?
A)0.0276 mol
B)0.0906 mol
C)11.0 mol
D)35.2 mol
E)50.6 mol
A)0.0276 mol
B)0.0906 mol
C)11.0 mol
D)35.2 mol
E)50.6 mol

Unlock Deck
Unlock for access to all 67 flashcards in this deck.
Unlock Deck
k this deck
29
What concentration term is defined as the number of moles of solute per kilogram of solvent in a solution?
A)normality
B)osmolarity
C)% (m/V)
D)molarity
E)molality
A)normality
B)osmolarity
C)% (m/V)
D)molarity
E)molality

Unlock Deck
Unlock for access to all 67 flashcards in this deck.
Unlock Deck
k this deck
30
Which statement is FALSE?
A)Water-soluble ionic compounds are electrolytes.
B)NaCl is an electrolyte.
C)Nonelectrolytes do not dissociate when dissolved in water.
D)Ionic compounds are typically insoluble in water.
E)When KI dissolves in water, the solution conducts electricity.
A)Water-soluble ionic compounds are electrolytes.
B)NaCl is an electrolyte.
C)Nonelectrolytes do not dissociate when dissolved in water.
D)Ionic compounds are typically insoluble in water.
E)When KI dissolves in water, the solution conducts electricity.

Unlock Deck
Unlock for access to all 67 flashcards in this deck.
Unlock Deck
k this deck
31
Calculate the molarity of 2.00 L of solution that contains 200.0 g of NaOH.[Formula weight: NaOH, 40.0 amu]
A)40.0 M
B)4.00 M
C)5.00 M
D)2.50 M
E)25.0 M
A)40.0 M
B)4.00 M
C)5.00 M
D)2.50 M
E)25.0 M

Unlock Deck
Unlock for access to all 67 flashcards in this deck.
Unlock Deck
k this deck
32
Which of the following is NOT a colligative property of a solution?
A)vapor pressure lowering
B)conductivity
C)boiling point elevation
D)freezing point depression
E)osmotic pressure
A)vapor pressure lowering
B)conductivity
C)boiling point elevation
D)freezing point depression
E)osmotic pressure

Unlock Deck
Unlock for access to all 67 flashcards in this deck.
Unlock Deck
k this deck
33
Which statement concerning solutions is FALSE?
A)Solutions are homogeneous mixtures.
B)Solutions consist of solutes and solvents.
C)Solutions are always liquids.
D)Aqueous solutions have water as the solvent.
E)Solutes cannot be filtered from a solution.
A)Solutions are homogeneous mixtures.
B)Solutions consist of solutes and solvents.
C)Solutions are always liquids.
D)Aqueous solutions have water as the solvent.
E)Solutes cannot be filtered from a solution.

Unlock Deck
Unlock for access to all 67 flashcards in this deck.
Unlock Deck
k this deck
34
How many grams of KCl are present in 250.0 mL of a 0.125 M solution? [Molar mass: KCl, 74.55 g/mol]
A)9.32 g KCl
B)0.0268 g KCl
C)37.3 g KCl
D)0.146 g KCl
E)2.33 g KCl
A)9.32 g KCl
B)0.0268 g KCl
C)37.3 g KCl
D)0.146 g KCl
E)2.33 g KCl

Unlock Deck
Unlock for access to all 67 flashcards in this deck.
Unlock Deck
k this deck
35
Increasing the pressure of a gas over a solution increases the solubility of the gas in the solution.This is an example corresponding to which law?
A)Dalton's Law
B)Henry's Law
C)Tyndall's Law
D)Raoult's Law
E)Boyle's Law
A)Dalton's Law
B)Henry's Law
C)Tyndall's Law
D)Raoult's Law
E)Boyle's Law

Unlock Deck
Unlock for access to all 67 flashcards in this deck.
Unlock Deck
k this deck
36
How are 0.15 M NaCl, 0.30 M glucose, and intracellular fluids similar?
A)They are all saturated.
B)They are all hypotonic.
C)They are all hypertonic.
D)They are all isotonic.
E)They are all supersaturated.
A)They are all saturated.
B)They are all hypotonic.
C)They are all hypertonic.
D)They are all isotonic.
E)They are all supersaturated.

Unlock Deck
Unlock for access to all 67 flashcards in this deck.
Unlock Deck
k this deck
37
Calculate the concentration (% m/V) of NaCl solution that was made by dissolving 15.0 g of sodium chloride in enough water to make 300.0 mL of solution.
A)50.0% (m/V)
B)0.0500% (m/V)
C)0.356% (m/V)
D)35.6% (m/V)
E)5.00% (m/V)
A)50.0% (m/V)
B)0.0500% (m/V)
C)0.356% (m/V)
D)35.6% (m/V)
E)5.00% (m/V)

Unlock Deck
Unlock for access to all 67 flashcards in this deck.
Unlock Deck
k this deck
38
What term describes a solution in which the solute concentration exceeds its equilibrium concentration under the prevailing conditions?
A)hypotonic
B)hypertonic
C)isotonic
D)supersaturated
E)saturated
A)hypotonic
B)hypertonic
C)isotonic
D)supersaturated
E)saturated

Unlock Deck
Unlock for access to all 67 flashcards in this deck.
Unlock Deck
k this deck
39
What is the law that states that vapor pressure of the solvent decreases in proportion to the concentration of the solute?
A)Dalton's Law
B)Henry's Law
C)Tyndall's Law
D)Raoult's Law
E)Boyle's Law
A)Dalton's Law
B)Henry's Law
C)Tyndall's Law
D)Raoult's Law
E)Boyle's Law

Unlock Deck
Unlock for access to all 67 flashcards in this deck.
Unlock Deck
k this deck
40
A student needs to prepare 250.0 mL of a 2.50 M HCl solution using the stock solution 12.0 M HCl.What volume of 12.0 M HCl is required for this dilution?
A)12.0 mL
B)25.0 mL
C)52.1 mL
D)5.21 mL
E)2.50 mL
A)12.0 mL
B)25.0 mL
C)52.1 mL
D)5.21 mL
E)2.50 mL

Unlock Deck
Unlock for access to all 67 flashcards in this deck.
Unlock Deck
k this deck
41
Which statement concerning solution concentration is TRUE?
A)A 1.0 ppm solution is more concentrated than a 1.0% (m/m) solution.
B)The ppm unit of concentration is typically used for very high concentrated solutions.
C)If a solution is 12% (m/m) it will also be 12% (m/V).
D)If a bottle is labeled 0.10 M Ba(OH)2, the concentration of OH- ions in solution is 0.20 M.
E)A solution that is 1 ppt is less concentrated than a solution that is 1 ppm.
A)A 1.0 ppm solution is more concentrated than a 1.0% (m/m) solution.
B)The ppm unit of concentration is typically used for very high concentrated solutions.
C)If a solution is 12% (m/m) it will also be 12% (m/V).
D)If a bottle is labeled 0.10 M Ba(OH)2, the concentration of OH- ions in solution is 0.20 M.
E)A solution that is 1 ppt is less concentrated than a solution that is 1 ppm.

Unlock Deck
Unlock for access to all 67 flashcards in this deck.
Unlock Deck
k this deck
42
An unknown amount of water is added to 75 mL of a 3.5 M sodium chloride solution.What can be said for certain about the concentration of the solution that results?
A)The concentration of the resulting solution will be less than 3.5 M.
B)The concentration of the resulting solution will be greater than 3.5 M.
C)The concentration of the resulting solution will be 0.26 M.
D)The concentration of the resulting solution will be 3.5 M because the amount of glucose has not changed.
E)It is impossible to say anything for certain about the concentration because the amount of water that was added is unknown.
A)The concentration of the resulting solution will be less than 3.5 M.
B)The concentration of the resulting solution will be greater than 3.5 M.
C)The concentration of the resulting solution will be 0.26 M.
D)The concentration of the resulting solution will be 3.5 M because the amount of glucose has not changed.
E)It is impossible to say anything for certain about the concentration because the amount of water that was added is unknown.

Unlock Deck
Unlock for access to all 67 flashcards in this deck.
Unlock Deck
k this deck
43
The label on a jar of jam says it contains 13 g of sucrose per tablespoon (15 mL).What is the molarity of sucrose in the jam? [Molar mass: sucrose, 342.3 g/mol]
A)0.0025 M
B)0.57 M
C)0.867 M
D)2.5 M
E)297 M
A)0.0025 M
B)0.57 M
C)0.867 M
D)2.5 M
E)297 M

Unlock Deck
Unlock for access to all 67 flashcards in this deck.
Unlock Deck
k this deck
44
One liter of alcohol combined with one liter of water does NOT produce two liters of solution.

Unlock Deck
Unlock for access to all 67 flashcards in this deck.
Unlock Deck
k this deck
45
An alloy, such as brass, is an example of a solution in the solid state.

Unlock Deck
Unlock for access to all 67 flashcards in this deck.
Unlock Deck
k this deck
46
An unopened soda bottle at 25°C contains CO2 gas at 6.2 atm.Calculate the equilibrium concentration of CO2 in the unopened soda bottle.[ ![<strong>An unopened soda bottle at 25°C contains CO<sub>2</sub> gas at 6.2 atm.Calculate the equilibrium concentration of CO<sub>2</sub> in the unopened soda bottle.[ = 3.1 × 10<sup>-2</sup> mol/(L•atm)]</strong> A)0.19 mol/L B)200 mol/L C)0.005 mol/L D)19.2 mol/L E)3.1 mol/L](https://storage.examlex.com/TB7200/11ead7b7_475b_1634_ad99_c3c3d2fd0391_TB7200_11.jpg) = 3.1 × 10-2 mol/(L•atm)]
= 3.1 × 10-2 mol/(L•atm)]
A)0.19 mol/L
B)200 mol/L
C)0.005 mol/L
D)19.2 mol/L
E)3.1 mol/L
![<strong>An unopened soda bottle at 25°C contains CO<sub>2</sub> gas at 6.2 atm.Calculate the equilibrium concentration of CO<sub>2</sub> in the unopened soda bottle.[ = 3.1 × 10<sup>-2</sup> mol/(L•atm)]</strong> A)0.19 mol/L B)200 mol/L C)0.005 mol/L D)19.2 mol/L E)3.1 mol/L](https://storage.examlex.com/TB7200/11ead7b7_475b_1634_ad99_c3c3d2fd0391_TB7200_11.jpg) = 3.1 × 10-2 mol/(L•atm)]
= 3.1 × 10-2 mol/(L•atm)]A)0.19 mol/L
B)200 mol/L
C)0.005 mol/L
D)19.2 mol/L
E)3.1 mol/L

Unlock Deck
Unlock for access to all 67 flashcards in this deck.
Unlock Deck
k this deck
47
The solid material that separates from solution when its solubility is exceeded is called a precipitate.

Unlock Deck
Unlock for access to all 67 flashcards in this deck.
Unlock Deck
k this deck
48
The solubility of gases in liquids increases with increasing pressure.

Unlock Deck
Unlock for access to all 67 flashcards in this deck.
Unlock Deck
k this deck
49
A patient requires 100.0 g of glucose in the next 12 hours.What volume of a 5.0% (m/V) glucose solution should be administered?
A)2.0 L
B)5.0 L
C)200 mL
D)20 L
E)5 mL
A)2.0 L
B)5.0 L
C)200 mL
D)20 L
E)5 mL

Unlock Deck
Unlock for access to all 67 flashcards in this deck.
Unlock Deck
k this deck
50
Cardiac arrest victims are sometimes treated by injection of a calcium chloride solution directly into the heart muscle.If 2.0 mL of a 5.0% (m/V) CaCl2 solution is administered to a patient, what mass of CaCl2 was provided?
A)1.0 mg
B)10 mg
C)0.10 g
D)10 g
E)2.5 g
A)1.0 mg
B)10 mg
C)0.10 g
D)10 g
E)2.5 g

Unlock Deck
Unlock for access to all 67 flashcards in this deck.
Unlock Deck
k this deck
51
In normal room lighting, the eye cannot distinguish a solution from a colloidal one.

Unlock Deck
Unlock for access to all 67 flashcards in this deck.
Unlock Deck
k this deck
52
If a solvent is colorless, all of its solutions will also be colorless.

Unlock Deck
Unlock for access to all 67 flashcards in this deck.
Unlock Deck
k this deck
53
What is the molarity of a solution prepared by diluting 10.0 mL of 18.0 M HCl with enough water to make 500.0 mL of solution?
A)0.360 M
B)1.80 M
C)9.25 M
D)1.11 M
E)2.27 M
A)0.360 M
B)1.80 M
C)9.25 M
D)1.11 M
E)2.27 M

Unlock Deck
Unlock for access to all 67 flashcards in this deck.
Unlock Deck
k this deck
54
What is the molarity of a solution that contains 4.50 mol of NaF dissolved in 750.0 mL of solution?
A)166 M
B)0.167 M
C)0.006 M
D)6.00 M
E)3.38 M
A)166 M
B)0.167 M
C)0.006 M
D)6.00 M
E)3.38 M

Unlock Deck
Unlock for access to all 67 flashcards in this deck.
Unlock Deck
k this deck
55
A chemist needs 0.725 moles of acetic acid for a particular reaction.What volume of a 1.50 M acetic acid solution is needed to provide this amount?
A)967 mL
B)1.09 L
C)0.967 L
D)0.483 L
E)1.09 mL
A)967 mL
B)1.09 L
C)0.967 L
D)0.483 L
E)1.09 mL

Unlock Deck
Unlock for access to all 67 flashcards in this deck.
Unlock Deck
k this deck
56
The solubility of KBr at 20°C is 60 g/100 g H2O.Increasing the temperature of the solution will likely increase the solubility of KBr.

Unlock Deck
Unlock for access to all 67 flashcards in this deck.
Unlock Deck
k this deck
57
The solubility of KBr at 20°C is 60 g/100 g H2O.Increasing the pressure above the solution will significantly increase the solubility of KBr.

Unlock Deck
Unlock for access to all 67 flashcards in this deck.
Unlock Deck
k this deck
58
What is the boiling point of a 1.5 m solution of NaCl? [kb = 0.52°C/m]
A)100.78°C
B)99.22°C
C)98.44°C
D)100.52°C
E)101.56°C
A)100.78°C
B)99.22°C
C)98.44°C
D)100.52°C
E)101.56°C

Unlock Deck
Unlock for access to all 67 flashcards in this deck.
Unlock Deck
k this deck
59
Which statement concerning solutions is FALSE?
A)Colloidal suspensions are solutions.
B)Solutions consist of solutes and solvents.
C)Solutions can have the solid, liquid, or gas state.
D)Solutions are homogeneous mixtures.
E)Solutes can be ionic or covalent.
A)Colloidal suspensions are solutions.
B)Solutions consist of solutes and solvents.
C)Solutions can have the solid, liquid, or gas state.
D)Solutions are homogeneous mixtures.
E)Solutes can be ionic or covalent.

Unlock Deck
Unlock for access to all 67 flashcards in this deck.
Unlock Deck
k this deck
60
A solution in equilibrium with undissolved solute is said to be unsaturated.

Unlock Deck
Unlock for access to all 67 flashcards in this deck.
Unlock Deck
k this deck
61
The heavier the solute molecule is, the greater its effect on the freezing point depression of a solution.

Unlock Deck
Unlock for access to all 67 flashcards in this deck.
Unlock Deck
k this deck
62
A solution that is 1 ppm contains more dissolved solute than one that is 1 ppt.

Unlock Deck
Unlock for access to all 67 flashcards in this deck.
Unlock Deck
k this deck
63
Osmosis may be defined as the movement of a solvent through a semipermeable membrane from a region of higher solute concentration to one of lower solute concentration.

Unlock Deck
Unlock for access to all 67 flashcards in this deck.
Unlock Deck
k this deck
64
An aqueous solution containing a nonvolatile solute such as NaCl will have a freezing point above 0°C at 1 atm pressure.

Unlock Deck
Unlock for access to all 67 flashcards in this deck.
Unlock Deck
k this deck
65
An aqueous solution containing a nonvolatile solute such as sucrose will boil above 100°C at 1 atm pressure.

Unlock Deck
Unlock for access to all 67 flashcards in this deck.
Unlock Deck
k this deck
66
Colligative properties depend only on the concentration of solute particles, not on their identity.

Unlock Deck
Unlock for access to all 67 flashcards in this deck.
Unlock Deck
k this deck
67
Osmosis is the process that regulates the sodium/potassium ratio in living cells.

Unlock Deck
Unlock for access to all 67 flashcards in this deck.
Unlock Deck
k this deck


