Deck 7: Energy, Rate and Equilibrium
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/61
Play
Full screen (f)
Deck 7: Energy, Rate and Equilibrium
1
The formation of rust (Fe2O3) on an exposed piece of metal typically takes several months.Which of the following statements is NOT a reasonable assumption about this chemical reaction? 4Fe(s) + 3O2(g) → 2Fe2O3(s)
A)The reaction has a low reaction rate.
B)The reaction has low activation energy.
C)The rusting of iron would occur faster in the warmer summer months than in the cooler winter months.
D)The rusting of iron would occur slower at higher altitudes where the concentration of oxygen is lower.
E)Adding a catalyst would increase the rate of the formation of rust.
A)The reaction has a low reaction rate.
B)The reaction has low activation energy.
C)The rusting of iron would occur faster in the warmer summer months than in the cooler winter months.
D)The rusting of iron would occur slower at higher altitudes where the concentration of oxygen is lower.
E)Adding a catalyst would increase the rate of the formation of rust.
The reaction has low activation energy.
2
A candy sample is completely combusted in a bomb calorimeter.The calorimeter contains 982 g of water, and the measured temperature increase is 2.62°C.What is the fuel value of the candy sample in nutritional Calories? [SHw = 1.00 cal/g•°C]
A)2573 Cal
B)2570 Cal
C)2.57 Cal
D)0.00267 Cal
E)2.67 × 10−6 Cal
A)2573 Cal
B)2570 Cal
C)2.57 Cal
D)0.00267 Cal
E)2.67 × 10−6 Cal
2.57 Cal
3
What is the name and symbol of the thermodynamic quantity that represents the heat absorbed or liberated in a chemical reaction at constant pressure?
A)Enthalpy Change, ∆H
B)Entropy Change, ∆S
C)Free Energy Change, ∆G
D)Enthalpy Change, ∆H and Entropy Change, ∆S.
E)None of the choices are correct.
A)Enthalpy Change, ∆H
B)Entropy Change, ∆S
C)Free Energy Change, ∆G
D)Enthalpy Change, ∆H and Entropy Change, ∆S.
E)None of the choices are correct.
Enthalpy Change, ∆H
4
What thermodynamic quantity is the ultimate predictor of reaction spontaneity?
A)Enthalpy Change, ∆H
B)Entropy Change, ∆S
C)Free Energy Change, ∆G
D)Entropy Change, ∆S and Free Energy Change, ∆G.
E)None of the choices are correct.
A)Enthalpy Change, ∆H
B)Entropy Change, ∆S
C)Free Energy Change, ∆G
D)Entropy Change, ∆S and Free Energy Change, ∆G.
E)None of the choices are correct.

Unlock Deck
Unlock for access to all 61 flashcards in this deck.
Unlock Deck
k this deck
5
The amount of heat necessary to raise 1 gram of a substance by 1 degree Celsius is known as which of the following?
A)enthalpy
B)activation energy
C)specific heat
D)heat of vaporization
E)free energy
A)enthalpy
B)activation energy
C)specific heat
D)heat of vaporization
E)free energy

Unlock Deck
Unlock for access to all 61 flashcards in this deck.
Unlock Deck
k this deck
6
Which of the following statements concerning the reaction below is FALSE? 2HgO(s) → 2Hg(l) + O2(g) ∆H = 182 kJ
A)There is an increase in entropy in this reaction.
B)This reaction is endothermic.
C)182 kJ of energy are required for every two moles of HgO that react.
D)The energy of the reactants is greater than the energy of the products.
E)The system absorbs energy from the surroundings in this reaction.
A)There is an increase in entropy in this reaction.
B)This reaction is endothermic.
C)182 kJ of energy are required for every two moles of HgO that react.
D)The energy of the reactants is greater than the energy of the products.
E)The system absorbs energy from the surroundings in this reaction.

Unlock Deck
Unlock for access to all 61 flashcards in this deck.
Unlock Deck
k this deck
7
Which statement concerning energy changes in chemical reactions is FALSE?
A)In an exothermic reaction, the system loses heat to the surroundings.
B)In an endothermic reaction, the system absorbs heat from the surroundings.
C)In an endothermic reaction, the reactants have a lower energy than the products.
D)In an exothermic reaction, the products have a higher energy than the reactants.
E)Exothermic reactions are characterized by a negative ∆H.
A)In an exothermic reaction, the system loses heat to the surroundings.
B)In an endothermic reaction, the system absorbs heat from the surroundings.
C)In an endothermic reaction, the reactants have a lower energy than the products.
D)In an exothermic reaction, the products have a higher energy than the reactants.
E)Exothermic reactions are characterized by a negative ∆H.

Unlock Deck
Unlock for access to all 61 flashcards in this deck.
Unlock Deck
k this deck
8
Which of the following is the equilibrium constant expression for the reaction? 2NO(g) + O2(g) → 2NO2(g)
A)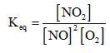
B)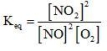
C)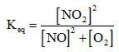
D)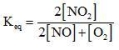
E)None of the choices are correct.
A)
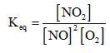
B)
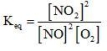
C)
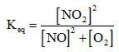
D)
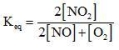
E)None of the choices are correct.

Unlock Deck
Unlock for access to all 61 flashcards in this deck.
Unlock Deck
k this deck
9
Consider the decomposition of calcium carbonate shown below.Which of the following statements correctly describes this reaction as it occurs in a test tube? CaCO3(s) + 42.5 kcal → CaO(s) + CO2(g)
A)The test tube would feel warm, since the reaction would absorb heat from the surroundings.
B)The test tube would feel cold, since the reaction would absorb heat from the surroundings.
C)The test tube would feel cold, since the reaction would release heat to the surroundings.
D)The test tube would feel warm, since the reaction would release heat to the surroundings.
E)It is impossible to predict without actually performing the experiment.
A)The test tube would feel warm, since the reaction would absorb heat from the surroundings.
B)The test tube would feel cold, since the reaction would absorb heat from the surroundings.
C)The test tube would feel cold, since the reaction would release heat to the surroundings.
D)The test tube would feel warm, since the reaction would release heat to the surroundings.
E)It is impossible to predict without actually performing the experiment.

Unlock Deck
Unlock for access to all 61 flashcards in this deck.
Unlock Deck
k this deck
10
For the reaction shown below, Keq=2×1011.Which of the following statements concerning this system at equilibrium is true? 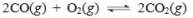
A)The equilibrium lies to the left.
B)The equilibrium solution contains equal amounts of CO, O2, and CO2.
C)The reaction is very fast, due to the high value for Keq.
D)The equilibrium solution contains predominantly CO2.
E)The equilibrium system contains almost twice as many reactant molecules as product molecules.
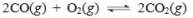
A)The equilibrium lies to the left.
B)The equilibrium solution contains equal amounts of CO, O2, and CO2.
C)The reaction is very fast, due to the high value for Keq.
D)The equilibrium solution contains predominantly CO2.
E)The equilibrium system contains almost twice as many reactant molecules as product molecules.

Unlock Deck
Unlock for access to all 61 flashcards in this deck.
Unlock Deck
k this deck
11
What is the term used to describe the energy barrier the reactants must overcome in order to form products in a chemical reaction?
A)energy of inception
B)activation energy
C)enthalpy
D)free energy
E)energy of formation
A)energy of inception
B)activation energy
C)enthalpy
D)free energy
E)energy of formation

Unlock Deck
Unlock for access to all 61 flashcards in this deck.
Unlock Deck
k this deck
12
The rate of a chemical reaction increases with an increase in concentration of one or more of the reactants.This is best explained by which of the following statements?
A)The increased concentration of the reactants increases the temperature of the molecules.
B)The increased concentration of the reactants increases the activation energy of the reaction.
C)The increased concentration of the reactants increases the speed of the molecules.
D)The increased concentration of the reactants increases the frequency of effective collisions.
E)The increased concentration of the reactants decreases the activation energy.
A)The increased concentration of the reactants increases the temperature of the molecules.
B)The increased concentration of the reactants increases the activation energy of the reaction.
C)The increased concentration of the reactants increases the speed of the molecules.
D)The increased concentration of the reactants increases the frequency of effective collisions.
E)The increased concentration of the reactants decreases the activation energy.

Unlock Deck
Unlock for access to all 61 flashcards in this deck.
Unlock Deck
k this deck
13
A carbohydrate sample weighing 0.235 g was found to have a fuel value of 3.84 kJ.What is the fuel value of one gram of this carbohydrate, in nutritional Calories?
A)3,910 Cal
B)0.535 Cal
C)16.3 Cal
D)643 Cal
E)3.91 Cal
A)3,910 Cal
B)0.535 Cal
C)16.3 Cal
D)643 Cal
E)3.91 Cal

Unlock Deck
Unlock for access to all 61 flashcards in this deck.
Unlock Deck
k this deck
14
Which of the following is FALSE about the energy diagram shown? 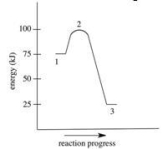
A)The activation energy is 100 kJ.
B)Point 2 on the diagram represents the activated complex.
C)This reaction is exothermic.
D)The energy of the products is lower than the energy of the reactants.
E)∆H = −50 kJ

A)The activation energy is 100 kJ.
B)Point 2 on the diagram represents the activated complex.
C)This reaction is exothermic.
D)The energy of the products is lower than the energy of the reactants.
E)∆H = −50 kJ

Unlock Deck
Unlock for access to all 61 flashcards in this deck.
Unlock Deck
k this deck
15
Which statement concerning energy changes in chemical reactions is FALSE?
A)The breaking of bonds requires energy, and the formation of bonds releases energy.
B)Activation energy is the minimum amount of energy required to initiate a chemical reaction.
C)A reaction is exothermic if the products have a lower energy than the reactants.
D)A reaction is endothermic if the reactants have a lower energy than the products.
E)The overall change in enthalpy (∆H) for a reaction is determined by the difference in energy between the products and the activated complex.
A)The breaking of bonds requires energy, and the formation of bonds releases energy.
B)Activation energy is the minimum amount of energy required to initiate a chemical reaction.
C)A reaction is exothermic if the products have a lower energy than the reactants.
D)A reaction is endothermic if the reactants have a lower energy than the products.
E)The overall change in enthalpy (∆H) for a reaction is determined by the difference in energy between the products and the activated complex.

Unlock Deck
Unlock for access to all 61 flashcards in this deck.
Unlock Deck
k this deck
16
Which statement concerning a reversible reaction at equilibrium is FALSE?
A)The concentration of the products is equal to the concentration of the reactants.
B)The forward and reverse reaction rates are equal.
C)There is no further change in the amount of reactants and products.
D)A stress to the system would cause the system to shift in the direction that best relieves the stress.
E)All of the statements are true for a reversible reaction at equilibrium.
A)The concentration of the products is equal to the concentration of the reactants.
B)The forward and reverse reaction rates are equal.
C)There is no further change in the amount of reactants and products.
D)A stress to the system would cause the system to shift in the direction that best relieves the stress.
E)All of the statements are true for a reversible reaction at equilibrium.

Unlock Deck
Unlock for access to all 61 flashcards in this deck.
Unlock Deck
k this deck
17
Ammonia is synthesized industrially by the reaction of nitrogen and hydrogen in the presence of an iron catalyst according to the reaction equation below.What effect would removing the iron catalyst have on this reaction? 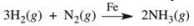
A)The reaction would shift to the right, producing more ammonia.
B)The reaction rate would increase.
C)The reaction would shift to the left, and consume ammonia.
D)The reaction rate would decrease.
E)There would be no effect on the reaction since Fe is not consumed in the reaction.
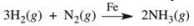
A)The reaction would shift to the right, producing more ammonia.
B)The reaction rate would increase.
C)The reaction would shift to the left, and consume ammonia.
D)The reaction rate would decrease.
E)There would be no effect on the reaction since Fe is not consumed in the reaction.

Unlock Deck
Unlock for access to all 61 flashcards in this deck.
Unlock Deck
k this deck
18
What instrument is used for measuring the heat energy absorbed or released in a chemical reaction?
A)thermometer
B)calorimeter
C)thermos
D)balance
E)scale
A)thermometer
B)calorimeter
C)thermos
D)balance
E)scale

Unlock Deck
Unlock for access to all 61 flashcards in this deck.
Unlock Deck
k this deck
19
How many small calories are equivalent to one nutritional Calorie?
A)10
B)100
C)1,000
D)10,000
E)100,000
A)10
B)100
C)1,000
D)10,000
E)100,000

Unlock Deck
Unlock for access to all 61 flashcards in this deck.
Unlock Deck
k this deck
20
A reversible reaction is said to have reached equilibrium when which of the following conditions is established?
A)The reverse reaction begins to occur.
B)The rates of the forward and reverse reactions become equal.
C)The rate of the forward reaction diminishes to zero.
D)The concentrations of reactants and products become equal.
E)The reactants are completely consumed.
A)The reverse reaction begins to occur.
B)The rates of the forward and reverse reactions become equal.
C)The rate of the forward reaction diminishes to zero.
D)The concentrations of reactants and products become equal.
E)The reactants are completely consumed.

Unlock Deck
Unlock for access to all 61 flashcards in this deck.
Unlock Deck
k this deck
21
Under normal conditions, which of the following would have the highest entropy?
A)steam
B)liquid water
C)ice
D)solid sodium chloride
E)solid iron
A)steam
B)liquid water
C)ice
D)solid sodium chloride
E)solid iron

Unlock Deck
Unlock for access to all 61 flashcards in this deck.
Unlock Deck
k this deck
22
A reaction will be spontaneous at all temperatures under which conditions?
A)The reaction is endothermic and accompanied by an increase of disorder.
B)The reaction is endothermic and accompanied by a decrease in disorder.
C)The reaction is exothermic and accompanied by an increase in disorder.
D)The reaction is exothermic and accompanied by a decrease in disorder.
E)The reaction has a positive value for free energy change.
A)The reaction is endothermic and accompanied by an increase of disorder.
B)The reaction is endothermic and accompanied by a decrease in disorder.
C)The reaction is exothermic and accompanied by an increase in disorder.
D)The reaction is exothermic and accompanied by a decrease in disorder.
E)The reaction has a positive value for free energy change.

Unlock Deck
Unlock for access to all 61 flashcards in this deck.
Unlock Deck
k this deck
23
Which one of the following actions can alter the activation energy of a reaction?
A)changing the temperature
B)changing the concentration of reactants
C)changing the concentration of products
D)changing the size of the reaction vessel
E)adding a catalyst
A)changing the temperature
B)changing the concentration of reactants
C)changing the concentration of products
D)changing the size of the reaction vessel
E)adding a catalyst

Unlock Deck
Unlock for access to all 61 flashcards in this deck.
Unlock Deck
k this deck
24
Which one of the following, if changed, would change the value of the equilibrium constant, Keq, of a reaction?
A)concentration of reactants
B)concentration of the products
C)temperature
D)catalyst
E)size of reaction vessel
A)concentration of reactants
B)concentration of the products
C)temperature
D)catalyst
E)size of reaction vessel

Unlock Deck
Unlock for access to all 61 flashcards in this deck.
Unlock Deck
k this deck
25
Thermodynamics is best described as which of the following?
A)the study of the rates at which reactions take place
B)the field of chemistry that determines the mechanisms of reactions
C)the study of LeChatelier's Principle
D)the study of energy, work, and heat
E)the study of the mass relationships in chemical reactions
A)the study of the rates at which reactions take place
B)the field of chemistry that determines the mechanisms of reactions
C)the study of LeChatelier's Principle
D)the study of energy, work, and heat
E)the study of the mass relationships in chemical reactions

Unlock Deck
Unlock for access to all 61 flashcards in this deck.
Unlock Deck
k this deck
26
One effect of a catalyst being added to a reaction mixture is
A)to increase the equilibrium constant for the reaction.
B)to slow down the rate of the reverse reaction.
C)to raise the temperature of the mixture.
D)to provide a new pathway for the reaction.
E)None of the choices are correct.
A)to increase the equilibrium constant for the reaction.
B)to slow down the rate of the reverse reaction.
C)to raise the temperature of the mixture.
D)to provide a new pathway for the reaction.
E)None of the choices are correct.

Unlock Deck
Unlock for access to all 61 flashcards in this deck.
Unlock Deck
k this deck
27
When a sample of aqueous hydrochloric acid was neutralized with aqueous sodium hydroxide in a calorimeter, the temperature of 100.0 g of water surrounding the reaction increased from 25.0°C to 31.5°C.If the specific heat of water is 1.00 cal/(g•°C), calculate the quantity of energy in calories involved in this neutralization reaction.
A)1000 cal
B)100.0 cal
C)6.50 cal
D)1250 cal
E)650 cal
A)1000 cal
B)100.0 cal
C)6.50 cal
D)1250 cal
E)650 cal

Unlock Deck
Unlock for access to all 61 flashcards in this deck.
Unlock Deck
k this deck
28
A granola bar contains 185 nutritional Calories.How many kilojoules is this? [1 cal = 4.18 J]
A)7.74 × 10-1 kJ
B)4.42 × 10-2 kJ
C)442 kJ
D)773 kJ
E)1.85 × 105 kJ
A)7.74 × 10-1 kJ
B)4.42 × 10-2 kJ
C)442 kJ
D)773 kJ
E)1.85 × 105 kJ

Unlock Deck
Unlock for access to all 61 flashcards in this deck.
Unlock Deck
k this deck
29
The reaction shown below is at equilibrium.Use LeChatelier's principle to predict the effect of adding ammonia gas to the equilibrium reaction mixture. 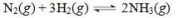
A)The equilibrium position will remain unchanged.
B)The equilibrium position will shift to the right.
C)The equilibrium position will shift to the left.
D)The equilibrium constant will increase.
E)All of the nitrogen gas will be used up.
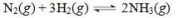
A)The equilibrium position will remain unchanged.
B)The equilibrium position will shift to the right.
C)The equilibrium position will shift to the left.
D)The equilibrium constant will increase.
E)All of the nitrogen gas will be used up.

Unlock Deck
Unlock for access to all 61 flashcards in this deck.
Unlock Deck
k this deck
30
Consider the reversible reaction:
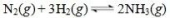 What is the correct expression for the equilibrium constant, Keq, for this reaction?
What is the correct expression for the equilibrium constant, Keq, for this reaction?
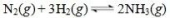 What is the correct expression for the equilibrium constant, Keq, for this reaction?
What is the correct expression for the equilibrium constant, Keq, for this reaction?
Unlock Deck
Unlock for access to all 61 flashcards in this deck.
Unlock Deck
k this deck
31
Ethylene glycol has a specific heat of 0.578 cal/(g•°C).If 23.2 g of ethylene glycol absorbs 75.6 cal of heat energy, what will the temperature increase be?
A)0.177°C
B)1.88°C
C)5.64°C
D)1.01 × 103 °C
E)3.03 × 103 °C
A)0.177°C
B)1.88°C
C)5.64°C
D)1.01 × 103 °C
E)3.03 × 103 °C

Unlock Deck
Unlock for access to all 61 flashcards in this deck.
Unlock Deck
k this deck
32
Which of the following is a process with a negative ∆S?
A)H2O(l) → H2(s)
B)CO2(s) → CO2(g)
C)CaCO3(s) → CaO(s) + CO2(g)
D)dissolving sugar in water
E)None of the choices are correct; ∆S cannot be negative.
A)H2O(l) → H2(s)
B)CO2(s) → CO2(g)
C)CaCO3(s) → CaO(s) + CO2(g)
D)dissolving sugar in water
E)None of the choices are correct; ∆S cannot be negative.

Unlock Deck
Unlock for access to all 61 flashcards in this deck.
Unlock Deck
k this deck
33
Which term describes the measure of the randomness or disorder of a chemical system?
A)energy
B)calorimetry
C)entropy
D)enthalpy
E)free energy
A)energy
B)calorimetry
C)entropy
D)enthalpy
E)free energy

Unlock Deck
Unlock for access to all 61 flashcards in this deck.
Unlock Deck
k this deck
34
In kinetics, the order of a reaction
A)is the inverse of the entropy of the system.
B)must be measured experimentally.
C)can be deduced from the balanced equation for the reaction.
D)depends on the rate constant.
E)depends on the concentrations of reactants.
A)is the inverse of the entropy of the system.
B)must be measured experimentally.
C)can be deduced from the balanced equation for the reaction.
D)depends on the rate constant.
E)depends on the concentrations of reactants.

Unlock Deck
Unlock for access to all 61 flashcards in this deck.
Unlock Deck
k this deck
35
When a cold-pack is activated, a chemical reaction occurs and the temperature of the pack contents drops sharply.Which of the following is a correct description of the reaction occurring in the pack?
A)The reaction is exothermic; ∆H ° > 0.
B)The reaction is exothermic; ∆H ° < 0.
C)The reaction is endothermic; ∆H ° > 0.
D)The reaction is endothermic; ∆H ° < 0.
E)None of the choices are correct.
A)The reaction is exothermic; ∆H ° > 0.
B)The reaction is exothermic; ∆H ° < 0.
C)The reaction is endothermic; ∆H ° > 0.
D)The reaction is endothermic; ∆H ° < 0.
E)None of the choices are correct.

Unlock Deck
Unlock for access to all 61 flashcards in this deck.
Unlock Deck
k this deck
36
In the industrial synthesis of ammonia, the equilibrium constant expression may be written as: ![<strong>In the industrial synthesis of ammonia, the equilibrium constant expression may be written as: Calculate the value of this equilibrium constant, if the equilibrium concentration of nitrogen in the reaction mixture at 600°C if [N<sub>2</sub>] = 4.53 M; [H<sub>2</sub>] = 2)49 M; and [NH<sub>3</sub>] = 7.62 M.</strong> A)5.15 B)2.07 C)0.830 D)0.676 E)1.44](https://storage.examlex.com/TB7200/11ead7b7_4758_08dc_ad99_2906842230cc_TB7200_00.jpg) Calculate the value of this equilibrium constant, if the equilibrium concentration of nitrogen in the reaction mixture at 600°C if [N2] = 4.53 M; [H2]
Calculate the value of this equilibrium constant, if the equilibrium concentration of nitrogen in the reaction mixture at 600°C if [N2] = 4.53 M; [H2]
= 2)49 M; and [NH3] = 7.62 M.
A)5.15
B)2.07
C)0.830
D)0.676
E)1.44
![<strong>In the industrial synthesis of ammonia, the equilibrium constant expression may be written as: Calculate the value of this equilibrium constant, if the equilibrium concentration of nitrogen in the reaction mixture at 600°C if [N<sub>2</sub>] = 4.53 M; [H<sub>2</sub>] = 2)49 M; and [NH<sub>3</sub>] = 7.62 M.</strong> A)5.15 B)2.07 C)0.830 D)0.676 E)1.44](https://storage.examlex.com/TB7200/11ead7b7_4758_08dc_ad99_2906842230cc_TB7200_00.jpg) Calculate the value of this equilibrium constant, if the equilibrium concentration of nitrogen in the reaction mixture at 600°C if [N2] = 4.53 M; [H2]
Calculate the value of this equilibrium constant, if the equilibrium concentration of nitrogen in the reaction mixture at 600°C if [N2] = 4.53 M; [H2]= 2)49 M; and [NH3] = 7.62 M.
A)5.15
B)2.07
C)0.830
D)0.676
E)1.44

Unlock Deck
Unlock for access to all 61 flashcards in this deck.
Unlock Deck
k this deck
37
Which of the following statements concerning the reversible reaction below is FALSE? 
A)The forward reaction produces NO2, and the reverse reaction produces N2O4.
B)At the start of the reaction, the forward reaction rate is greater than the reverse reaction rate.
C)As the reaction progresses and the concentration of N2O4 decreases, the rate of the forward reaction decreases.
D)As the reaction progresses and the concentration of NO2 increases, the rate of the reverse reaction increases.
E)When the reaction reaches equilibrium, the concentrations of NO2 and N2O4 are equal.

A)The forward reaction produces NO2, and the reverse reaction produces N2O4.
B)At the start of the reaction, the forward reaction rate is greater than the reverse reaction rate.
C)As the reaction progresses and the concentration of N2O4 decreases, the rate of the forward reaction decreases.
D)As the reaction progresses and the concentration of NO2 increases, the rate of the reverse reaction increases.
E)When the reaction reaches equilibrium, the concentrations of NO2 and N2O4 are equal.

Unlock Deck
Unlock for access to all 61 flashcards in this deck.
Unlock Deck
k this deck
38
Which of the following represents an exothermic reaction?
A)A(g) + B(g) → 2C(g) ∆H = +100 kcal
B)A(g) → B(g) + 33 kcal
C)50 kcal + 2A(g) → C(g) + D(s)
D)A(g) + B(g) → 2C(g) ∆H = +100 kcal and A(g) → B(g) + 33 kcal are exothermic reactions.
E)A(g) + B(g) → 2C(g) ∆H = +100 kcal and 50 kcal + 2A(g) → C(g) + D(s) are exothermic reactions.
A)A(g) + B(g) → 2C(g) ∆H = +100 kcal
B)A(g) → B(g) + 33 kcal
C)50 kcal + 2A(g) → C(g) + D(s)
D)A(g) + B(g) → 2C(g) ∆H = +100 kcal and A(g) → B(g) + 33 kcal are exothermic reactions.
E)A(g) + B(g) → 2C(g) ∆H = +100 kcal and 50 kcal + 2A(g) → C(g) + D(s) are exothermic reactions.

Unlock Deck
Unlock for access to all 61 flashcards in this deck.
Unlock Deck
k this deck
39
In the potential energy diagram for a chemical reaction, what is the name used for the unstable species corresponding to the top of the energy barrier separating reactants from products?
A)catalyst
B)activated complex
C)intermediate
D)free radical
E)substrate
A)catalyst
B)activated complex
C)intermediate
D)free radical
E)substrate

Unlock Deck
Unlock for access to all 61 flashcards in this deck.
Unlock Deck
k this deck
40
In general, which state of matter has the lowest entropy?
A)solid
B)liquid
C)gas
D)plasma
E)supercritical fluid
A)solid
B)liquid
C)gas
D)plasma
E)supercritical fluid

Unlock Deck
Unlock for access to all 61 flashcards in this deck.
Unlock Deck
k this deck
41
According to the second law of thermodynamics, a system and its surroundings spontaneously tend toward increasing order.

Unlock Deck
Unlock for access to all 61 flashcards in this deck.
Unlock Deck
k this deck
42
Which of the following is a TRUE statement concerning the specific heat of a substance?
A)Specific heat is the amount of heat required to raise one gram of a substance by one degree Celsius.
B)Specific heat is 1.00 cal for all substances.
C)Specific heat is the change in the temperature of a substance as it absorbs heat.
D)Specific heat is the amount of heat required to raise one mole of a substance by ten degrees.
E)Specific heat is the heat given off in a chemical reaction.
A)Specific heat is the amount of heat required to raise one gram of a substance by one degree Celsius.
B)Specific heat is 1.00 cal for all substances.
C)Specific heat is the change in the temperature of a substance as it absorbs heat.
D)Specific heat is the amount of heat required to raise one mole of a substance by ten degrees.
E)Specific heat is the heat given off in a chemical reaction.

Unlock Deck
Unlock for access to all 61 flashcards in this deck.
Unlock Deck
k this deck
43
The equilibrium constant expression for a reaction can be written if the balanced equation is known.

Unlock Deck
Unlock for access to all 61 flashcards in this deck.
Unlock Deck
k this deck
44
Lowering the activation energy of a reaction will increase its rate.

Unlock Deck
Unlock for access to all 61 flashcards in this deck.
Unlock Deck
k this deck
45
In general, a liquid state will have higher entropy than a solid state.

Unlock Deck
Unlock for access to all 61 flashcards in this deck.
Unlock Deck
k this deck
46
Exothermic reactions are always spontaneous.

Unlock Deck
Unlock for access to all 61 flashcards in this deck.
Unlock Deck
k this deck
47
10.0 grams of octane are burned in a bomb calorimeter containing 2.00 × 102 g H2O.If the water temperature increases from 25.00oC to 37.00oC, how much energy was released by the system?
A)7.40 kcal
B)3.00 × 103 kcal
C)2.52 kcal
D)1.65 kcal
E)2.40 kcal
A)7.40 kcal
B)3.00 × 103 kcal
C)2.52 kcal
D)1.65 kcal
E)2.40 kcal

Unlock Deck
Unlock for access to all 61 flashcards in this deck.
Unlock Deck
k this deck
48
Which of the following pairs of substances has the substance with the highest entropy listed first?
A)Ni(s), Br2(l)
B)Hg(l), He(g)
C)Br2(l), H2O(g)
D)Ne(g), H2O(l)
E)Ni(s), Br2(l) and Ne(g), H2O(l) are correct.
A)Ni(s), Br2(l)
B)Hg(l), He(g)
C)Br2(l), H2O(g)
D)Ne(g), H2O(l)
E)Ni(s), Br2(l) and Ne(g), H2O(l) are correct.

Unlock Deck
Unlock for access to all 61 flashcards in this deck.
Unlock Deck
k this deck
49
A reaction that leads to an increase in the entropy of the system is always spontaneous.

Unlock Deck
Unlock for access to all 61 flashcards in this deck.
Unlock Deck
k this deck
50
The order of a reaction with respect to any reactant is shown in the rate equation.

Unlock Deck
Unlock for access to all 61 flashcards in this deck.
Unlock Deck
k this deck
51
Endothermic reactions are never spontaneous.

Unlock Deck
Unlock for access to all 61 flashcards in this deck.
Unlock Deck
k this deck
52
Reactants and products that are aqueous solutions do not appear in the equilibrium constant expression.

Unlock Deck
Unlock for access to all 61 flashcards in this deck.
Unlock Deck
k this deck
53
LeChatelier's principle states that if a stress is placed on a system at equilibrium, the system will respond by altering the equilibrium composition in such a way as to relieve the stress.

Unlock Deck
Unlock for access to all 61 flashcards in this deck.
Unlock Deck
k this deck
54
A reaction that leads to a decrease in the free energy of the system is always spontaneous.

Unlock Deck
Unlock for access to all 61 flashcards in this deck.
Unlock Deck
k this deck
55
For the reaction A(g) → 2B(g), Keq = 4.5 × 105.Which statement is TRUE for the system at equilibrium?
A)[A] >> [B]
B)There is twice as much B as there is A in the reaction vessel.
C)There is more than 100 times more B than there is A in the reaction vessel.
D)There is twice as much A as there is B in the reaction vessel.
E)Changing the temperature will have no affect on the equilibrium.
A)[A] >> [B]
B)There is twice as much B as there is A in the reaction vessel.
C)There is more than 100 times more B than there is A in the reaction vessel.
D)There is twice as much A as there is B in the reaction vessel.
E)Changing the temperature will have no affect on the equilibrium.

Unlock Deck
Unlock for access to all 61 flashcards in this deck.
Unlock Deck
k this deck
56
Changing the temperature alters the value of the equilibrium constant for a reaction.

Unlock Deck
Unlock for access to all 61 flashcards in this deck.
Unlock Deck
k this deck
57
Removal of a gaseous product from an equilibrium reaction mixture shifts the equilibrium toward formation of more products.

Unlock Deck
Unlock for access to all 61 flashcards in this deck.
Unlock Deck
k this deck
58
Which of the following will NOT increase the rate of a reaction?
A)increasing the size of the reaction vessel
B)adding a catalyst
C)increasing the temperature
D)increasing the concentration of the reactants
E)All of the above will increase the rate of a reaction.
A)increasing the size of the reaction vessel
B)adding a catalyst
C)increasing the temperature
D)increasing the concentration of the reactants
E)All of the above will increase the rate of a reaction.

Unlock Deck
Unlock for access to all 61 flashcards in this deck.
Unlock Deck
k this deck
59
A collision between reactant molecules that produces one or more product molecules is called an effective collision.

Unlock Deck
Unlock for access to all 61 flashcards in this deck.
Unlock Deck
k this deck
60
A reaction that leads to a decrease in the enthalpy of the system is always spontaneous.

Unlock Deck
Unlock for access to all 61 flashcards in this deck.
Unlock Deck
k this deck
61
A catalyst increases the equilibrium constant for a reaction.

Unlock Deck
Unlock for access to all 61 flashcards in this deck.
Unlock Deck
k this deck


