Deck 20: Dienes, Conjugated Systems, and Pericyclic Reactions
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/75
Play
Full screen (f)
Deck 20: Dienes, Conjugated Systems, and Pericyclic Reactions
1
What is the correct order of exothermicity for hydrogenation of the following hexadienes upon treatment with H2/Pd (more exothermic > less exothermic)? 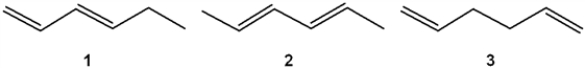
A) 1 > 2 > 3
B) 3 > 1 > 2
C) 2 > 1 > 3
D) 3 > 2 > 1

A) 1 > 2 > 3
B) 3 > 1 > 2
C) 2 > 1 > 3
D) 3 > 2 > 1
3 > 1 > 2
2
Which of the following alkenes undergoes the least exothermic hydrogenation upon treatment with H2/Pd 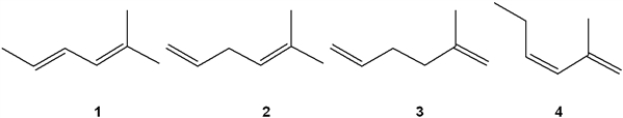
A) 1
B) 2
C) 3
D) 4

A) 1
B) 2
C) 3
D) 4
3
3
Which of the following is(are) formed upon addition of 1 mol of Br2 to 1,3-cyclohexane? 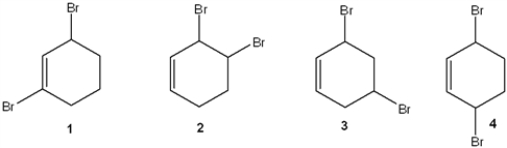
A) only 2
B) only 1 and 3
C) only 2 and 4
D) 1, 2 and 3

A) only 2
B) only 1 and 3
C) only 2 and 4
D) 1, 2 and 3
only 2 and 4
4
What is (are) the major organic product(s) formed in the following reaction? 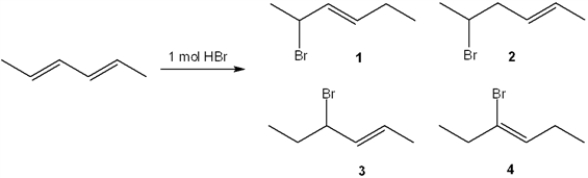
A) only 1
B) only 3
C) only 1 and 3
D) only 2 and 4

A) only 1
B) only 3
C) only 1 and 3
D) only 2 and 4

Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck
5
What is the IUPAC name of the following compound? 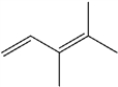
A) 2,3-dimethyl-2,4-pentadiene
B) (Z)-2,3-dimethyl-2,4-pentadiene
C) 3,4-dimethyl-1,3-pentadiene
D) (Z)-3,4-dimethyl-1,3-pentadiene

A) 2,3-dimethyl-2,4-pentadiene
B) (Z)-2,3-dimethyl-2,4-pentadiene
C) 3,4-dimethyl-1,3-pentadiene
D) (Z)-3,4-dimethyl-1,3-pentadiene

Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck
6
Which of the following are conjugated dienes? 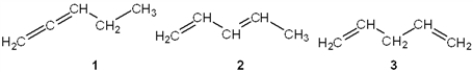
A) only 1
B) only 2
C) only 1 and 2
D) 1, 2 and 3

A) only 1
B) only 2
C) only 1 and 2
D) 1, 2 and 3

Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck
7
Which of the following terms describes the mechanism for the addition of Br2 to butadiene?
A) electrophilic addition
B) nucleophilic addition
C) pericyclic addition
D) conjugate addition
A) electrophilic addition
B) nucleophilic addition
C) pericyclic addition
D) conjugate addition

Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck
8
What is the kinetic product obtained from the addition of 1 mole of bromine to 1,3-butadiene?
A) 3,4-dibromo-1-butene
B) (E)-1,4-dibromo-2-butene
C) (Z)-1,4-dibromo-2-butene
D) (Z)-2,3-dibromo-2-butene
A) 3,4-dibromo-1-butene
B) (E)-1,4-dibromo-2-butene
C) (Z)-1,4-dibromo-2-butene
D) (Z)-2,3-dibromo-2-butene

Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck
9
Which of the following is not a major organic product obtained from the addition of 1 mole of bromine to (E)-1,3-pentadiene?
A) (E)-4,5-dibromo-2-pentene
B) 3,4-dibromo-1-pentene
C) (E)-1,4-dibromo-2-pentene
D) (E)-1,2-dibromo-2-pentene
A) (E)-4,5-dibromo-2-pentene
B) 3,4-dibromo-1-pentene
C) (E)-1,4-dibromo-2-pentene
D) (E)-1,2-dibromo-2-pentene

Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck
10
What is (are) the major organic product(s) formed in the following reaction? 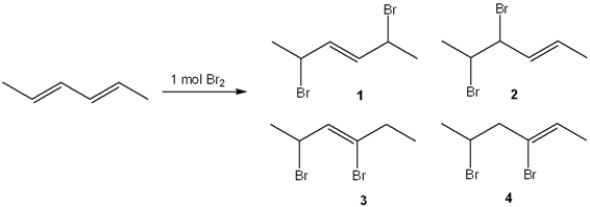
A) only 1
B) only 1 and 2
C) only 1 and 3
D) only 2 and 4

A) only 1
B) only 1 and 2
C) only 1 and 3
D) only 2 and 4

Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck
11
What is the reactive intermediate in the reaction of 1,3-butadiene with HBr resulting in 1,2-addition?
A) cyclic bromonium cation
B) allylic cation
C) allylic radical
D) dienophile
A) cyclic bromonium cation
B) allylic cation
C) allylic radical
D) dienophile

Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck
12
Which of the following arrangements of p atomic orbitals gives the highest energy π-antibonding molecular orbital of 1,3-butadiene? 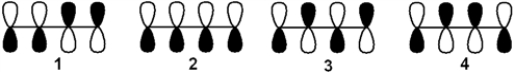
A) 1
B) 2
C) 3
D) 4

A) 1
B) 2
C) 3
D) 4

Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck
13
What is the systematic IUPAC name of the following compound? 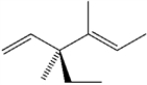
A) (3S,4E)-3-ethyl-3,4-dimethyl-1,4-hexadiene
B) (3R,4E)-3-ethyl-3,4-dimethyl-1,4-hexadiene
C) (3S,4Z)-3-ethyl-3,4-dimethyl-1,4-hexadiene
D) (3R,4Z)-3-ethyl-3,4-dimethyl-1,4-hexadiene

A) (3S,4E)-3-ethyl-3,4-dimethyl-1,4-hexadiene
B) (3R,4E)-3-ethyl-3,4-dimethyl-1,4-hexadiene
C) (3S,4Z)-3-ethyl-3,4-dimethyl-1,4-hexadiene
D) (3R,4Z)-3-ethyl-3,4-dimethyl-1,4-hexadiene

Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck
14
Which of the following arrangements of p atomic orbitals gives the lowest energy π-bonding molecular orbital of 1,3-butadiene? 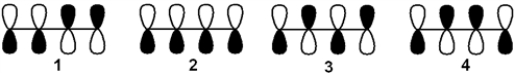
A) 1
B) 2
C) 3
D) 4

A) 1
B) 2
C) 3
D) 4

Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck
15
What is the thermodynamic product obtained from the addition of 1 mole of bromine to 1,3-butadiene?
A) 3,4-dibromo-1-butene
B) (E)-1,4-dibromo-2-butene
C) (Z)-1,4-dibromo-2-butene
D) (Z)-2,3-dibromo-2-butene
A) 3,4-dibromo-1-butene
B) (E)-1,4-dibromo-2-butene
C) (Z)-1,4-dibromo-2-butene
D) (Z)-2,3-dibromo-2-butene

Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck
16
What is (are) the major organic product(s) formed in the following reaction? 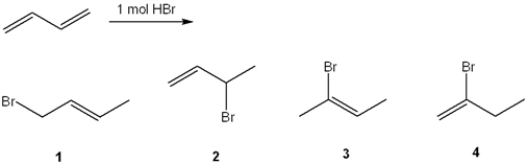
A) only 1
B) only 2
C) only 1 and 3
D) only 2 and 4

A) only 1
B) only 2
C) only 1 and 3
D) only 2 and 4

Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck
17
Which of the following are conjugated dienes? 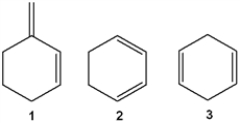
A) only 1
B) only 2
C) only 1 and 2
D) 1, 2 and 3

A) only 1
B) only 2
C) only 1 and 2
D) 1, 2 and 3

Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck
18
What is (are) the major organic product(s) formed in the following reaction? 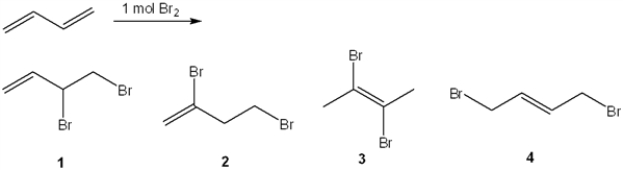
A) only 1
B) only 1 and 3
C) only 2 and 4
D) only 1 and 4

A) only 1
B) only 1 and 3
C) only 2 and 4
D) only 1 and 4

Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck
19
Which of the following arrangements of p atomic orbitals of 1,3-butadiene has the greatest number of nodes? 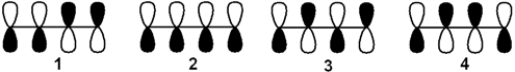
A) 1
B) 2
C) 3
D) 4

A) 1
B) 2
C) 3
D) 4

Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck
20
What is the IUPAC name of the following compound? 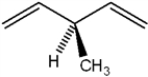
A) (R)-3-vinyl-1-butene
B) 3-methyl-1,5-pentadiene
C) 3-methyl-1,4-pentadiene
D) (R)-3-methyl-1,4-pentadiene

A) (R)-3-vinyl-1-butene
B) 3-methyl-1,5-pentadiene
C) 3-methyl-1,4-pentadiene
D) (R)-3-methyl-1,4-pentadiene

Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck
21
Which of the following has the highest value of λmax in the ultraviolet-visible spectrum? 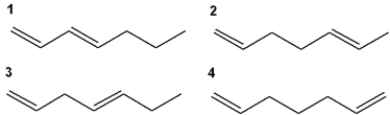
A) 1
B) 2
C) 3
D) 4

A) 1
B) 2
C) 3
D) 4

Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck
22
What are the units of λmax in ultraviolet-visible spectroscopy?
A) J⋅mol−1
B) M−1
C) nm
D) none, λmax is a dimensionless quantity
A) J⋅mol−1
B) M−1
C) nm
D) none, λmax is a dimensionless quantity

Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck
23
For a diene to undergo a Diels-Alder reaction it must be able to adopt an s-trans conformation.

Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck
24
Which of the following energy diagrams represents the electronic state of butadiene after absorption of radiation? 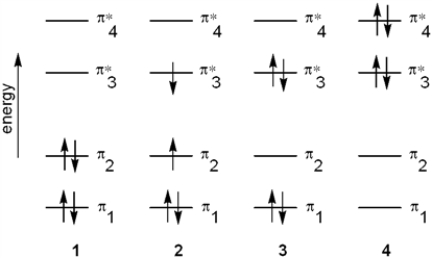
A) 1
B) 2
C) 3
D) 4

A) 1
B) 2
C) 3
D) 4

Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck
25
Which of the following relationships is not valid as applied to ultraviolet-visible spectroscopy?
A) A = log (Io/I)
B) A = εcl
C) ΔE = hν
D) A = λmaxl
A) A = log (Io/I)
B) A = εcl
C) ΔE = hν
D) A = λmaxl

Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck
26
The conjugated portion of the following molecule is circled. 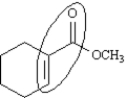


Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck
27
Electrophilic addition reaction of conjugated dienes that occur at high temperature and/or long reaction times (reversible conditions) are said to be under kinetic control.

Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck
28
Which of the following processes takes place upon absorption of ultraviolet-visible radiation?
A) bond vibration
B) electron excitation
C) nuclear spin flip
D) electron spin flip
A) bond vibration
B) electron excitation
C) nuclear spin flip
D) electron spin flip

Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck
29
What range of wavelengths corresponds to the ultraviolet region of the electromagnetic spectrum?
A) 200-400 nm
B) 400-700 nm
C) 2.5-25 μm
D) 2-10 mm
A) 200-400 nm
B) 400-700 nm
C) 2.5-25 μm
D) 2-10 mm

Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck
30
Which of the following has the highest value of λmax in the ultraviolet-visible spectrum? 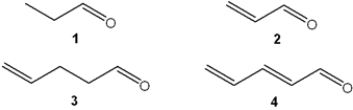
A) 1
B) 2
C) 3
D) 4

A) 1
B) 2
C) 3
D) 4

Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck
31
Which of the following reaction coordinate diagrams explains the formation of different thermodynamic (T) and kinetic products from the same reactants (R)? 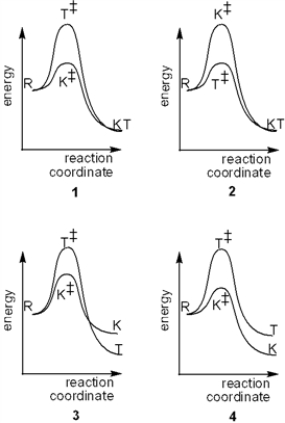
A) 1
B) 2
C) 3
D) 4

A) 1
B) 2
C) 3
D) 4

Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck
32
Consider the following structure. 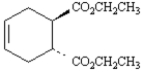 This substance could be produced by the reaction of the following diene and dienophile.
This substance could be produced by the reaction of the following diene and dienophile. 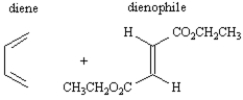
 This substance could be produced by the reaction of the following diene and dienophile.
This substance could be produced by the reaction of the following diene and dienophile. 

Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck
33
The conjugated portion of the following molecule is circled. 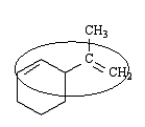


Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck
34
Which of the following energy diagrams represents the electronic state of the ground state of 1,3-butadiene? 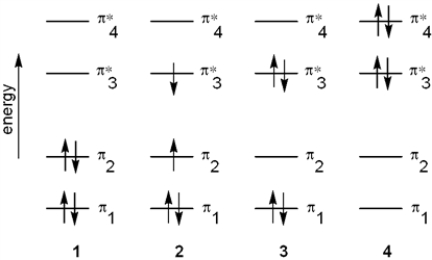
A) 1
B) 2
C) 3
D) 4

A) 1
B) 2
C) 3
D) 4

Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck
35
What are the units of ε in ultraviolet-visible spectroscopy?
A) M−1⋅cm−1
B) M⋅cm
C) J⋅mol−1⋅M−1
D) none, ε is a dimensionless quantity
A) M−1⋅cm−1
B) M⋅cm
C) J⋅mol−1⋅M−1
D) none, ε is a dimensionless quantity

Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck
36
What quantity is abbreviated as ε in ultraviolet-visible spectroscopy?
A) absorbance
B) molecular absorptivity
C) intensity of transmitted light
D) wavelength of maximum absorbance
A) absorbance
B) molecular absorptivity
C) intensity of transmitted light
D) wavelength of maximum absorbance

Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck
37
Which of the following laws relates absorbance to the concentration of a sample and the pathlength in ultraviolet-visible spectroscopy?
A) Buzz-Beer law
B) Beer-Lambert law
C) Duff-Beer law
D) Christopher-Lambert law
A) Buzz-Beer law
B) Beer-Lambert law
C) Duff-Beer law
D) Christopher-Lambert law

Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck
38
Which of the following electronic transitions is apparent in the ultraviolet-visible spectrum of 1,3-butadiene?
A) n → π*
B) π → π *
C) σ → π *
D) σ → π
A) n → π*
B) π → π *
C) σ → π *
D) σ → π

Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck
39
What is (are) the major organic product(s) formed in the following reaction? 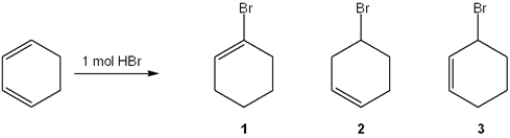
A) only 1
B) only 2
C) only 3
D) 1, 2 and 3

A) only 1
B) only 2
C) only 3
D) 1, 2 and 3

Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck
40
What are the units of absorbance, A, in ultraviolet-visible spectroscopy?
A) J⋅mol−1
B) nm⋅mol−1
C) J⋅mol−1⋅M−1
D) none, A is a dimensionless quantity
A) J⋅mol−1
B) nm⋅mol−1
C) J⋅mol−1⋅M−1
D) none, A is a dimensionless quantity

Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck
41
Consider the following reaction. ![Consider the following reaction. This is an example of a sigmatropic reaction with a [3,3]-shift.](https://storage.examlex.com/TB7078/11ead7c8_9d49_7798_84b0_09318ae3fea8_TB7078_00.jpg) This is an example of a sigmatropic reaction with a [3,3]-shift.
This is an example of a sigmatropic reaction with a [3,3]-shift.
![Consider the following reaction. This is an example of a sigmatropic reaction with a [3,3]-shift.](https://storage.examlex.com/TB7078/11ead7c8_9d49_7798_84b0_09318ae3fea8_TB7078_00.jpg) This is an example of a sigmatropic reaction with a [3,3]-shift.
This is an example of a sigmatropic reaction with a [3,3]-shift.
Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck
42
Consider the reaction below to answer the following question(s). 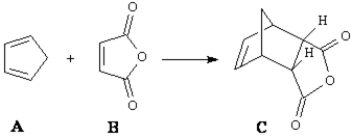 Fill in the blank in the question with the appropriate letter.
Fill in the blank in the question with the appropriate letter.
The dienophile in the reaction is ____.
 Fill in the blank in the question with the appropriate letter.
Fill in the blank in the question with the appropriate letter.The dienophile in the reaction is ____.

Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck
43
The following spectrum could arise from π to π* transitions. 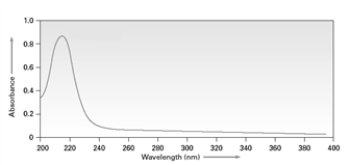


Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck
44
Consider the reaction below to answer the following question(s): 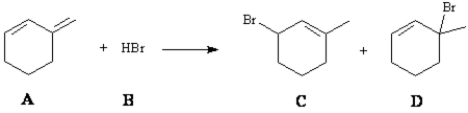 Enter the appropriate letter in the blank for each the following statements.
Enter the appropriate letter in the blank for each the following statements.
The product that results from following mechanistic step is _____.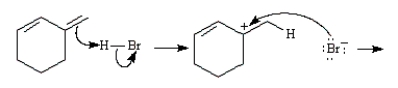
 Enter the appropriate letter in the blank for each the following statements.
Enter the appropriate letter in the blank for each the following statements.The product that results from following mechanistic step is _____.


Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck
45
Consider the reaction below to answer the following question(s).  Fill in the blank in the question with the appropriate letter.
Fill in the blank in the question with the appropriate letter.
This is an example of a(n) ____________ reaction.
A. sigmatropic reaction
B. electrophillic addition
C. Diels-Alder reaction
D. Cope rearrangement
 Fill in the blank in the question with the appropriate letter.
Fill in the blank in the question with the appropriate letter.This is an example of a(n) ____________ reaction.
A. sigmatropic reaction
B. electrophillic addition
C. Diels-Alder reaction
D. Cope rearrangement

Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck
46
What is the IUPAC name of the following compound? 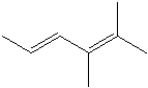


Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck
47
What is the IUPAC name of the following compound? 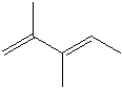


Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck
48
Consider the following spectrum for a conjugated diene. 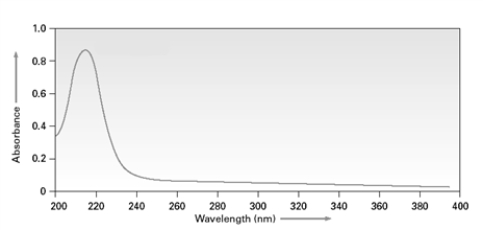 If a similar spectrum were taken for a conjugated tetraene, the λmax would be _________than 217 nm.
If a similar spectrum were taken for a conjugated tetraene, the λmax would be _________than 217 nm.
 If a similar spectrum were taken for a conjugated tetraene, the λmax would be _________than 217 nm.
If a similar spectrum were taken for a conjugated tetraene, the λmax would be _________than 217 nm.
Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck
49
Based on the following table.
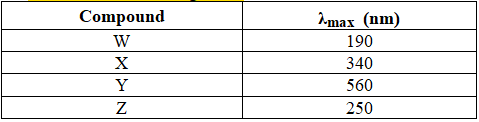
Compound ______ has the largest energy difference between the HOMO and LUMO.

Compound ______ has the largest energy difference between the HOMO and LUMO.

Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck
50
The following compound could act as a diene in a Diels-Alder reaction. 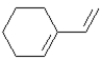


Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck
51
Sigmatropic shifts, cycloaddition reactions and anuulation reactions are all examples of pericyclic reactions.

Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck
52
The following two compounds could be easily distinguished by NMR, IR, UV or MS spectra. 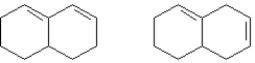


Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck
53
Consider the following reaction. ![Consider the following reaction. This is an example of a sigmatropic reaction with a [3,3]-shift.](https://storage.examlex.com/TB7078/11ead7c8_9d49_9ea9_84b0_37fc0d07e320_TB7078_00.jpg) This is an example of a sigmatropic reaction with a [3,3]-shift.
This is an example of a sigmatropic reaction with a [3,3]-shift.
![Consider the following reaction. This is an example of a sigmatropic reaction with a [3,3]-shift.](https://storage.examlex.com/TB7078/11ead7c8_9d49_9ea9_84b0_37fc0d07e320_TB7078_00.jpg) This is an example of a sigmatropic reaction with a [3,3]-shift.
This is an example of a sigmatropic reaction with a [3,3]-shift.
Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck
54
For Diels-Alder cycloaddition reactions to take place most rapidly and in highest yield a dienophile must be substituted with electron-donating groups.

Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck
55
Consider the reaction below to answer the following question(s):  Enter the appropriate letter in the blank for each the following statements.
Enter the appropriate letter in the blank for each the following statements.
The electrophile in this reaction is ____.
 Enter the appropriate letter in the blank for each the following statements.
Enter the appropriate letter in the blank for each the following statements.The electrophile in this reaction is ____.

Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck
56
Consider the reaction below to answer the following question(s).  Fill in the blank in the question with the appropriate letter.
Fill in the blank in the question with the appropriate letter.
The diene in the reaction is ____.
 Fill in the blank in the question with the appropriate letter.
Fill in the blank in the question with the appropriate letter.The diene in the reaction is ____.

Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck
57
Consider the reaction below to answer the following question(s):  Enter the appropriate letter in the blank for each the following statements.
Enter the appropriate letter in the blank for each the following statements.
The nucleophile in this reaction is ____.
 Enter the appropriate letter in the blank for each the following statements.
Enter the appropriate letter in the blank for each the following statements.The nucleophile in this reaction is ____.

Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck
58
Consider the reaction below to answer the following question(s):  Enter the appropriate letter in the blank for each the following statements.
Enter the appropriate letter in the blank for each the following statements.
The product that results from 1,4-addition is _____.
 Enter the appropriate letter in the blank for each the following statements.
Enter the appropriate letter in the blank for each the following statements.The product that results from 1,4-addition is _____.

Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck
59
Consider the reaction below to answer the following question(s):  Enter the appropriate letter in the blank for each the following statements.
Enter the appropriate letter in the blank for each the following statements.
The kinetically controlled product in this reaction is _____.
 Enter the appropriate letter in the blank for each the following statements.
Enter the appropriate letter in the blank for each the following statements.The kinetically controlled product in this reaction is _____.

Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck
60
When an organic molecule is irradiated with ultraviolet radiation, the energy absorbed by the molecule corresponds to the amount necessary to excite electrons from one molecular orbital to another.

Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck
61
Provide the structures of the constitutional isomers of the two major organic products obtained from the following reaction? 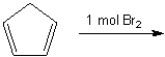
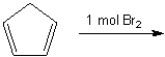

Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck
62
What are the two major organic products obtained from the following reaction? 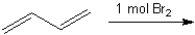
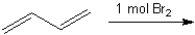

Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck
63
Why carbonyl groups of aldehydes and ketones on conjugation with carbon-carbon double bonds show strong absorption in the ultraviolet spectrum?

Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck
64
The molar absorptivity of ethylene in hexane is 15,000 L mol−1 cm−1. What is the maximum absorbance observed at a concentration of 1.5 x 10−4 mol L−1? (Path length = 1 cm)
A) 2.25
B) 1.25
C) 3.25
D) 2.52
A) 2.25
B) 1.25
C) 3.25
D) 2.52

Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck
65
Which of 1,3-cyclohexadiene and 1,4-cyclohexadiene has the greatest heat of hydrogenation (i.e., the most exothermic reaction with H2 in the presence of a catalyst). Provide a brief explanation for your choice ?

Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck
66
What is the major organic product obtained from the following reaction? 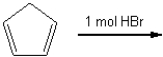
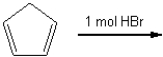

Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck
67
The [2+2] cycloaddition reaction of ethylene to form cyclobutane does not occur because _____. ![<strong>The [2+2] cycloaddition reaction of ethylene to form cyclobutane does not occur because _____. </strong> A) the reaction is forbidden as determined by the frontier molecular orbital analysis B) the reaction is allowed as determined by the frontier molecular orbital analysis C) the reaction is allowed, and ethylene collides in a suprafacial manner D) the reaction is allowed, and ethylene collides in an antarafacial manner](https://storage.examlex.com/TB7078/11ead7c8_9d4c_0fbc_84b0_fbdf5d201147_TB7078_00.jpg)
A) the reaction is forbidden as determined by the frontier molecular orbital analysis
B) the reaction is allowed as determined by the frontier molecular orbital analysis
C) the reaction is allowed, and ethylene collides in a suprafacial manner
D) the reaction is allowed, and ethylene collides in an antarafacial manner
![<strong>The [2+2] cycloaddition reaction of ethylene to form cyclobutane does not occur because _____. </strong> A) the reaction is forbidden as determined by the frontier molecular orbital analysis B) the reaction is allowed as determined by the frontier molecular orbital analysis C) the reaction is allowed, and ethylene collides in a suprafacial manner D) the reaction is allowed, and ethylene collides in an antarafacial manner](https://storage.examlex.com/TB7078/11ead7c8_9d4c_0fbc_84b0_fbdf5d201147_TB7078_00.jpg)
A) the reaction is forbidden as determined by the frontier molecular orbital analysis
B) the reaction is allowed as determined by the frontier molecular orbital analysis
C) the reaction is allowed, and ethylene collides in a suprafacial manner
D) the reaction is allowed, and ethylene collides in an antarafacial manner

Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck
68
How would you be able to distinguish between 1,3-cyclohexadiene and 1,4-cyclohexadiene using ultraviolet spectroscopy?

Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck
69
What are the two major organic products obtained from the following reaction? 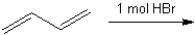
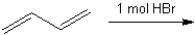

Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck
70
Draw two resonance structures of the reactive intermediate that accounts for the formation of 1,2- and 1,4-addition products upon reaction of 1,3-butadiene with one equivalent of HBr.

Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck
71
Pericyclic reactions occur in a single step with the _____.
A) formation of a radical
B) formation of an ionic intermediate
C) formation of a molecular ion
D) formation of a transition state
A) formation of a radical
B) formation of an ionic intermediate
C) formation of a molecular ion
D) formation of a transition state

Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck
72
Identify the product of the given reaction. 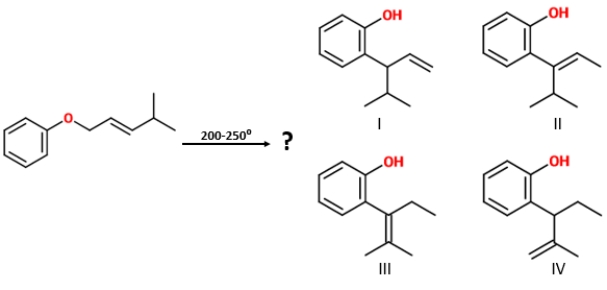
A) I
B) II
C) IV
D) III

A) I
B) II
C) IV
D) III

Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck
73
Identify the product of the given reaction. 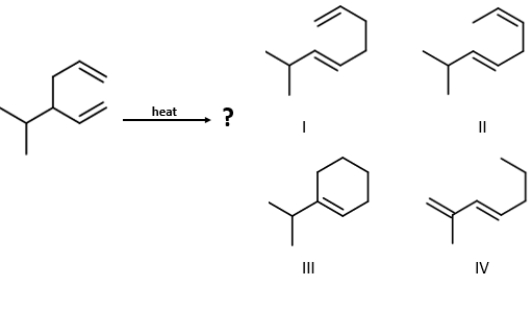
A) I
B) II
C) IV
D) III

A) I
B) II
C) IV
D) III

Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck
74
Draw two resonance structures of the reactive intermediate that accounts for the formation of 1,2- and 1,4-addition products upon reaction of 1,3-butadiene with one equivalent of bromine.

Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck
75
A reaction in which two reactants add together in a single step to form a cyclic product is known as a _____.

Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck


