Deck 14: Temperature and Heat
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/115
Play
Full screen (f)
Deck 14: Temperature and Heat
1
Normal body temperature for humans is 37 C. What is this temperature in kelvins?
A) 296
B) 310
C) 393
D) 273
A) 296
B) 310
C) 393
D) 273
310
2
Which of the following statements is true?
A) A hot object contains a lot of heat.
B) A cold object contains only a little heat.
C) Objects do not contain heat.
D) Statements a and b are true.
A) A hot object contains a lot of heat.
B) A cold object contains only a little heat.
C) Objects do not contain heat.
D) Statements a and b are true.
Objects do not contain heat.
3
Which best describes the relationship between two systems in thermal equilibrium?
A) No net energy is exchanged.
B) Volumes are equal.
C) Masses are equal.
D) The velocity is zero.
A) No net energy is exchanged.
B) Volumes are equal.
C) Masses are equal.
D) The velocity is zero.
No net energy is exchanged.
4
Arrange the following units, Calorie, calorie, and Joule from smallest to largest.
A) Joule, Calorie, calorie
B) calorie, Calorie, Joule
C) Joule, calorie, Calorie
D) Answers a and c are both correct, it apparently is a trick question.
A) Joule, Calorie, calorie
B) calorie, Calorie, Joule
C) Joule, calorie, Calorie
D) Answers a and c are both correct, it apparently is a trick question.

Unlock Deck
Unlock for access to all 115 flashcards in this deck.
Unlock Deck
k this deck
5
A temperature of 233 K equals which of the following?
A) 506 C
B) 40 C
C) -40 F
D) 40 F
A) 506 C
B) 40 C
C) -40 F
D) 40 F

Unlock Deck
Unlock for access to all 115 flashcards in this deck.
Unlock Deck
k this deck
6
A temperature change from 15 C to 45 C corresponds to what incremental change in F?
A) 54
B) 40
C) 36
D) 313
A) 54
B) 40
C) 36
D) 313

Unlock Deck
Unlock for access to all 115 flashcards in this deck.
Unlock Deck
k this deck
7
What is the temperature of a system in thermal equilibrium with another system made up of ice and water at one atmosphere of pressure?
A) 0 F
B) 273 K
C) 0 K
D) 100 C
A) 0 F
B) 273 K
C) 0 K
D) 100 C

Unlock Deck
Unlock for access to all 115 flashcards in this deck.
Unlock Deck
k this deck
8
Pennies used to be made of copper, but now they are made of copper-coated zinc. If one were to do a precise calorimetry experiment to determine the specific heat of the new pennies, what would be the result to be?
A) It would be that of copper since copper is on the outside.
B) It would be that of zinc since zinc is in the center.
C) It would be the sum of the copper and zinc specific heats.
D) It would be between that of copper and that of zinc, depending on coating thickness.
A) It would be that of copper since copper is on the outside.
B) It would be that of zinc since zinc is in the center.
C) It would be the sum of the copper and zinc specific heats.
D) It would be between that of copper and that of zinc, depending on coating thickness.

Unlock Deck
Unlock for access to all 115 flashcards in this deck.
Unlock Deck
k this deck
9
Carbon dioxide forms into a solid (dry ice) at approximately -157 F. What temperature in degrees Celsius does this correspond to?
A) "-157 C"
B) "-93 C"
C) "-105 C"
D) "-121 C"
A) "-157 C"
B) "-93 C"
C) "-105 C"
D) "-121 C"

Unlock Deck
Unlock for access to all 115 flashcards in this deck.
Unlock Deck
k this deck
10
Calories of the food type are equal to which of the following?
A) 4.186 J
B) 4186 J
C) 1 BTU
D) 1054 J
A) 4.186 J
B) 4186 J
C) 1 BTU
D) 1054 J

Unlock Deck
Unlock for access to all 115 flashcards in this deck.
Unlock Deck
k this deck
11
How many calories are equal to one BTU? (One calorie = 4.186 J, one BTU = 1054 J.)
A) 0.252
B) 3.97
C) 252
D) 397
A) 0.252
B) 3.97
C) 252
D) 397

Unlock Deck
Unlock for access to all 115 flashcards in this deck.
Unlock Deck
k this deck
12
Of the following systems, which contains the most heat?
A) 100 kg of water at 80°C
B) 250 kg of water at 40°C
C) 600 kg of ice at 0°C
D) Systems do not contain heat.
A) 100 kg of water at 80°C
B) 250 kg of water at 40°C
C) 600 kg of ice at 0°C
D) Systems do not contain heat.

Unlock Deck
Unlock for access to all 115 flashcards in this deck.
Unlock Deck
k this deck
13
If heat is flowing from a table to a block of ice moving across the table, which of the following must be true?
A) The table is rough, and there is friction between the table and ice.
B) The ice is cooler than the table.
C) The ice is changing phase.
D) All three are possible, but none is absolutely necessary.
A) The table is rough, and there is friction between the table and ice.
B) The ice is cooler than the table.
C) The ice is changing phase.
D) All three are possible, but none is absolutely necessary.

Unlock Deck
Unlock for access to all 115 flashcards in this deck.
Unlock Deck
k this deck
14
What is the temperature of a system in thermal equilibrium with another system made up of water and steam at one atmosphere of pressure?
A) 0 F
B) 273 K
C) 0 K
D) 100 C
A) 0 F
B) 273 K
C) 0 K
D) 100 C

Unlock Deck
Unlock for access to all 115 flashcards in this deck.
Unlock Deck
k this deck
15
A substance is heated from 15 C to 45 C. What would the same incremental change be when registered in kelvins?
A) 20
B) 40
C) 30
D) 313
A) 20
B) 40
C) 30
D) 313

Unlock Deck
Unlock for access to all 115 flashcards in this deck.
Unlock Deck
k this deck
16
An interval of one Celsius degree is equivalent to an interval of:
A) one Fahrenheit degree.
B) one kelvin.
C) 5/9 Fahrenheit degree.
D) 5/9 kelvin.
A) one Fahrenheit degree.
B) one kelvin.
C) 5/9 Fahrenheit degree.
D) 5/9 kelvin.

Unlock Deck
Unlock for access to all 115 flashcards in this deck.
Unlock Deck
k this deck
17
At what temperature is the same numerical value obtained in Celsius and Fahrenheit?
A) "-40 "
B) "0 "
C) "40 "
D) "-72 "
A) "-40 "
B) "0 "
C) "40 "
D) "-72 "

Unlock Deck
Unlock for access to all 115 flashcards in this deck.
Unlock Deck
k this deck
18
Arrange the temperatures 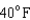 ,
, 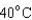 , and
, and 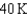 from highest to lowest.
from highest to lowest.
A)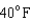 ,
, 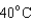 ,
, 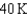
B)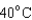 ,
, 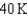 ,
, 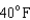
C)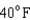 ,
, 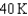 ,
, 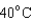
D)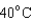 ,
, 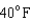 ,
, 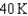
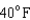 ,
, 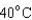 , and
, and 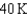 from highest to lowest.
from highest to lowest.A)
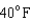 ,
, 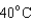 ,
, 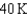
B)
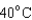 ,
, 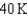 ,
, 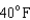
C)
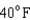 ,
, 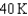 ,
, 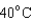
D)
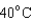 ,
, 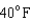 ,
, 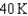

Unlock Deck
Unlock for access to all 115 flashcards in this deck.
Unlock Deck
k this deck
19
Which best describes a system made up of ice, water, and steam existing together?
A) absolute zero
B) triple point
C) ice point
D) steam point
A) absolute zero
B) triple point
C) ice point
D) steam point

Unlock Deck
Unlock for access to all 115 flashcards in this deck.
Unlock Deck
k this deck
20
If it is given that 546 K equals 273 C, then it follows that 423 K equals:
A) 127 C.
B) 150 C.
C) 473 C.
D) 1200 C.
A) 127 C.
B) 150 C.
C) 473 C.
D) 1200 C.

Unlock Deck
Unlock for access to all 115 flashcards in this deck.
Unlock Deck
k this deck
21
An 100-g piece of copper, initially at 295 C, is dropped into 250 g of water contained in a 300-g aluminum calorimeter; the water and calorimeter are initially at 10.0 C. What is the final temperature of the system? (Specific heats of copper and aluminum are 0.0920 and 0.215 cal/g· C, respectively. cw = 1.00 cal/g· C)
A) 12.8 C
B) 16.5 C
C) 18.1 C
D) 32.1 C
A) 12.8 C
B) 16.5 C
C) 18.1 C
D) 32.1 C

Unlock Deck
Unlock for access to all 115 flashcards in this deck.
Unlock Deck
k this deck
22
A 3.00 g lead bullet is traveling at a speed of 300 m/s when it embeds in a wood post. If we assume that half of the resultant heat energy generated remains with the bullet, what is the increase in temperature of the embedded bullet? (specific heat of lead = 0.0305 kcal/kg· C, 1 kcal = 4186 J)
A) 113 C
B) 137 C
C) 176 C
D) 259 C
A) 113 C
B) 137 C
C) 176 C
D) 259 C

Unlock Deck
Unlock for access to all 115 flashcards in this deck.
Unlock Deck
k this deck
23
A 0.2-kg aluminum plate, initially at 20 C, slides down a 10-m long surface, inclined at a 30 angle to the horizontal. The force of kinetic friction exactly balances the component of gravity down the plane so that the plate, once started, glides down at constant velocity. If 90% of the mechanical energy of the system is absorbed by the aluminum, what is its temperature increase at the bottom of the incline? (Specific heat for aluminum is 900 J/kg · C.)
A) 0.16 C
B) 0.07 C
C) 0.05 C
D) 0.03 C
A) 0.16 C
B) 0.07 C
C) 0.05 C
D) 0.03 C

Unlock Deck
Unlock for access to all 115 flashcards in this deck.
Unlock Deck
k this deck
24
What is the temperature increase of 3.0 kg of water when heated by an 800-W immersion heater for 10 min? (cw = 4186 J/kg · C)
A) 56 C
B) 38 C
C) 29 C
D) 14 C
A) 56 C
B) 38 C
C) 29 C
D) 14 C

Unlock Deck
Unlock for access to all 115 flashcards in this deck.
Unlock Deck
k this deck
25
An inventor develops a stationary cycling device by which an individual, while pedaling, can convert all of the energy expended into heat for warming water. What minimum power must be generated if 300 g of water (enough for 1 cup of coffee) is to be heated in 10 min from 30 C to 90 C? (1 cal = 4.186 J, the specific heat of water is 4 186 J/kg · C)
A) 9400 W
B) 590 W
C) 160 W
D) 130 W
A) 9400 W
B) 590 W
C) 160 W
D) 130 W

Unlock Deck
Unlock for access to all 115 flashcards in this deck.
Unlock Deck
k this deck
26
A plot of the temperature versus the energy per kg added to a piece of ice as it goes from below freezing at -10 C to becoming steam at 110 C consists of straight lines, some horizontal and some with an upward slope. What do the upward slopes represent?
A) specific heats
B) reciprocals of specific heats
C) latent heats
D) reciprocals of latent heats
A) specific heats
B) reciprocals of specific heats
C) latent heats
D) reciprocals of latent heats

Unlock Deck
Unlock for access to all 115 flashcards in this deck.
Unlock Deck
k this deck
27
A machine gear consists of 0.40 kg of iron and 0.16 kg of copper. How much total heat is generated in the part if its temperature increases by 35 C? (Specific heats of iron and copper are 450 and 390 J/kg · C, respectively.)
A) 910 J
B) 8500 J
C) 4000 J
D) 5300 J
A) 910 J
B) 8500 J
C) 4000 J
D) 5300 J

Unlock Deck
Unlock for access to all 115 flashcards in this deck.
Unlock Deck
k this deck
28
A solar heated house loses about 7.4 *107 cal through its outer surfaces on a typical 24-hour winter day. What mass of storage rock is needed to provide this amount of heat if it is brought up to initial temperature of 62 C by the solar collectors, and the house is maintained at 20 C? (Specific heat of rock is 0.21 cal/g · C.)
A) 160 kg
B) 1200 kg
C) 6100 kg
D) 8400 kg
A) 160 kg
B) 1200 kg
C) 6100 kg
D) 8400 kg

Unlock Deck
Unlock for access to all 115 flashcards in this deck.
Unlock Deck
k this deck
29
A swimming pool heater has to be able to raise the temperature of the 30,000 gallons of water in the pool by 10.0 C. How many kilowatt-hours of energy are required? (One gallon of water has a mass of approximately 3.79 kg and the specific heat of water is 4186 J/kg · C.)
A) 1960 kWh
B) 1320 kWh
C) 1130 kWh
D) 216 kWh
A) 1960 kWh
B) 1320 kWh
C) 1130 kWh
D) 216 kWh

Unlock Deck
Unlock for access to all 115 flashcards in this deck.
Unlock Deck
k this deck
30
A hot (70 C) lump of metal has a mass of 250 g and a specific heat of 0.25 cal/g· C. John drops the metal into a 500-g calorimeter containing 40 g of water at 20 C. The calorimeter is constructed of a material that has a specific heat of 0.10 cal/ g· C. When equilibrium is reached, what will be the final temperature? (cwater = 1.00 cal/g· C.)
A) 114 C
B) 72 C
C) 40 C
D) 37 C
A) 114 C
B) 72 C
C) 40 C
D) 37 C

Unlock Deck
Unlock for access to all 115 flashcards in this deck.
Unlock Deck
k this deck
31
A 100-g block of copper is taken from a kiln and quickly placed into a beaker of negligible heat capacity containing 300 g of water. The water temperature rises from 15 C to 40 C. Given cCu = 0.10 cal/g· C, and cwater = 1.00 cal/g· C, what was the temperature of the kiln?
A) 500 C
B) 635 C
C) 790 C
D) 535 C
A) 500 C
B) 635 C
C) 790 C
D) 535 C

Unlock Deck
Unlock for access to all 115 flashcards in this deck.
Unlock Deck
k this deck
32
If a 2100-kg car was moving at 30 m/s, what would be its kinetic energy expressed in the unusual (for kinetic energy) units of calories? (1 cal = 4.186 J)
A) 3.0 * 104
B) 2.3 * 105
C) 3.8 * 106
D) 1.1 *105
A) 3.0 * 104
B) 2.3 * 105
C) 3.8 * 106
D) 1.1 *105

Unlock Deck
Unlock for access to all 115 flashcards in this deck.
Unlock Deck
k this deck
33
Marc attaches a falling 250-kg object with a rope through a pulley to a paddle wheel shaft. He places the system in a well-insulated tank holding 12 kg of water. When the object falls, it causes the paddle wheel to rotate and churn the water. If the object falls a vertical distance of 100 m at constant speed, what is the temperature change of the water? (1 kcal = 4186 J, the specific heat of water is 4186 J/kg · C, and g = 9.8 m/s2)
A) 19,600 C
B) 4700 C
C) 4.9 C
D) 2.3 C
A) 19,600 C
B) 4700 C
C) 4.9 C
D) 2.3 C

Unlock Deck
Unlock for access to all 115 flashcards in this deck.
Unlock Deck
k this deck
34
A solar heating system has a 25.0% conversion efficiency; the solar radiation incident on the panels is 1000 W/m2. What is the increase in temperature of 40.0-kg of water in a 1.00-h period by a 4.00 m2 area collector? (cw = 4186 J/kg · C)
A) 14.3 C
B) 21.5 C
C) 28.7 C
D) 44.3 C
A) 14.3 C
B) 21.5 C
C) 28.7 C
D) 44.3 C

Unlock Deck
Unlock for access to all 115 flashcards in this deck.
Unlock Deck
k this deck
35
A piece of copper of mass 100 g is being drilled through with a 1/2" electric drill. The drill operates at 50.0 W and takes 30.0 s to bore through the copper. If all the energy from the drill heats the copper, find the copper's increase in temperature. (ccopper = 387 J/kg · C.)
A) 40.6 C
B) 38.8 C
C) 31.0 C
D) 27.3 C
A) 40.6 C
B) 38.8 C
C) 31.0 C
D) 27.3 C

Unlock Deck
Unlock for access to all 115 flashcards in this deck.
Unlock Deck
k this deck
36
Find the final equilibrium temperature when 20.0 g of milk at 10.0 C is added to 160 g of coffee at 90.0 C. (Assume the specific heats of coffee and milk are the same as water and neglect the heat capacity of the container, and cwater = 1.00 cal/g· C = 4186 J/kg· C)
A) 85.3 C
B) 81.1 C
C) 71.4 C
D) 66.7 C
A) 85.3 C
B) 81.1 C
C) 71.4 C
D) 66.7 C

Unlock Deck
Unlock for access to all 115 flashcards in this deck.
Unlock Deck
k this deck
37
A slice of bread contains about 90 kcal. If specific heat of a person were 1.00 kcal/kg· C, by how many C would the temperature of a 70.0-kg person increase if all the energy in the bread were converted to heat?
A) 2.25 C
B) 1.86 C
C) 1.43 C
D) 1.29 C
A) 2.25 C
B) 1.86 C
C) 1.43 C
D) 1.29 C

Unlock Deck
Unlock for access to all 115 flashcards in this deck.
Unlock Deck
k this deck
38
An inventor develops a stationary cycling device by which an individual, while pedaling, can convert all of the energy expended into heat for warming water. How much mechanical energy is required to increase the temperature of 300 g of water (enough for 1 cup of coffee) from 30 C to 90 C? (1 cal = 4.186 J, the specific heat of water is 4186 J/kg · C)
A) 94,000 J
B) 75,000 J
C) 5400 J
D) 14 J
A) 94,000 J
B) 75,000 J
C) 5400 J
D) 14 J

Unlock Deck
Unlock for access to all 115 flashcards in this deck.
Unlock Deck
k this deck
39
A 20-kg piece of aluminum (which has a specific heat of 900 J/kg · C) is warmed so that its temperature increases by 5.0 C. How much heat was transferred into it?
A) 4.5 *104 J
B) 9.0 *104 J
C) 1.4 * 105 J
D) 2.0 * 105 J
A) 4.5 *104 J
B) 9.0 *104 J
C) 1.4 * 105 J
D) 2.0 * 105 J

Unlock Deck
Unlock for access to all 115 flashcards in this deck.
Unlock Deck
k this deck
40
A waterfall is 70 m high. What is the increase in water temperature at the bottom of the falls if all the initial potential energy goes into heating the water? (g = 9.8 m/s2, cw = 4186 J/kg· C)
A) 0.16 C
B) 0.34 C
C) 0.69 C
D) 1.04 C
A) 0.16 C
B) 0.34 C
C) 0.69 C
D) 1.04 C

Unlock Deck
Unlock for access to all 115 flashcards in this deck.
Unlock Deck
k this deck
41
A 0.0030-kg lead bullet is traveling at a speed of 300 m/s when it embeds in a block of ice at 0 C. If all the heat generated goes into melting ice, what quantity of ice is melted? (Lf = 80 kcal/kg, the specific heat of lead = 0.03 kcal/kg· C, and 1 kcal = 4186 J)
A) 1.47 *10 - 2 kg
B) 4.0 *10 - 4 kg
C) 3.2 *10-3 kg
D) 2.6 *10-4 kg
A) 1.47 *10 - 2 kg
B) 4.0 *10 - 4 kg
C) 3.2 *10-3 kg
D) 2.6 *10-4 kg

Unlock Deck
Unlock for access to all 115 flashcards in this deck.
Unlock Deck
k this deck
42
In typical calorimetry problems, what is the usual conservation law being used?
A) energy
B) heat
C) temperature
D) None since nothing is being conserved.
A) energy
B) heat
C) temperature
D) None since nothing is being conserved.

Unlock Deck
Unlock for access to all 115 flashcards in this deck.
Unlock Deck
k this deck
43
How much heat energy is required to vaporize a 1.0-g ice cube at 0 C? The heat of fusion of ice is 80 cal/g. The heat of vaporization of water is 540 cal/g, and cwater = 1.00 cal/g· C.
A) 620 cal
B) 720 cal
C) 820 cal
D) 1 kcal
A) 620 cal
B) 720 cal
C) 820 cal
D) 1 kcal

Unlock Deck
Unlock for access to all 115 flashcards in this deck.
Unlock Deck
k this deck
44
Iced tea is made by adding ice to 3.0 kg of hot tea, initially at 80 C. How many kg of ice, initially at 0 C, are required to bring the mixture to 10 C? (Lf = 3.33 x 105 J/kg, cw = 4186 J/kg· C)
A) 2.8 kg
B) 1.6 kg
C) 1.4 kg
D) 2.3 kg
A) 2.8 kg
B) 1.6 kg
C) 1.4 kg
D) 2.3 kg

Unlock Deck
Unlock for access to all 115 flashcards in this deck.
Unlock Deck
k this deck
45
In cloud formation, water vapor turns into water droplets which get bigger and bigger until it rains. This will cause the temperature of the air in the clouds to:
A) get warmer.
B) get cooler.
C) will not affect the temperature of the air in the clouds.
D) There is no air in clouds.
A) get warmer.
B) get cooler.
C) will not affect the temperature of the air in the clouds.
D) There is no air in clouds.

Unlock Deck
Unlock for access to all 115 flashcards in this deck.
Unlock Deck
k this deck
46
The zeroth law of thermodynamics pertains to what relational condition that may exist between two systems?
A) zero net forces
B) zero velocities
C) zero temperature
D) thermal equilibrium
A) zero net forces
B) zero velocities
C) zero temperature
D) thermal equilibrium

Unlock Deck
Unlock for access to all 115 flashcards in this deck.
Unlock Deck
k this deck
47
Which of the following involves the greatest heat transfer?
A) One gram of steam at 100 C changing to water at 100 C.
B) One gram of ice at 0 C changing to water at 0 C.
C) One gram of water cooling from 100 C to 0 C.
D) One gram of ice heating from -100 C to 0 C.
A) One gram of steam at 100 C changing to water at 100 C.
B) One gram of ice at 0 C changing to water at 0 C.
C) One gram of water cooling from 100 C to 0 C.
D) One gram of ice heating from -100 C to 0 C.

Unlock Deck
Unlock for access to all 115 flashcards in this deck.
Unlock Deck
k this deck
48
Metal lids on glass jars can often be loosened by running them under hot water. Why is this?
A) The hot water is a lubricant.
B) The metal and glass expand due to the heating, and the glass being of smaller radius expands less than the metal.
C) The metal has a higher coefficient of thermal expansion than glass so the metal expands more than the glass, thus loosening the connection.
D) This is just folklore.
A) The hot water is a lubricant.
B) The metal and glass expand due to the heating, and the glass being of smaller radius expands less than the metal.
C) The metal has a higher coefficient of thermal expansion than glass so the metal expands more than the glass, thus loosening the connection.
D) This is just folklore.

Unlock Deck
Unlock for access to all 115 flashcards in this deck.
Unlock Deck
k this deck
49
A 50-g cube of ice, initially at 0.0 C, is dropped into 200 g of water in an 80-g aluminum container, both initially at 40 C. What is the final equilibrium temperature? (Specific heat for aluminum is 900 J/kg· C, the specific heat of water is 4186 J/kg· C, and Lf = 3.33 * 105 J/kg.)
A) 17.6 C
B) 9.5 C
C) 12.1 C
D) 20.6 C
A) 17.6 C
B) 9.5 C
C) 12.1 C
D) 20.6 C

Unlock Deck
Unlock for access to all 115 flashcards in this deck.
Unlock Deck
k this deck
50
A steel wire, 100 m long at 10 C, has a coefficient of linear expansion of 11*10 - 6/ C. Give its change in length as the temperature changes from 10 C to 45 C.
A) 0.65 cm
B) 3.8 cm
C) 5.8 cm
D) 12 cm
A) 0.65 cm
B) 3.8 cm
C) 5.8 cm
D) 12 cm

Unlock Deck
Unlock for access to all 115 flashcards in this deck.
Unlock Deck
k this deck
51
What happens to a given mass of water as it is cooled from 4 C to zero?
A) expands
B) contracts
C) vaporizes
D) Neither expands, contracts, nor vaporizes.
A) expands
B) contracts
C) vaporizes
D) Neither expands, contracts, nor vaporizes.

Unlock Deck
Unlock for access to all 115 flashcards in this deck.
Unlock Deck
k this deck
52
A puddle holds 150 g of water. If 0.75 g of water evaporates from the surface, what is the approximate temperature change of the remaining water? (Lv = 540 cal/g)
A) "-2.7 C"
B) "+2.7 C"
C) "-0.27 C"
D) "+0.27 C"
A) "-2.7 C"
B) "+2.7 C"
C) "-0.27 C"
D) "+0.27 C"

Unlock Deck
Unlock for access to all 115 flashcards in this deck.
Unlock Deck
k this deck
53
I take 2.0 kg of ice and dump it into 1.0 kg of water and, when equilibrium is reached, I have 3.0 kg of ice at 0 C. The water was originally at 0 C. The specific heat of water = 1.00 kcal/kg· C, the specific heat of ice = 0.50 kcal/kg· C, and the latent heat of fusion of water is 80 kcal/kg. The original temperature of the ice was:
A) "one or two degrees below 0 C."
B) "-80 C."
C) "-120 C."
D) "The whole experiment is impossible."
A) "one or two degrees below 0 C."
B) "-80 C."
C) "-120 C."
D) "The whole experiment is impossible."

Unlock Deck
Unlock for access to all 115 flashcards in this deck.
Unlock Deck
k this deck
54
When heating 2 kg of ice from 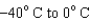 , 2 kg of water from
, 2 kg of water from 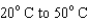 , and 2 kg of steam from
, and 2 kg of steam from 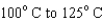 , which requires the most energy?
, which requires the most energy?
A) the ice
B) the water
C) The steam, it's obvious since it is the hottest.
D) All three take the same amount.
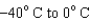 , 2 kg of water from
, 2 kg of water from 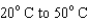 , and 2 kg of steam from
, and 2 kg of steam from 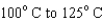 , which requires the most energy?
, which requires the most energy?A) the ice
B) the water
C) The steam, it's obvious since it is the hottest.
D) All three take the same amount.

Unlock Deck
Unlock for access to all 115 flashcards in this deck.
Unlock Deck
k this deck
55
A 6-g lead bullet traveling in 20 C air at 300 m/s strikes a flat steel plate and stops. What is the final temperature of the lead bullet? (Assume the bullet retains all heat.) The melting point of lead is 327 C. The specific heat of lead is 0.128 J/g· C. The heat of fusion of lead is 24.5 J/g.
A) 227 C
B) 260 C
C) 293 C
D) 327 C
A) 227 C
B) 260 C
C) 293 C
D) 327 C

Unlock Deck
Unlock for access to all 115 flashcards in this deck.
Unlock Deck
k this deck
56
Twenty grams of a solid at 70 C is place in 100 grams of a fluid at 20 C. Thermal equilibrium is reached at 30 C. The specific heat of the solid:
A) is equal to that of the fluid.
B) is less than that of the fluid.
C) is more than that of the fluid.
D) cannot be compared to that of a material in a different phase.
A) is equal to that of the fluid.
B) is less than that of the fluid.
C) is more than that of the fluid.
D) cannot be compared to that of a material in a different phase.

Unlock Deck
Unlock for access to all 115 flashcards in this deck.
Unlock Deck
k this deck
57
Which of the following has the highest specific heat?
A) ice
B) water
C) steam
D) They are all water, so they all have the same specific heat.
A) ice
B) water
C) steam
D) They are all water, so they all have the same specific heat.

Unlock Deck
Unlock for access to all 115 flashcards in this deck.
Unlock Deck
k this deck
58
How much heat energy must be removed from 150 g of oxygen at 22 C to liquefy it at -183 C? (The specific heat of oxygen gas is 0.218 cal/g· C, and its heat of vaporization is 50.9 cal/g.)
A) 14,300 cal
B) 9560 cal
C) 4320 cal
D) 2160 cal
A) 14,300 cal
B) 9560 cal
C) 4320 cal
D) 2160 cal

Unlock Deck
Unlock for access to all 115 flashcards in this deck.
Unlock Deck
k this deck
59
A rectangular steel plate with dimensions of 60 cm *25 cm is heated from 20 C to 150 C. What is its change in area? (Coefficient of linear expansion for steel is 11 *10 - 6/ C.)
A) 0.82 cm2
B) 1.1 cm2
C) 4.3 cm2
D) 6.6 cm2
A) 0.82 cm2
B) 1.1 cm2
C) 4.3 cm2
D) 6.6 cm2

Unlock Deck
Unlock for access to all 115 flashcards in this deck.
Unlock Deck
k this deck
60
Which of the following best describes a substance in which the temperature remains constant while at the same time it is experiencing an inward heat flow?
A) gas
B) liquid
C) solid
D) substance undergoing a change of state
A) gas
B) liquid
C) solid
D) substance undergoing a change of state

Unlock Deck
Unlock for access to all 115 flashcards in this deck.
Unlock Deck
k this deck
61
What happens to a volume of water when its temperature is reduced from 8 C to 4 C?
A) density increases
B) density decreases
C) density remains constant
D) vaporizes
A) density increases
B) density decreases
C) density remains constant
D) vaporizes

Unlock Deck
Unlock for access to all 115 flashcards in this deck.
Unlock Deck
k this deck
62
What happens to a given volume of water when heated from 0 C to 4 C?
A) density increases
B) density decreases
C) density remains constant
D) vaporizes
A) density increases
B) density decreases
C) density remains constant
D) vaporizes

Unlock Deck
Unlock for access to all 115 flashcards in this deck.
Unlock Deck
k this deck
63
At 20 C an aluminum ring has an inner diameter of 5.000 cm, and a brass rod has a diameter of 5.020 cm. Keeping the brass rod at 20°C, which of the following temperatures of the ring will allow the ring to just slip over the brass rod? ( Al = 2.4 *10 - 5 / C, brass = 1.9 *10 - 5/ C )
A) 111 C
B) 187 C
C) 228 C
D) 437 C
A) 111 C
B) 187 C
C) 228 C
D) 437 C

Unlock Deck
Unlock for access to all 115 flashcards in this deck.
Unlock Deck
k this deck
64
A steel plate has a hole drilled through it. The plate is put into a furnace and heated. What happens to the size of the inside diameter of a hole as its temperature increases?
A) increases
B) decreases
C) remains constant
D) becomes elliptical
A) increases
B) decreases
C) remains constant
D) becomes elliptical

Unlock Deck
Unlock for access to all 115 flashcards in this deck.
Unlock Deck
k this deck
65
In the text there is a table of coefficients of linear ( 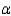 ) and volume (
) and volume ( 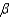 ) expansions due to temperature changes. For the solid materials in this list, there is a relationship between
) expansions due to temperature changes. For the solid materials in this list, there is a relationship between 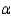 and
and 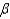 which allows you to find the value of
which allows you to find the value of 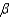 if you know
if you know 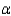 . Which of the following best describes that relationship?
. Which of the following best describes that relationship?
A)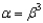
B)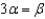
C)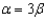
D)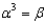
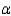 ) and volume (
) and volume ( 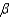 ) expansions due to temperature changes. For the solid materials in this list, there is a relationship between
) expansions due to temperature changes. For the solid materials in this list, there is a relationship between 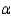 and
and 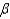 which allows you to find the value of
which allows you to find the value of 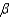 if you know
if you know 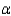 . Which of the following best describes that relationship?
. Which of the following best describes that relationship?A)
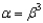
B)
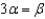
C)
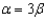
D)
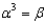

Unlock Deck
Unlock for access to all 115 flashcards in this deck.
Unlock Deck
k this deck
66
A steel piano string is attached, under tension, at each end to secure posts. When gently struck, a faint note can be detected coming from the string. The string is then cooled by blowing cold air over it and then is struck again. How does the note now detected compare to the previous one.
A) It is the same since the posts don't move.
B) It is at a higher frequency than the original one.
C) It is at a lower frequency than the original one.
D) More than one answer is correct.
A) It is the same since the posts don't move.
B) It is at a higher frequency than the original one.
C) It is at a lower frequency than the original one.
D) More than one answer is correct.

Unlock Deck
Unlock for access to all 115 flashcards in this deck.
Unlock Deck
k this deck
67
A steel sphere sits on top of an aluminum ring. The steel sphere ( = 1.10 *10-5/ C) has a diameter of 4.0000 cm at 0 C. The aluminum ring ( = 2.40 * 10 - 5/ C) has an inside diameter of 3.9900 cm at 0 C. Closest to which temperature given will the sphere just fall through the ring?
A) 462 C
B) 193 C
C) 116 C
D) 57.7 C
A) 462 C
B) 193 C
C) 116 C
D) 57.7 C

Unlock Deck
Unlock for access to all 115 flashcards in this deck.
Unlock Deck
k this deck
68
The observation that materials expand in size with an increase in temperature can be applied to what proportion of existing substances?
A) 100%
B) most
C) few
D) none
A) 100%
B) most
C) few
D) none

Unlock Deck
Unlock for access to all 115 flashcards in this deck.
Unlock Deck
k this deck
69
At room temperature, the coefficient of linear expansion for Pyrex glass is _____ that for ordinary glass.
A) the same as
B) more than
C) less than
D) stronger than
A) the same as
B) more than
C) less than
D) stronger than

Unlock Deck
Unlock for access to all 115 flashcards in this deck.
Unlock Deck
k this deck
70
The thermal expansion of a solid is caused by:
A) the breaking of bonds between atoms.
B) increasing the amplitude of the atoms' vibration.
C) increasing the distance between equilibrium positions for the vibrating atoms.
D) all of the above.
A) the breaking of bonds between atoms.
B) increasing the amplitude of the atoms' vibration.
C) increasing the distance between equilibrium positions for the vibrating atoms.
D) all of the above.

Unlock Deck
Unlock for access to all 115 flashcards in this deck.
Unlock Deck
k this deck
71
A brass cube, 10 cm on a side, is raised in temperature by 150 C. The coefficient of volume expansion of brass is 57 *10 - 6/ C. By what percentage is any one of the 10-cm edges increased in length?
A) 4%
B) 2.8%
C) 0.38%
D) 0.29%
A) 4%
B) 2.8%
C) 0.38%
D) 0.29%

Unlock Deck
Unlock for access to all 115 flashcards in this deck.
Unlock Deck
k this deck
72
A long steel beam has a length of fifty meters on a cold day when the temperature is 0 C. What is the length of the beam on a hot day when T = 40 C? ( steel= 1.1 *10 - 5/ C)
A) 50.044 m
B) 50.022 m
C) 50.088 m
D) 50.0044 m
A) 50.044 m
B) 50.022 m
C) 50.088 m
D) 50.0044 m

Unlock Deck
Unlock for access to all 115 flashcards in this deck.
Unlock Deck
k this deck
73
A pipe of length 10.0 m increases in length by 2.0 cm when its temperature is increased by 90 F. What is its coefficient of linear expansion?
A) 22* 10-6/ C
B) 17* 10-6/ C
C) 13 * 10-6/ C
D) 40 * 10-6/ C
A) 22* 10-6/ C
B) 17* 10-6/ C
C) 13 * 10-6/ C
D) 40 * 10-6/ C

Unlock Deck
Unlock for access to all 115 flashcards in this deck.
Unlock Deck
k this deck
74
Suppose the ends of a 20-m long steel beam are rigidly clamped at 0 C to prevent expansion. The rail has a cross-sectional area of 15 cm2. What force does the beam exert when it is heated to 40 C? ( steel = 1.1 * 10 - 5/C , Ysteel = 2.0 * 1011 N/m2)
A) 2.6 *0 105 N
B) 5.6 * 104 N
C) 1.3 * 105 N
D) 6.5 * 102 N
A) 2.6 *0 105 N
B) 5.6 * 104 N
C) 1.3 * 105 N
D) 6.5 * 102 N

Unlock Deck
Unlock for access to all 115 flashcards in this deck.
Unlock Deck
k this deck
75
Between 0 C and 4 C, the volume coefficient of expansion for water:
A) is positive.
B) is zero.
C) is becoming less dense.
D) is negative.
A) is positive.
B) is zero.
C) is becoming less dense.
D) is negative.

Unlock Deck
Unlock for access to all 115 flashcards in this deck.
Unlock Deck
k this deck
76
As a copper wire is heated, its length increases by 0.200%. What is the change of the temperature of the wire? ( Cu = 16.6 *10 -6/C )
A) 120 C
B) 60.2 C
C) 30.1 C
D) 6.0 C
A) 120 C
B) 60.2 C
C) 30.1 C
D) 6.0 C

Unlock Deck
Unlock for access to all 115 flashcards in this deck.
Unlock Deck
k this deck
77
A 2.00-kg copper rod is 40.00 cm long at 23°C. If 50,000 J are transferred to the rod by heat, what is its change in length? (ccopper = 387 J/kg·°C and copper = 17 *10-6/°C)
A) 0.022 cm
B) 0.044 cm
C) 0.055 cm
D) More information is needed.
A) 0.022 cm
B) 0.044 cm
C) 0.055 cm
D) More information is needed.

Unlock Deck
Unlock for access to all 115 flashcards in this deck.
Unlock Deck
k this deck
78
A brass cube, 10 cm on a side, is raised in temperature by 150 C. The coefficient of volume expansion of brass is 57 *10 - 6/ C. By what percentage does volume increase?
A) 12%
B) 2.8%
C) 1.1%
D) 0.86%
A) 12%
B) 2.8%
C) 1.1%
D) 0.86%

Unlock Deck
Unlock for access to all 115 flashcards in this deck.
Unlock Deck
k this deck
79
An automobile gas tank is filled to its capacity of 20.00 gallons with the gasoline at an initial temperature of 10 C. The automobile is parked in the Sun causing the gasoline's temperature to rise to 60 C. If the coefficient of volume expansion for gasoline is 9.6 *10-4/ C, what volume runs out the overflow tube? Assume the change in volume of the tank is negligible.
A) 1.74 gallons
B) 0.96 gallon
C) 0.72 gallon
D) 0.30 gallon
A) 1.74 gallons
B) 0.96 gallon
C) 0.72 gallon
D) 0.30 gallon

Unlock Deck
Unlock for access to all 115 flashcards in this deck.
Unlock Deck
k this deck
80
What happens to its moment of inertia when a steel disk is heated?
A) It increases.
B) It decreases.
C) It stays the same.
D) It increases for half the temperature increase and then decreases for the rest of the temperature increase.
A) It increases.
B) It decreases.
C) It stays the same.
D) It increases for half the temperature increase and then decreases for the rest of the temperature increase.

Unlock Deck
Unlock for access to all 115 flashcards in this deck.
Unlock Deck
k this deck


