Deck 28: Quantum Theory
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/63
Play
Full screen (f)
Deck 28: Quantum Theory
1
According to Einstein, as the wavelength of the incident monochromatic light beam becomes shorter, the work function of a target material in a phototube:
A) increases.
B) is directly proportional to wavelength.
C) decreases.
D) remains constant.
A) increases.
B) is directly proportional to wavelength.
C) decreases.
D) remains constant.
remains constant.
2
The ultraviolet catastrophe predicts that:
A) a black body can absorb an infinite amount of radiation if the radiation is in the ultraviolet region.
B) the radiated energy approaches zero as the wavelength approaches zero.
C) as an object gets hotter its light will change from dull red to blue white.
D) all objects should radiate extreme amounts of ultraviolet light.
A) a black body can absorb an infinite amount of radiation if the radiation is in the ultraviolet region.
B) the radiated energy approaches zero as the wavelength approaches zero.
C) as an object gets hotter its light will change from dull red to blue white.
D) all objects should radiate extreme amounts of ultraviolet light.
all objects should radiate extreme amounts of ultraviolet light.
3
If a monochromatic light beam with quantum energy value of 4.0 eV is incident upon a photocell where the work function of the target metal is 1.60 eV, what is the maximum kinetic energy of ejected electrons?
A) 4.6 eV
B) 4.8 eV
C) 2.4 eV
D) 1.4 eV
A) 4.6 eV
B) 4.8 eV
C) 2.4 eV
D) 1.4 eV
2.4 eV
4
What is the wavelength of a monochromatic light beam, where the photon energy is 2.00 eV? (h = 6.63 *10-34 J.s, c = 3.00 *108 m/s, 1 nm = 10-9 m, and 1 eV = 1.6 *10-19 J)
A) 414 nm
B) 1243 nm
C) 311 nm
D) 622 nm
A) 414 nm
B) 1243 nm
C) 311 nm
D) 622 nm

Unlock Deck
Unlock for access to all 63 flashcards in this deck.
Unlock Deck
k this deck
5
Light of wavelength 6.1 *10-7 m has an energy of: (h = 6.63 *10-34 J.s, c = 3.00 * 108 m/s)
A) 3.1 *10-19 J
B) 1.5 *10-19 J
C) 1.7 *10-19 J
D) 3.3 *10-19 J
A) 3.1 *10-19 J
B) 1.5 *10-19 J
C) 1.7 *10-19 J
D) 3.3 *10-19 J

Unlock Deck
Unlock for access to all 63 flashcards in this deck.
Unlock Deck
k this deck
6
According to Wien's displacement law, if the absolute temperature of a radiating blackbody is doubled, then the peak wavelength emitted will change by what factor?
A) 4
B) 2
C) 1
D) 1/2
A) 4
B) 2
C) 1
D) 1/2

Unlock Deck
Unlock for access to all 63 flashcards in this deck.
Unlock Deck
k this deck
7
What is the wavelength of a monochromatic light beam, where the photon energy is 4.81 *10-19 J? (h = 6.63 *10-34 J.s, c = 3.00 *108 m/s, and 1 nm = 10-9 m)
A) 354 nm
B) 414 nm
C) 787 nm
D) 398 nm
A) 354 nm
B) 414 nm
C) 787 nm
D) 398 nm

Unlock Deck
Unlock for access to all 63 flashcards in this deck.
Unlock Deck
k this deck
8
Of photons of red, yellow, light, and blue light, which photons have the least energy?
A) red
B) blue
C) green
D) yellow
A) red
B) blue
C) green
D) yellow

Unlock Deck
Unlock for access to all 63 flashcards in this deck.
Unlock Deck
k this deck
9
If a blackbody is at 2000° C, what will be the peak wavelength emitted?
A) 1.67 m
B) 1.28 m
C) 0.679 m
D) 1.45 m
A) 1.67 m
B) 1.28 m
C) 0.679 m
D) 1.45 m

Unlock Deck
Unlock for access to all 63 flashcards in this deck.
Unlock Deck
k this deck
10
If a quantum of radiation has an energy of 3.0 keV, what is its wavelength? (h = 6.63 *10-34 J.s, 1 eV = 1.60 *10-19 J, c = 3.00 *108 m/s, and 1 nm = 10-9 m)
A) 0.62 nm
B) 0.32 nm
C) 0.41 nm
D) 1.02 nm
A) 0.62 nm
B) 0.32 nm
C) 0.41 nm
D) 1.02 nm

Unlock Deck
Unlock for access to all 63 flashcards in this deck.
Unlock Deck
k this deck
11
Surface #1 has work function 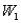 , and when bombarded with photons of wavelength
, and when bombarded with photons of wavelength 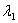 emits photoelectrons with maximum energy
emits photoelectrons with maximum energy 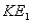 . Surface #2 has work function
. Surface #2 has work function 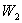 , and when bombarded by photons of wavelength
, and when bombarded by photons of wavelength 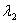 emits photoelectrons with maximum energy
emits photoelectrons with maximum energy 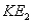 . If
. If 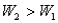 , then which of the following must be true?
, then which of the following must be true?
A)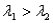
B)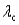 for surface #1 is greater than
for surface #1 is greater than 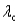 for surface #2.
for surface #2.
C)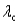 for surface #2 is greater than
for surface #2 is greater than 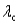 for surface #1.
for surface #1.
D)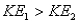
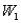 , and when bombarded with photons of wavelength
, and when bombarded with photons of wavelength 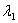 emits photoelectrons with maximum energy
emits photoelectrons with maximum energy 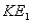 . Surface #2 has work function
. Surface #2 has work function 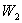 , and when bombarded by photons of wavelength
, and when bombarded by photons of wavelength 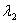 emits photoelectrons with maximum energy
emits photoelectrons with maximum energy 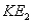 . If
. If 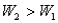 , then which of the following must be true?
, then which of the following must be true?A)
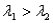
B)
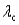 for surface #1 is greater than
for surface #1 is greater than 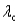 for surface #2.
for surface #2.C)
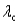 for surface #2 is greater than
for surface #2 is greater than 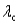 for surface #1.
for surface #1.D)
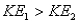

Unlock Deck
Unlock for access to all 63 flashcards in this deck.
Unlock Deck
k this deck
12
What is the surface temperature of a distant star (which emits light as if it were a blackbody) where the peak wavelength is 456 nm? (Hint: The surface of the human body at 35° C has a peak wavelength of 9.41 m). (1 nm = 10 - 9 m = 10-3 m)
A) 5100 K
B) 4510 K
C) 6040 K
D) 6360 K
A) 5100 K
B) 4510 K
C) 6040 K
D) 6360 K

Unlock Deck
Unlock for access to all 63 flashcards in this deck.
Unlock Deck
k this deck
13
Blue light will not eject electrons from a certain metal; however, which one of the following may possibly eject electrons from that metal?
A) red
B) green
C) infrared
D) ultraviolet
A) red
B) green
C) infrared
D) ultraviolet

Unlock Deck
Unlock for access to all 63 flashcards in this deck.
Unlock Deck
k this deck
14
As the temperature of a radiation emitting blackbody becomes lower, what happens to the peak wavelength of the radiation?
A) remains constant
B) increases
C) decreases
D) is directly proportional to temperature
A) remains constant
B) increases
C) decreases
D) is directly proportional to temperature

Unlock Deck
Unlock for access to all 63 flashcards in this deck.
Unlock Deck
k this deck
15
What is the frequency of monochromatic light where the photon energy is 6.5 *10-19 J? (h = 6.63 *10-34 J.s)
A) 2.2 *1014 Hz
B) 9.8 * 1014 Hz
C) 4.4 *1014 Hz
D) 8.3 *1014 Hz
A) 2.2 *1014 Hz
B) 9.8 * 1014 Hz
C) 4.4 *1014 Hz
D) 8.3 *1014 Hz

Unlock Deck
Unlock for access to all 63 flashcards in this deck.
Unlock Deck
k this deck
16
Which of the following is an indication that one is dealing with a wave property instead of a particle property?
A) energy
B) quanta
C) interference
D) momentum
A) energy
B) quanta
C) interference
D) momentum

Unlock Deck
Unlock for access to all 63 flashcards in this deck.
Unlock Deck
k this deck
17
A quantum of radiation has an energy of 1.3 keV. What is its frequency? (h = 6.63 *10-34 J.s and 1 eV = 1.60 *10-19 J)
A) 6.3 *1017 Hz
B) 3.1 * 1017 Hz
C) 4.8 * 1017 Hz
D) 7.2 * 1017 Hz
A) 6.3 *1017 Hz
B) 3.1 * 1017 Hz
C) 4.8 * 1017 Hz
D) 7.2 * 1017 Hz

Unlock Deck
Unlock for access to all 63 flashcards in this deck.
Unlock Deck
k this deck
18
Star A has the peak of its blackbody radiation at A. Star B has its peak at B, which is one-third that of A. If Star A's surface temperature is TA, how does the surface temperature TB of Star B compare?
A) TB = TA/9
B) TB = 3 TA
C) TB = 9 TA
D) TB = TA/3
A) TB = TA/9
B) TB = 3 TA
C) TB = 9 TA
D) TB = TA/3

Unlock Deck
Unlock for access to all 63 flashcards in this deck.
Unlock Deck
k this deck
19
Classical theories predict that most of the energy from a blackbody should be radiated:
A) at the wavelength given by Wien's displacement law.
B) as thermal radiation in the infrared region.
C) a blackbody should not radiate.
D) as ultraviolet light.
A) at the wavelength given by Wien's displacement law.
B) as thermal radiation in the infrared region.
C) a blackbody should not radiate.
D) as ultraviolet light.

Unlock Deck
Unlock for access to all 63 flashcards in this deck.
Unlock Deck
k this deck
20
Planck's quantum theory is compatible with the experimental data related to which of the following?
A) line spectra emitted by hydrogen gas
B) the photoelectric effect
C) blackbody radiation
D) all of these choices
A) line spectra emitted by hydrogen gas
B) the photoelectric effect
C) blackbody radiation
D) all of these choices

Unlock Deck
Unlock for access to all 63 flashcards in this deck.
Unlock Deck
k this deck
21
Who was the first to successfully explain the photoelectric effect?
A) Young
B) Einstein
C) Planck
D) Bohr
A) Young
B) Einstein
C) Planck
D) Bohr

Unlock Deck
Unlock for access to all 63 flashcards in this deck.
Unlock Deck
k this deck
22
A light-emitting diode has a power output of 0.3 W. If 590 nm is the average wavelength of the source, about how many photons are emitted per second? (h = 6.63 *10-34 J.s, c = 3.00 *108 m/s, and 1 nm = 10-9 m)
A) 1021
B) 1025
C) 1018
D) 1029
A) 1021
B) 1025
C) 1018
D) 1029

Unlock Deck
Unlock for access to all 63 flashcards in this deck.
Unlock Deck
k this deck
23
According to Einstein, increasing the brightness of a beam of light without changing its color will increase:
A) the frequency of the photons.
B) the energy of each photon.
C) the speed of the photons.
D) the number of photons.
A) the frequency of the photons.
B) the energy of each photon.
C) the speed of the photons.
D) the number of photons.

Unlock Deck
Unlock for access to all 63 flashcards in this deck.
Unlock Deck
k this deck
24
Sources of red, blue, and yellow light each emit light with a power of 50 mW. Which source emits the least photons per second?
A) the red source
B) They all emit the same number per second.
C) the blue source
D) the yellow source
A) the red source
B) They all emit the same number per second.
C) the blue source
D) the yellow source

Unlock Deck
Unlock for access to all 63 flashcards in this deck.
Unlock Deck
k this deck
25
Which change will not affect the kinetic energy of the most energetic electrons emitted in the photoelectric effect?
A) changing the frequency of the light
B) changing the metal the light is hitting
C) changing the brightness of the light
D) All of the above will affect the electron's kinetic energy.
A) changing the frequency of the light
B) changing the metal the light is hitting
C) changing the brightness of the light
D) All of the above will affect the electron's kinetic energy.

Unlock Deck
Unlock for access to all 63 flashcards in this deck.
Unlock Deck
k this deck
26
The microwave in the kitchen has a label that indicates it produces its microwaves at a frequency of 2450 MHz. What is the energy associated with one of these microwave photons? (h = 6.63 *10-34 J.s)
A)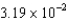 eV
eV
B) 2.45 eV
C)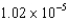 eV
eV
D) 1.62 eV
A)
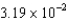 eV
eVB) 2.45 eV
C)
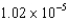 eV
eVD) 1.62 eV

Unlock Deck
Unlock for access to all 63 flashcards in this deck.
Unlock Deck
k this deck
27
Blue light ( = 460 nm) is incident on a piece of potassium (W = 2.45 eV). What is the maximum kinetic energy of the ejected photoelectrons? (h = 6.63 * 10-34 J.s, c = 3.00 * 108 m/s, 1 eV = 1.60 *10-19 J, and 1 nm = 10-9 m)
A) 1.0 eV
B) 0.25 eV
C) 0.50 eV
D) 4.9 eV
A) 1.0 eV
B) 0.25 eV
C) 0.50 eV
D) 4.9 eV

Unlock Deck
Unlock for access to all 63 flashcards in this deck.
Unlock Deck
k this deck
28
What is the maximum velocity of a photoelectron emitted from a surface with work function 5.22 eV when illuminated by 200-nm ultraviolet light? (melectron = 9.11 *10-31 kg, h = 6.63 *10-34 J.s, 1 eV = 1.60 *10-19 J, and 1 nm = 10-9 m)
A) 800,000 m/s
B) 212,000 m/s
C) 653,000 m/s
D) 591,000 m/s
A) 800,000 m/s
B) 212,000 m/s
C) 653,000 m/s
D) 591,000 m/s

Unlock Deck
Unlock for access to all 63 flashcards in this deck.
Unlock Deck
k this deck
29
According to the de Broglie hypothesis, which of the following statements is applicable to the wavelength of a moving particle?
A) directly proportional to its momentum
B) inversely proportional to its energy
C) inversely proportional to its momentum
D) directly proportional to its energy
A) directly proportional to its momentum
B) inversely proportional to its energy
C) inversely proportional to its momentum
D) directly proportional to its energy

Unlock Deck
Unlock for access to all 63 flashcards in this deck.
Unlock Deck
k this deck
30
Which of the following statements best describes the relation between quantum theory and the photoelectric effect experiment?
A) The photoelectric effect contradicts quantum theory.
B) Quantum theory explains the photoelectric effect.
C) Quantum theory has no bearing on the photoelectric effect.
D) The photoelectric effect explains quantum theory.
A) The photoelectric effect contradicts quantum theory.
B) Quantum theory explains the photoelectric effect.
C) Quantum theory has no bearing on the photoelectric effect.
D) The photoelectric effect explains quantum theory.

Unlock Deck
Unlock for access to all 63 flashcards in this deck.
Unlock Deck
k this deck
31
Of the following energies for photons, which is the least energy that could result in photoelectron production if the work function is 1.60 eV?
A) 1.50 eV
B) 6.01 eV
C) 2.90 eV
D) 3.50 eV
A) 1.50 eV
B) 6.01 eV
C) 2.90 eV
D) 3.50 eV

Unlock Deck
Unlock for access to all 63 flashcards in this deck.
Unlock Deck
k this deck
32
A proton and an electron each have the same de Broglie wavelength. (i) Which has the lower speed, and (ii) which has the lower kinetic energy?
A) (i) the electron, (ii) the electron
B) (i) the electron, (ii) Either one can have the greater kinetic energy.
C) (i) the proton, (ii) Either one can have the greater kinetic energy.
D) (i) the proton, (ii) the proton
A) (i) the electron, (ii) the electron
B) (i) the electron, (ii) Either one can have the greater kinetic energy.
C) (i) the proton, (ii) Either one can have the greater kinetic energy.
D) (i) the proton, (ii) the proton

Unlock Deck
Unlock for access to all 63 flashcards in this deck.
Unlock Deck
k this deck
33
A helium-neon laser emits red light having a wavelength of 632.8 nm and a power of 10 mW. How many photons are emitted each second? (h = 6.63 *10-34 J.s, c = 3.00 * 108 m/s, and 1 nm = 10-9 m)
A) 4.8 * 1017
B) 1.6 *1015
C) 3.2 * 1016
D) 2.6 *1018
A) 4.8 * 1017
B) 1.6 *1015
C) 3.2 * 1016
D) 2.6 *1018

Unlock Deck
Unlock for access to all 63 flashcards in this deck.
Unlock Deck
k this deck
34
What is the energy of a photon whose frequency is 7.9 *1020 Hz? (h = 6.63 *10 - 34 J.s and 1 eV = 1.60*10 - 19 J)
A) 5.0 MeV
B) 3.3 MeV
C) 1.6 MeV
D) 2.5 MeV
A) 5.0 MeV
B) 3.3 MeV
C) 1.6 MeV
D) 2.5 MeV

Unlock Deck
Unlock for access to all 63 flashcards in this deck.
Unlock Deck
k this deck
35
If a 1H nucleus, a 2H nucleus, and a 3H nucleus all had the same momentum, which one has the lowest de Broglie wavelength?
A) "3H"
B) "2H"
C) "1H"
D) "All three have the same de Broglie wavelength."
A) "3H"
B) "2H"
C) "1H"
D) "All three have the same de Broglie wavelength."

Unlock Deck
Unlock for access to all 63 flashcards in this deck.
Unlock Deck
k this deck
36
A detector absorbs 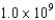 photons of monochromatic light and their total energy is found to be 33 nJ. What is the wavelength associated with these photons? (h = 6.63 *10-34 J.s, c = 3.00 * 108 m/s)
photons of monochromatic light and their total energy is found to be 33 nJ. What is the wavelength associated with these photons? (h = 6.63 *10-34 J.s, c = 3.00 * 108 m/s)
A) 600 nm
B) 540 nm
C) 400 nm
D) 500 nm
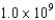 photons of monochromatic light and their total energy is found to be 33 nJ. What is the wavelength associated with these photons? (h = 6.63 *10-34 J.s, c = 3.00 * 108 m/s)
photons of monochromatic light and their total energy is found to be 33 nJ. What is the wavelength associated with these photons? (h = 6.63 *10-34 J.s, c = 3.00 * 108 m/s)A) 600 nm
B) 540 nm
C) 400 nm
D) 500 nm

Unlock Deck
Unlock for access to all 63 flashcards in this deck.
Unlock Deck
k this deck
37
How much energy (in eV) does a photon of 500-nm light have? (h = 6.63 * 10-34 J.s, c = 3.00 * 108 m/s, 1 eV = 1.60 *10-19 J, and 1 nm = 10-9 m)
A) 1.78 eV
B) 2.48 eV
C) 1.24 eV
D) 3.11 eV
A) 1.78 eV
B) 2.48 eV
C) 1.24 eV
D) 3.11 eV

Unlock Deck
Unlock for access to all 63 flashcards in this deck.
Unlock Deck
k this deck
38
According to de Broglie, as the momentum of a moving particle is quartered, the corresponding wavelength changes by what factor?
A) 1/4
B) 1/16
C) 16
D) 4
A) 1/4
B) 1/16
C) 16
D) 4

Unlock Deck
Unlock for access to all 63 flashcards in this deck.
Unlock Deck
k this deck
39
Of the following photons, which has the lowest energy?
A) visible
B) ultraviolet
C) infrared
D) microwave
A) visible
B) ultraviolet
C) infrared
D) microwave

Unlock Deck
Unlock for access to all 63 flashcards in this deck.
Unlock Deck
k this deck
40
What is the de Broglie wavelength for a proton (m = 1.67 * 10-27 kg) moving at a speed of 3.0 * 106 m/s? (h = 6.63 *10-34 J.s)
A) 0.33 *10-13 m
B) 1.3 *10-13 m
C) 0.66 *10-13 m
D) 2.0 *10-13 m
A) 0.33 *10-13 m
B) 1.3 *10-13 m
C) 0.66 *10-13 m
D) 2.0 *10-13 m

Unlock Deck
Unlock for access to all 63 flashcards in this deck.
Unlock Deck
k this deck
41
If the measured momentum of an electron is 3.20 *10-27 kg.m/s with an uncertainty of 4.8 *10-28 kg.m/s, what is the minimum uncertainty in the position? (h = 6.63 * 10-34 J.s)
A) 0.63 * 10-4 m
B) 2.2 *10-7 m
C) 3.3 *10-6 m
D) 1.1 * 10-7 m
A) 0.63 * 10-4 m
B) 2.2 *10-7 m
C) 3.3 *10-6 m
D) 1.1 * 10-7 m

Unlock Deck
Unlock for access to all 63 flashcards in this deck.
Unlock Deck
k this deck
42
What is the energy of a photon that has the same wavelength as a 24 eV electron? (h = 6.63 *10-34 J.s, me = 9.11*10-31 kg)
A) 24 eV
B) 14 * 10-16 eV
C) 3.5 keV
D) 5.0 keV
A) 24 eV
B) 14 * 10-16 eV
C) 3.5 keV
D) 5.0 keV

Unlock Deck
Unlock for access to all 63 flashcards in this deck.
Unlock Deck
k this deck
43
That light has a dual nature is referring to light:
A) having energy and momentum.
B) acting as waves and particles.
C) having high- or low-energy photons.
D) undergoing pair production.
A) having energy and momentum.
B) acting as waves and particles.
C) having high- or low-energy photons.
D) undergoing pair production.

Unlock Deck
Unlock for access to all 63 flashcards in this deck.
Unlock Deck
k this deck
44
According to Heisenberg, as the uncertainty in the measurement of a particle's momentum is increased by a factor of 2, by what factor is the uncertainty in that particle's position changed?
A) 1
B) 4
C) 1/2
D) 2
A) 1
B) 4
C) 1/2
D) 2

Unlock Deck
Unlock for access to all 63 flashcards in this deck.
Unlock Deck
k this deck
45
When 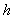 stands for Planck's constant, what does
stands for Planck's constant, what does 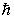 (read as "h bar") stand for?
(read as "h bar") stand for?
A) The bar means that we are only referring to spin angular momentum.
B) The bar means that the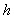 is divided by
is divided by 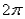 .
.
C) The bar means that it is negative, i.e., the same as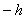 .
.
D) The bar means that we are talking about angular momentum.
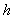 stands for Planck's constant, what does
stands for Planck's constant, what does 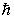 (read as "h bar") stand for?
(read as "h bar") stand for?A) The bar means that we are only referring to spin angular momentum.
B) The bar means that the
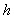 is divided by
is divided by 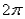 .
.C) The bar means that it is negative, i.e., the same as
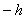 .
.D) The bar means that we are talking about angular momentum.

Unlock Deck
Unlock for access to all 63 flashcards in this deck.
Unlock Deck
k this deck
46
Due to the dual nature of light and matter, either can act in an experiment as if it is a wave or a particle. In which experiment is the wave aspect exhibited for matter?
A) the Davisson and Germer experiment
B) Wien's law determinations
C) the Stern-Gerlach experiment
D) the photoelectric effect
A) the Davisson and Germer experiment
B) Wien's law determinations
C) the Stern-Gerlach experiment
D) the photoelectric effect

Unlock Deck
Unlock for access to all 63 flashcards in this deck.
Unlock Deck
k this deck
47
A proton (mass = 1.67 *10 - 27 kg) has a kinetic energy of 1.00 MeV. If its momentum is measured with an uncertainty of 2.00%, what is the minimum uncertainty in its position? (h = 6.63 *10 - 34 J.s and 1 eV = 1.6 *10 - 19 J)
A) 9.08 * 10 - 14 m
B) 1.14 *10 - 13 m
C) 5.64 *10 - 14 m
D) 2.28 * 10 - 13 m
A) 9.08 * 10 - 14 m
B) 1.14 *10 - 13 m
C) 5.64 *10 - 14 m
D) 2.28 * 10 - 13 m

Unlock Deck
Unlock for access to all 63 flashcards in this deck.
Unlock Deck
k this deck
48
If an electron has a measured wavelength of 1.20 *10 - 10 m, what is its kinetic energy? (h = 6.63 * 10 - 34 J.s, 1 eV = 1.6 *10 - 19 J, and me = 9.11 *10 - 31 kg)
A) 209 eV
B) 55.0 eV
C) 105 eV
D) 147 eV
A) 209 eV
B) 55.0 eV
C) 105 eV
D) 147 eV

Unlock Deck
Unlock for access to all 63 flashcards in this deck.
Unlock Deck
k this deck
49
When an electron is in a magnetic field, different spin states associated with the electron's magnetic moment will occur. What are these states called?
A) up, down
B) plus, minus
C) clockwise, counterclockwise
D) north, south
A) up, down
B) plus, minus
C) clockwise, counterclockwise
D) north, south

Unlock Deck
Unlock for access to all 63 flashcards in this deck.
Unlock Deck
k this deck
50
Starting from rest, an electron accelerates through a potential difference of 67 V. What is its de Broglie wavelength? (h = 6.63 *10 - 34 J.s, me = 9.11 *10 - 31 kg, and 1 eV = 1.60 *10 - 19 J)
A) 1.1 *10 - 10 m
B) 1.5 *10 - 10 m
C) 1.9 *10 - 10 m
D) 2.3 *10 - 10 m
A) 1.1 *10 - 10 m
B) 1.5 *10 - 10 m
C) 1.9 *10 - 10 m
D) 2.3 *10 - 10 m

Unlock Deck
Unlock for access to all 63 flashcards in this deck.
Unlock Deck
k this deck
51
When electrons are placed in a magnetic field, their spin orientations are quantized. How many orientations with respect to the field may result?
A) 1
B) 2
C) 3
D) 4 or more
A) 1
B) 2
C) 3
D) 4 or more

Unlock Deck
Unlock for access to all 63 flashcards in this deck.
Unlock Deck
k this deck
52
The wave function as derived in Schrödinger's equation is best described as being a measure of which of the following?
A) particle wavelength
B) photon wavelength
C) probability
D) photon beam frequency
A) particle wavelength
B) photon wavelength
C) probability
D) photon beam frequency

Unlock Deck
Unlock for access to all 63 flashcards in this deck.
Unlock Deck
k this deck
53
An electron and an alpha particle (a helium-4 nucleus) each have the same non-relativistic speed. Which has the greatest kinetic energy, and which has the shortest de Broglie wavelength?
A) the alpha particle, the alpha particle
B) the alpha particle, the electron
C) the electron, the alpha particle
D) the electron, the electron
A) the alpha particle, the alpha particle
B) the alpha particle, the electron
C) the electron, the alpha particle
D) the electron, the electron

Unlock Deck
Unlock for access to all 63 flashcards in this deck.
Unlock Deck
k this deck
54
In the STM, what are the S and T?
A) scattering, transparent
B) superconducting, trans-barrier
C) standard, transmission
D) scanning, tunneling
A) scattering, transparent
B) superconducting, trans-barrier
C) standard, transmission
D) scanning, tunneling

Unlock Deck
Unlock for access to all 63 flashcards in this deck.
Unlock Deck
k this deck
55
The "seeing" ability or resolution of radiation is determined by its wavelength. If the size of an atom is approximately 10-10 m, how fast must an electron travel to have a wavelength smaller than that of an atom? (me = 9.11 *10-31 kg and h = 6.63 *10-34 J.s)
A) 1.0 *106 m/s
B) 7.3 *106 m/s
C) 5.4 *105 m/s
D) 3.4 * 106 m/s
A) 1.0 *106 m/s
B) 7.3 *106 m/s
C) 5.4 *105 m/s
D) 3.4 * 106 m/s

Unlock Deck
Unlock for access to all 63 flashcards in this deck.
Unlock Deck
k this deck
56
The uncertainty principle was derived by whom?
A) Heisenberg
B) Schrödinger
C) Gerlach
D) de Broglie
A) Heisenberg
B) Schrödinger
C) Gerlach
D) de Broglie

Unlock Deck
Unlock for access to all 63 flashcards in this deck.
Unlock Deck
k this deck
57
What potential difference is needed to accelerate a proton from rest to give it a wavelength of 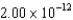 m?
m?
A) 411 V
B) 206 V
C) 1650 V
D) 617 V
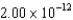 m?
m?A) 411 V
B) 206 V
C) 1650 V
D) 617 V

Unlock Deck
Unlock for access to all 63 flashcards in this deck.
Unlock Deck
k this deck
58
The Heisenberg uncertainty principle places restriction on the precision of simultaneously measuring both position and momentum. This principle can also be applied to the simultaneous measurement of two other variables, which are:
A) mass and charge.
B) torque and frequency.
C) force and color.
D) energy and time interval.
A) mass and charge.
B) torque and frequency.
C) force and color.
D) energy and time interval.

Unlock Deck
Unlock for access to all 63 flashcards in this deck.
Unlock Deck
k this deck
59
The de Broglie wavelength of a 0.060-kg golf ball is 3.56 *10-34 m. What is its speed? (h = 6.63 *10-34 J.s)
A) 31 m/s
B) 15 m/s
C) 26 m/s
D) 48 m/s
A) 31 m/s
B) 15 m/s
C) 26 m/s
D) 48 m/s

Unlock Deck
Unlock for access to all 63 flashcards in this deck.
Unlock Deck
k this deck
60
An electron microscope operates with electrons of kinetic energy 100 keV. What is the wavelength of these electrons? Assume this speed is not relativistic. (h = 6.63 *10 - 34 J.s, c = 3.00 * 108 m/s, 1 eV = 1.60 *10 - 19 J, and me = 9.11 *10 - 31 kg)
A) 5.49 *10 - 12 m
B) 7.14 *10 - 11 m
C) 9.28*10 - 10 m
D) 3.88 *10 - 12 m
A) 5.49 *10 - 12 m
B) 7.14 *10 - 11 m
C) 9.28*10 - 10 m
D) 3.88 *10 - 12 m

Unlock Deck
Unlock for access to all 63 flashcards in this deck.
Unlock Deck
k this deck
61
If the barrier width for an STM is 0.1 nm, what would be the corresponding minimum momentum uncertainty of an electron traversing this boundary?
A) 10-25 kg·m/s
B) 10-23 kg·m/s
C) 10-24 kg·m/s
D) 10-22 kg·m/s
A) 10-25 kg·m/s
B) 10-23 kg·m/s
C) 10-24 kg·m/s
D) 10-22 kg·m/s

Unlock Deck
Unlock for access to all 63 flashcards in this deck.
Unlock Deck
k this deck
62
Which of the following is most sensitive to low-intensity light?
A) the green cones
B) the blue cones
C) the red cones
D) the rods
A) the green cones
B) the blue cones
C) the red cones
D) the rods

Unlock Deck
Unlock for access to all 63 flashcards in this deck.
Unlock Deck
k this deck
63
If it takes 50 photons per 0.1 s entering the eye so that the brain senses the light, which of the following colors of light would be delivering the most power to the eye at this minimum perception level?
A) red
B) violet
C) blue
D) All would be delivering the same power.
A) red
B) violet
C) blue
D) All would be delivering the same power.

Unlock Deck
Unlock for access to all 63 flashcards in this deck.
Unlock Deck
k this deck


