Deck 29: Atomic Theory
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/63
Play
Full screen (f)
Deck 29: Atomic Theory
1
What is the wavelength of the line in the hydrogen spectrum that is comprised of transitions from the n = 5 to the n = 3 levels?
A) 1880 nm
B) 1290 nm
C) 2250 nm
D) 1920 nm
A) 1880 nm
B) 1290 nm
C) 2250 nm
D) 1920 nm
1290 nm
2
The ionization energy of the hydrogen atom is 13.6 eV. What is the energy of the n = 5 state?
A) -0.544 eV
B) 0.378 eV
C) 0.544 eV
D) -0.378 eV
A) -0.544 eV
B) 0.378 eV
C) 0.544 eV
D) -0.378 eV
-0.544 eV
3
The four visible colors emitted by hydrogen atoms are produced by electrons:
A) that start in the ground state.
B) that end up in the ground state.
C) that end up in the level with n = 2.
D) that start in the level with n = 2.
A) that start in the ground state.
B) that end up in the ground state.
C) that end up in the level with n = 2.
D) that start in the level with n = 2.
that end up in the level with n = 2.
4
The Paschen series of hydrogen corresponds to electron transitions from higher levels to n = 3. What is the longest wavelength in that series? (h = 6.63 * 10-34 J.s. The ground state of hydrogen is at -13.6 eV.)
A) 822 nm
B) 365 nm
C) 1880 nm
D) 1094 nm
A) 822 nm
B) 365 nm
C) 1880 nm
D) 1094 nm

Unlock Deck
Unlock for access to all 63 flashcards in this deck.
Unlock Deck
k this deck
5
If the radius of the electron orbit in the n = 1 level of the hydrogen atoms is 0.0529 nm, what is its radius for the n = 4 level? (Assume the Bohr model is valid).
A) 1.32 nm
B) 0.265 nm
C) 0.106 nm
D) 0.846 nm
A) 1.32 nm
B) 0.265 nm
C) 0.106 nm
D) 0.846 nm

Unlock Deck
Unlock for access to all 63 flashcards in this deck.
Unlock Deck
k this deck
6
In the Bohr model of the atom, the orbits where electrons move slowest:
A) have the least radius.
B) have the highest energy.
C) have the least angular momentum.
D) have the lowest energy.
A) have the least radius.
B) have the highest energy.
C) have the least angular momentum.
D) have the lowest energy.

Unlock Deck
Unlock for access to all 63 flashcards in this deck.
Unlock Deck
k this deck
7
The Lyman series of hydrogen is made up of those transitions made from higher levels to n = 1. If the first line (n = 2 to n = 1) in this series has a wavelength of 122 nm, what is the wavelength of the third line (n = 4 to n = 1)?
A) 364 nm
B) 97.6 nm
C) 103 nm
D) 486 nm
A) 364 nm
B) 97.6 nm
C) 103 nm
D) 486 nm

Unlock Deck
Unlock for access to all 63 flashcards in this deck.
Unlock Deck
k this deck
8
Of the wavelengths emitted from a hydrogen gas discharge tube, those that are associated with transitions from higher levels down to the n = 1 level produce which of the following?
A) infrared
B) visible
C) ultraviolet
D) mixture of infrared and visible
A) infrared
B) visible
C) ultraviolet
D) mixture of infrared and visible

Unlock Deck
Unlock for access to all 63 flashcards in this deck.
Unlock Deck
k this deck
9
The Lyman series of hydrogen corresponds to electron transitions from higher levels to n = 1. What is the longest wavelength in that series? (h = 6.63 *10 - 34 J.s. The ground state of hydrogen is at -13.6 eV.)
A) 91.4 nm
B) 273 nm
C) 456 nm
D) 122 nm
A) 91.4 nm
B) 273 nm
C) 456 nm
D) 122 nm

Unlock Deck
Unlock for access to all 63 flashcards in this deck.
Unlock Deck
k this deck
10
If a hydrogen atom, originally in its ground state of energy -13.6 eV, absorbs a photon of energy 22.0 eV, what is the resulting kinetic energy of the electron if the proton has negligible kinetic energy?
A)" Such a photon cannot be absorbed in this case."
B) "8.4 eV"
C) "2.4 eV"
D) "16.0 eV"
A)" Such a photon cannot be absorbed in this case."
B) "8.4 eV"
C) "2.4 eV"
D) "16.0 eV"

Unlock Deck
Unlock for access to all 63 flashcards in this deck.
Unlock Deck
k this deck
11
The ionization energy of the hydrogen atom is 13.6 eV. What is the energy of a photon emitted corresponding to a transition from the n = 6 to n = 2 state?
A) 7.9 eV
B) 2.9 eV
C) 3.0 eV
D) 4.0 eV
A) 7.9 eV
B) 2.9 eV
C) 3.0 eV
D) 4.0 eV

Unlock Deck
Unlock for access to all 63 flashcards in this deck.
Unlock Deck
k this deck
12
Of the various wavelengths emitted from a hydrogen gas discharge tube, those associated with transitions from higher levels down to the n = 2 level produce which of the following?
A) visible
B) infrared
C) ultraviolet
D) a mixture of visible and ultraviolet
A) visible
B) infrared
C) ultraviolet
D) a mixture of visible and ultraviolet

Unlock Deck
Unlock for access to all 63 flashcards in this deck.
Unlock Deck
k this deck
13
When a high voltage is applied to a low-pressure gas causing it to glow, it will emit which type of spectrum?
A) line emission
B) line absorption
C) continuous
D) monochromatic
A) line emission
B) line absorption
C) continuous
D) monochromatic

Unlock Deck
Unlock for access to all 63 flashcards in this deck.
Unlock Deck
k this deck
14
The ionization energy of the hydrogen atom is 13.6 eV. What is the wavelength of a photon having this much energy? (h = 6.63 * 10 - 34 J.s, c = 3.00 *108 m/s, 1 eV = 1.6 *10 - 19 J, and 1 nm = 10 - 9 m)
A) 91.4 nm
B) 360 nm
C) 136 nm
D) 273 nm
A) 91.4 nm
B) 360 nm
C) 136 nm
D) 273 nm

Unlock Deck
Unlock for access to all 63 flashcards in this deck.
Unlock Deck
k this deck
15
In contrast to the plum-pudding model of the atom, Rutherford's model:
A) was first to explain atoms emitting discrete frequencies.
B) had the positive charge concentrated in a small region.
C) eliminated radiation from accelerating charges.
D) had the positive charge spread uniformly through the atom.
A) was first to explain atoms emitting discrete frequencies.
B) had the positive charge concentrated in a small region.
C) eliminated radiation from accelerating charges.
D) had the positive charge spread uniformly through the atom.

Unlock Deck
Unlock for access to all 63 flashcards in this deck.
Unlock Deck
k this deck
16
The ionization energy for the hydrogen atom is 13.6 eV. What is the energy of a photon that is emitted as a hydrogen atom makes a transition between the n = 4 and n = 3 states?
A) 3.40 eV
B) 0.66 eV
C) 6.80 eV
D) 2.55 eV
A) 3.40 eV
B) 0.66 eV
C) 6.80 eV
D) 2.55 eV

Unlock Deck
Unlock for access to all 63 flashcards in this deck.
Unlock Deck
k this deck
17
When a cool gas is placed between a glowing wire filament source and a diffraction grating, the resultant spectrum from the grating is which one of the following?
A) line emission
B) continuous
C) line absorption
D) monochromatic
A) line emission
B) continuous
C) line absorption
D) monochromatic

Unlock Deck
Unlock for access to all 63 flashcards in this deck.
Unlock Deck
k this deck
18
When a wire carries high current causing it to glow, it will emit which type of spectrum?
A) line absorption
B) monochromatic
C) continuous
D) line emission
A) line absorption
B) monochromatic
C) continuous
D) line emission

Unlock Deck
Unlock for access to all 63 flashcards in this deck.
Unlock Deck
k this deck
19
An alpha particle is:
A) any positively charged nucleus.
B) an X-ray.
C) a neutral helium atom.
D) None of these choices.
A) any positively charged nucleus.
B) an X-ray.
C) a neutral helium atom.
D) None of these choices.

Unlock Deck
Unlock for access to all 63 flashcards in this deck.
Unlock Deck
k this deck
20
According to the Rutherford model of the atom, most of the volume of an atom:
A) is empty space.
B) was occupied by the nucleus.
C) excluded electrons.
D) contained positive charges.
A) is empty space.
B) was occupied by the nucleus.
C) excluded electrons.
D) contained positive charges.

Unlock Deck
Unlock for access to all 63 flashcards in this deck.
Unlock Deck
k this deck
21
The quantum mechanical model of the hydrogen atom requires that if the principal quantum number is 5, there will be how many different permitted orbital quantum number(s)?
A) two
B) five
C) one
D) four
A) two
B) five
C) one
D) four

Unlock Deck
Unlock for access to all 63 flashcards in this deck.
Unlock Deck
k this deck
22
Characteristic K X-rays are the result of:
A) inner electron transitions.
B) outer electron transitions.
C) nuclear electron states.
D) buckytubes.
A) inner electron transitions.
B) outer electron transitions.
C) nuclear electron states.
D) buckytubes.

Unlock Deck
Unlock for access to all 63 flashcards in this deck.
Unlock Deck
k this deck
23
A muon behaves like an electron except that it has 207 times the mass of the electron. If a muon were bound to a proton, how would the energy levels in the Bohr model compare to those for a bound electron?
A) They would be 207 times as much as those for the electron.
B) They would be (207)2 times as much as those for the electron.
C) They would be the same.
D) They would be (1/207) times as much as those for the electron.
A) They would be 207 times as much as those for the electron.
B) They would be (207)2 times as much as those for the electron.
C) They would be the same.
D) They would be (1/207) times as much as those for the electron.

Unlock Deck
Unlock for access to all 63 flashcards in this deck.
Unlock Deck
k this deck
24
A photon is emitted from a hydrogen atom that undergoes a transition from n = 3 to n = 2. Calculate the energy and wavelength of the photon. (The ionization energy of hydrogen is 13.6 eV, and h = 6.63 *10-34 J.s, c = 3.00 * 108 m/s, 1 eV = 1.60 *10-19 J, and 1 nm = 10-9 m)
A) 2.21 eV, 563 nm
B) 1.89 eV, 460 nm
C) 2.58 * 10-19 J, 658 nm
D) 3.02 * 10-19 J, 658 nm
A) 2.21 eV, 563 nm
B) 1.89 eV, 460 nm
C) 2.58 * 10-19 J, 658 nm
D) 3.02 * 10-19 J, 658 nm

Unlock Deck
Unlock for access to all 63 flashcards in this deck.
Unlock Deck
k this deck
25
In an analysis relating Bohr's theory to the de Broglie wavelength of electrons, when an electron moves from the n = 1 level to the n = 3 level, the circumference for its orbit becomes 9 times greater. This occurs because:
A) the wavelength of the electron becomes nine times as long.
B) there are triple as many wavelengths, and each wavelength is triple in length.
C) there are nine times as many wavelengths in the new orbit.
D) the electron is moving nine times as fast.
A) the wavelength of the electron becomes nine times as long.
B) there are triple as many wavelengths, and each wavelength is triple in length.
C) there are nine times as many wavelengths in the new orbit.
D) the electron is moving nine times as fast.

Unlock Deck
Unlock for access to all 63 flashcards in this deck.
Unlock Deck
k this deck
26
When a hydrogen atom absorbs a photon that raises it to the n = 5 state, how many different energies are possible for the photon(s) that may be emitted as the atom eventually returns to the ground state?
A) 5
B) 3
C) 4
D) The correct value is not given.
A) 5
B) 3
C) 4
D) The correct value is not given.

Unlock Deck
Unlock for access to all 63 flashcards in this deck.
Unlock Deck
k this deck
27
In the n = 5 shell, how many distinct values of 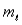 are possible?
are possible?
A) 5
B) 15
C) 10
D) The correct value is not given.
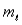 are possible?
are possible?A) 5
B) 15
C) 10
D) The correct value is not given.

Unlock Deck
Unlock for access to all 63 flashcards in this deck.
Unlock Deck
k this deck
28
When an electron moves from the n = 1 to the n = 3 orbit:
A) the radius doubles and the angular momentum increases by a factor of 9.
B) the radius increases by a factor of 9, and the angular momentum triples.
C) both the radius and the angular momentum increase by a factor of 9.
D) both the radius and the angular momentum triple.
A) the radius doubles and the angular momentum increases by a factor of 9.
B) the radius increases by a factor of 9, and the angular momentum triples.
C) both the radius and the angular momentum increase by a factor of 9.
D) both the radius and the angular momentum triple.

Unlock Deck
Unlock for access to all 63 flashcards in this deck.
Unlock Deck
k this deck
29
The Bohr theory does not predict that:
A) the approximate radius of a hydrogen atom is 5.3 *10-11 m.
B) hydrogen atoms will give off the lines from the series ending in the n = 2 state.
C) the ground state of hydrogen is spherically symmetric.
D) it requires 13.6 eV to ionize hydrogen.
A) the approximate radius of a hydrogen atom is 5.3 *10-11 m.
B) hydrogen atoms will give off the lines from the series ending in the n = 2 state.
C) the ground state of hydrogen is spherically symmetric.
D) it requires 13.6 eV to ionize hydrogen.

Unlock Deck
Unlock for access to all 63 flashcards in this deck.
Unlock Deck
k this deck
30
The quantum mechanical model of the hydrogen atom requires that if the principal quantum number = 6, there will be how many permitted orbital quantum numbers?
A) 3
B) 16
C) 6
D) 5
A) 3
B) 16
C) 6
D) 5

Unlock Deck
Unlock for access to all 63 flashcards in this deck.
Unlock Deck
k this deck
31
In the hydrogen atom the potential energy is negative, but the absolute value of the potential energy:
A) is half the kinetic energy of the electron.
B) is equal to n2 times the kinetic energy of the electron.
C) is equal to the kinetic energy of the electron.
D) is twice the kinetic energy of the electron.
A) is half the kinetic energy of the electron.
B) is equal to n2 times the kinetic energy of the electron.
C) is equal to the kinetic energy of the electron.
D) is twice the kinetic energy of the electron.

Unlock Deck
Unlock for access to all 63 flashcards in this deck.
Unlock Deck
k this deck
32
When a hydrogen atom absorbs a photon that raises it to the n = 4 state, what is the least number of photons that can be emitted by that atom as it returns to the ground state?
A) 4
B) 3
C) 5
D) The correct value is not given.
A) 4
B) 3
C) 5
D) The correct value is not given.

Unlock Deck
Unlock for access to all 63 flashcards in this deck.
Unlock Deck
k this deck
33
The speed of the electron in the Bohr theory of hydrogen is:
A) inversely proportional to n.
B) inversely proportional to n2.
C) proportional to n.
D) proportional to n2.
A) inversely proportional to n.
B) inversely proportional to n2.
C) proportional to n.
D) proportional to n2.

Unlock Deck
Unlock for access to all 63 flashcards in this deck.
Unlock Deck
k this deck
34
How many possible substates are available in a hydrogen atom where the principal quantum number is 2?
A) 18
B) 8
C) 36
D) 9
A) 18
B) 8
C) 36
D) 9

Unlock Deck
Unlock for access to all 63 flashcards in this deck.
Unlock Deck
k this deck
35
The quantum mechanical model of the hydrogen atom requires that if the orbital quantum number = 5, there will be how many permitted orbital magnetic quantum numbers allowed?
A) 7
B) 15
C) 5
D) 11
A) 7
B) 15
C) 5
D) 11

Unlock Deck
Unlock for access to all 63 flashcards in this deck.
Unlock Deck
k this deck
36
The quantum mechanical model of the hydrogen atom requires that if the orbital quantum number of the hydrogen atom is 3, there will be how many permitted orbital magnetic quantum numbers?
A) three
B) nine
C) four
D) seven
A) three
B) nine
C) four
D) seven

Unlock Deck
Unlock for access to all 63 flashcards in this deck.
Unlock Deck
k this deck
37
The Bohr model of the hydrogen atom accounts for which quantum number?
A) orbital
B) principal
C) orbital magnetic
D) All of the above.
A) orbital
B) principal
C) orbital magnetic
D) All of the above.

Unlock Deck
Unlock for access to all 63 flashcards in this deck.
Unlock Deck
k this deck
38
The quantum mechanical model of the hydrogen atom requires that if the orbital magnetic quantum number is 4, there will be how many permitted spin magnetic quantum numbers?
A) four
B) seven
C) three
D) two
A) four
B) seven
C) three
D) two

Unlock Deck
Unlock for access to all 63 flashcards in this deck.
Unlock Deck
k this deck
39
Which of the following transitions in hydrogen from an initial state (ni) to a final state (nf) results in the least energy emitted?
A) ni = 3, nf = 95
B) ni = 80, nf = 2
C) ni = 2, nf = 1
D) ni = 1, nf = 3
A) ni = 3, nf = 95
B) ni = 80, nf = 2
C) ni = 2, nf = 1
D) ni = 1, nf = 3

Unlock Deck
Unlock for access to all 63 flashcards in this deck.
Unlock Deck
k this deck
40
A hydrogen atom in the ground state absorbs a 12.75-eV photon. To what level is the electron promoted? (The ionization energy of hydrogen is 13.6 eV).
A) n = 2
B) n = 3
C) n = 5
D) n = 4
A) n = 2
B) n = 3
C) n = 5
D) n = 4

Unlock Deck
Unlock for access to all 63 flashcards in this deck.
Unlock Deck
k this deck
41
The quantum mechanical model of the hydrogen atom requires that if the orbital quantum number = 4, there are permitted how many possible substates?
A) 22
B) 18
C) 8
D) 32
A) 22
B) 18
C) 8
D) 32

Unlock Deck
Unlock for access to all 63 flashcards in this deck.
Unlock Deck
k this deck
42
What is the maximum number of electrons that can occupy a 3d subshell?
A) 5
B) 14
C) 10
D) 18
A) 5
B) 14
C) 10
D) 18

Unlock Deck
Unlock for access to all 63 flashcards in this deck.
Unlock Deck
k this deck
43
What is the lowest value for n of a shell that can contain a g electron?
A) 5
B) 4
C) 3
D) 6
A) 5
B) 4
C) 3
D) 6

Unlock Deck
Unlock for access to all 63 flashcards in this deck.
Unlock Deck
k this deck
44
How many electrons are in chlorine's (atomic number 17) next to outer shell (n = 2)?
A) 18
B) 4
C) 2
D) 8
A) 18
B) 4
C) 2
D) 8

Unlock Deck
Unlock for access to all 63 flashcards in this deck.
Unlock Deck
k this deck
45
The quantum mechanical model of the hydrogen atom requires that if the principal quantum number = 3, there will be permitted how many orbital magnetic quantum numbers?
A) 5
B) 8
C) 4
D) 7
A) 5
B) 8
C) 4
D) 7

Unlock Deck
Unlock for access to all 63 flashcards in this deck.
Unlock Deck
k this deck
46
For n = 5, how many allowed values are possible for the orbital quantum number and the orbital magnetic quantum number?
A) 5, 11
B) 6, 12
C) 5, 9
D) 4, 9
A) 5, 11
B) 6, 12
C) 5, 9
D) 4, 9

Unlock Deck
Unlock for access to all 63 flashcards in this deck.
Unlock Deck
k this deck
47
The ground state configuration for an element is 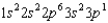 . What is the atomic number of the element?
. What is the atomic number of the element?
A) 11
B) 9
C) 24
D) 13
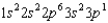 . What is the atomic number of the element?
. What is the atomic number of the element?A) 11
B) 9
C) 24
D) 13

Unlock Deck
Unlock for access to all 63 flashcards in this deck.
Unlock Deck
k this deck
48
Magnesium has atomic number 12. In its ground state, how many electrons are in its n = 3 shell?
A) 2
B) 8
C) 16
D) 10
A) 2
B) 8
C) 16
D) 10

Unlock Deck
Unlock for access to all 63 flashcards in this deck.
Unlock Deck
k this deck
49
What is the next energy level that fills after the 4d energy level is full?
A) 5p
B) 4s
C) 6s
D) 4f
A) 5p
B) 4s
C) 6s
D) 4f

Unlock Deck
Unlock for access to all 63 flashcards in this deck.
Unlock Deck
k this deck
50
The ground state electronic configuration for silicon is 1s22s22p63s23p2. Which of the following elements would be expected to be chemically similar to silicon?
A) boron with configuration 1s22s22p1
B) lithium with configuration 1s22s1
C) carbon with configuration 1s22s22p2
D) sodium with configuration 1s22s22p63s1
A) boron with configuration 1s22s22p1
B) lithium with configuration 1s22s1
C) carbon with configuration 1s22s22p2
D) sodium with configuration 1s22s22p63s1

Unlock Deck
Unlock for access to all 63 flashcards in this deck.
Unlock Deck
k this deck
51
For n = 6, what are the maximum orbital quantum number and the maximum orbital magnetic quantum number?
A) 7, 6
B) 5, 4
C) 6, 5
D) 5, 5
A) 7, 6
B) 5, 4
C) 6, 5
D) 5, 5

Unlock Deck
Unlock for access to all 63 flashcards in this deck.
Unlock Deck
k this deck
52
The quantum mechanical model of the hydrogen atom suggests a visual picture of the electron as which of the following?
A) planetary orbiting body
B) probability cloud
C) raisin in pudding
D) light quantum
A) planetary orbiting body
B) probability cloud
C) raisin in pudding
D) light quantum

Unlock Deck
Unlock for access to all 63 flashcards in this deck.
Unlock Deck
k this deck
53
If the principal quantum number for hydrogen is 4, which one of the following is not a permitted orbital magnetic quantum number for that atom?
A) "-2"
B) "0"
C) "3"
D) "5"
A) "-2"
B) "0"
C) "3"
D) "5"

Unlock Deck
Unlock for access to all 63 flashcards in this deck.
Unlock Deck
k this deck
54
The stimulated emission of photons from the excited atoms in a gas laser is prompted by which of the following?
A) nearby presence of photons of same wavelength as those emitted
B) high flux of electrons
C) high voltage
D) high temperature
A) nearby presence of photons of same wavelength as those emitted
B) high flux of electrons
C) high voltage
D) high temperature

Unlock Deck
Unlock for access to all 63 flashcards in this deck.
Unlock Deck
k this deck
55
The red light from a HeNe laboratory laser results from a transition in Ne. Determine the energy difference between the states if the wavelength of the light given off is 634 nm. (h = 6.63 *10-34 J.s, c = 3.00 *108 m/s, 1 eV = 1.60 *10-19 J, and 1 nm = 10-9 m)
A) 2.22 eV
B) 1.43 eV
C) 1.96 eV
D) 2.04 eV
A) 2.22 eV
B) 1.43 eV
C) 1.96 eV
D) 2.04 eV

Unlock Deck
Unlock for access to all 63 flashcards in this deck.
Unlock Deck
k this deck
56
The restriction that no more than one electron may occupy a given quantum state in an atom was first stated by which of the following scientists?
A) Bohr
B) Pauli
C) de Broglie
D) Heisenberg
A) Bohr
B) Pauli
C) de Broglie
D) Heisenberg

Unlock Deck
Unlock for access to all 63 flashcards in this deck.
Unlock Deck
k this deck
57
For the n = 5 shell, what are the lowest values possible for 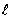 and
and 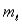 respectively?
respectively?
A) "-5, -5"
B) "-4, -4"
C) "0, -4"
D) "0, 0"
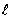 and
and 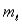 respectively?
respectively?A) "-5, -5"
B) "-4, -4"
C) "0, -4"
D) "0, 0"

Unlock Deck
Unlock for access to all 63 flashcards in this deck.
Unlock Deck
k this deck
58
The quantum number that can have only two possible values is the:
A) orbital quantum number.
B) principal quantum number.
C) spin magnetic quantum number.
D) orbital magnetic quantum number.
A) orbital quantum number.
B) principal quantum number.
C) spin magnetic quantum number.
D) orbital magnetic quantum number.

Unlock Deck
Unlock for access to all 63 flashcards in this deck.
Unlock Deck
k this deck
59
The ground state electronic configuration for sodium is 1s22s22p63s1. What is the orbital quantum number of the last (3s1) electron?
A) 3
B) 1
C) 2
D) 0
A) 3
B) 1
C) 2
D) 0

Unlock Deck
Unlock for access to all 63 flashcards in this deck.
Unlock Deck
k this deck
60
The ground state electronic configuration for magnesium is 1s22s22p63s2. Which of the following would most likely result in an element being chemically similar to magnesium?
A) the valence subshell having 2 electrons
B) the valence electron being an s2 electron
C) the valence electron being a p2 electron
D) the valence electron having principal quantum number 3
A) the valence subshell having 2 electrons
B) the valence electron being an s2 electron
C) the valence electron being a p2 electron
D) the valence electron having principal quantum number 3

Unlock Deck
Unlock for access to all 63 flashcards in this deck.
Unlock Deck
k this deck
61
A ruby laser delivers a 2.28-J pulse in approximately 50 nanoseconds. The wavelength of the light is 694.4 nm. At least how many atoms within the ruby rod had to be excited to allow this high-energy laser pulse? (h = 6.63 *10-34 J.s, c = 3.00* 108 m/s, 1 eV = 1.6 *10-19 J, and 1 nm = 10-9 m)
A) 3 * 1019
B) 4 * 1018
C) 8 *1018
D) 6 * 1020
A) 3 * 1019
B) 4 * 1018
C) 8 *1018
D) 6 * 1020

Unlock Deck
Unlock for access to all 63 flashcards in this deck.
Unlock Deck
k this deck
62
In neon, the 20.66-eV level can undergo lasing action to the 18.70-eV level. What is the energy of the resulting photons?
A) 18.70 eV
B) 20.66 eV
C) 1.96 eV
D) 39.36 eV
A) 18.70 eV
B) 20.66 eV
C) 1.96 eV
D) 39.36 eV

Unlock Deck
Unlock for access to all 63 flashcards in this deck.
Unlock Deck
k this deck
63
The wavelength of a coherent laser is 628 nm. What energy difference exists between the upper excited state involved and the lower unexcited ground state? (h = 6.63 *10-34 J.s, c = 3.00 *108 m/s, 1 eV = 1.60 *10-19 J, and 1 nm = 10-9 m)
A) 1.86 eV
B) 1.81 eV
C) 1.75 eV
D) 1.98 eV
A) 1.86 eV
B) 1.81 eV
C) 1.75 eV
D) 1.98 eV

Unlock Deck
Unlock for access to all 63 flashcards in this deck.
Unlock Deck
k this deck


