Deck 11: Introduction to Organic Molecules and Functional Groups
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/123
Play
Full screen (f)
Deck 11: Introduction to Organic Molecules and Functional Groups
1
Which structure has all of the hydrogens and lone pairs correctly added to the compound shown below? 
A)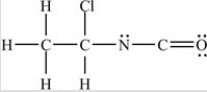
B)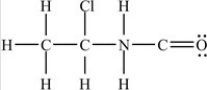
C)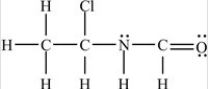
D)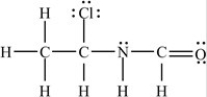

A)

B)

C)

D)


2
What is the correct bond angle for the bond indicated in the following structure? 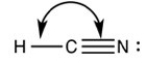
A)109.5º
B)180º
C)120º
D)90º
E)60º

A)109.5º
B)180º
C)120º
D)90º
E)60º
180º
3
Which of the following correctly describes the bond angles indicated in the structure below? 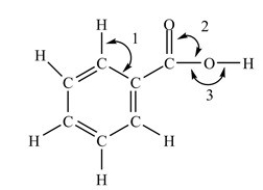
A)Angle 1 = 109.5°,Angle 2 = 109.5°,and Angle 3 = 180°
B)Angle 1 = 120°,Angle 2 = 120°,and Angle 3 = 180°
C)Angle 1 = 120°,Angle 2 = 120°,and Angle 3 = ~109.5°

A)Angle 1 = 109.5°,Angle 2 = 109.5°,and Angle 3 = 180°
B)Angle 1 = 120°,Angle 2 = 120°,and Angle 3 = 180°
C)Angle 1 = 120°,Angle 2 = 120°,and Angle 3 = ~109.5°
Angle 1 = 120°,Angle 2 = 120°,and Angle 3 = ~109.5°
4
Which formula represents an inorganic compound?
A)CH4
B)C2H5OH
C)Br2
D)C4H10
A)CH4
B)C2H5OH
C)Br2
D)C4H10

Unlock Deck
Unlock for access to all 123 flashcards in this deck.
Unlock Deck
k this deck
5
Which structure has all of the hydrogen atoms and lone pairs correctly added to the compound shown below? 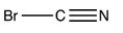
A)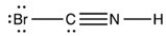
B)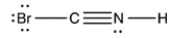
C)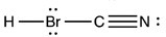
D)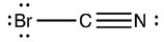
E)The compound given is not missing any hydrogen atoms nor any lone pairs.
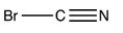
A)
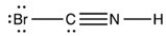
B)
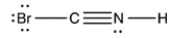
C)
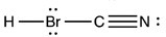
D)
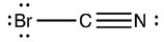
E)The compound given is not missing any hydrogen atoms nor any lone pairs.

Unlock Deck
Unlock for access to all 123 flashcards in this deck.
Unlock Deck
k this deck
6
What is the correct bond angle for the bond indicated in the following structure? 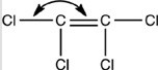
A)109.5º
B)180º
C)120º
D)90º
E)60º

A)109.5º
B)180º
C)120º
D)90º
E)60º

Unlock Deck
Unlock for access to all 123 flashcards in this deck.
Unlock Deck
k this deck
7
What is the bond angle associated with a tetrahedral shape?
A)109.5º
B)180º
C)120º
D)90º
E)60º
A)109.5º
B)180º
C)120º
D)90º
E)60º

Unlock Deck
Unlock for access to all 123 flashcards in this deck.
Unlock Deck
k this deck
8
What is the most common multiple bond between carbon and a heteroatom?
A)A carbon-oxygen double bond (C=O)
B)A carbon-nitrogen double bond (C=N)
C)A carbon-nitrogen single bond (C-N)
D)A carbon-sulfur double bond (C=S)
A)A carbon-oxygen double bond (C=O)
B)A carbon-nitrogen double bond (C=N)
C)A carbon-nitrogen single bond (C-N)
D)A carbon-sulfur double bond (C=S)

Unlock Deck
Unlock for access to all 123 flashcards in this deck.
Unlock Deck
k this deck
9
A carbon atom surrounded by two other atoms generally forms ________ triple bond(s)?
A)0
B)1
C)2
D)3
A)0
B)1
C)2
D)3

Unlock Deck
Unlock for access to all 123 flashcards in this deck.
Unlock Deck
k this deck
10
Which formula represents an organic compound?
A)NaCl
B)BaSO4
C)PH3
D)C4H10
A)NaCl
B)BaSO4
C)PH3
D)C4H10

Unlock Deck
Unlock for access to all 123 flashcards in this deck.
Unlock Deck
k this deck
11
What is the shape around the C (1)atom and around the O(2)atom in the structure of diethyl ether given below? 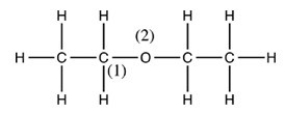
A)The shape around both C (1)and O (2)is tetrahedral.
B)The shape around C (1)is tetrahedral; the shape around O (2)is bent.
C)The shape around both C (1)and O (2)is bent.
D)The shape around C(1)is trigonal pyramidal; the shape around O (2)is linear.
E)The shape around both C(1)and O (2)is linear.

A)The shape around both C (1)and O (2)is tetrahedral.
B)The shape around C (1)is tetrahedral; the shape around O (2)is bent.
C)The shape around both C (1)and O (2)is bent.
D)The shape around C(1)is trigonal pyramidal; the shape around O (2)is linear.
E)The shape around both C(1)and O (2)is linear.

Unlock Deck
Unlock for access to all 123 flashcards in this deck.
Unlock Deck
k this deck
12
According to the common bonding patters for atoms,halogen atoms typically have ________ lone pairs of electrons when they are bonded in organic compounds.
A)4
B)3
C)2
D)1
A)4
B)3
C)2
D)1

Unlock Deck
Unlock for access to all 123 flashcards in this deck.
Unlock Deck
k this deck
13
Which structure has all of the hydrogen atoms correctly added to the compound shown below? 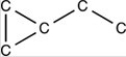
A)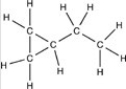
B)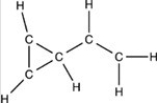
C)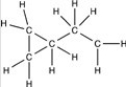
D)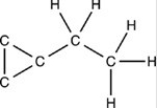

A)

B)

C)

D)


Unlock Deck
Unlock for access to all 123 flashcards in this deck.
Unlock Deck
k this deck
14
What is the shape around each carbon atom in the structure shown below? 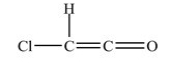
A)The shape around each carbon atom is tetrahedral.
B)The shape around each carbon atom is bent.
C)The shape around the left carbon atom is tetrahedral and bent around the right carbon atom.
D)The shape around the left carbon atom is trigonal planar and bent around the right carbon atom.
E)The shape around the left carbon atom is trigonal planar and linear around the right carbon atom.

A)The shape around each carbon atom is tetrahedral.
B)The shape around each carbon atom is bent.
C)The shape around the left carbon atom is tetrahedral and bent around the right carbon atom.
D)The shape around the left carbon atom is trigonal planar and bent around the right carbon atom.
E)The shape around the left carbon atom is trigonal planar and linear around the right carbon atom.

Unlock Deck
Unlock for access to all 123 flashcards in this deck.
Unlock Deck
k this deck
15
How many covalent bonds does carbon generally form in organic compounds?
A)2
B)3
C)4
D)8
A)2
B)3
C)4
D)8

Unlock Deck
Unlock for access to all 123 flashcards in this deck.
Unlock Deck
k this deck
16
Which of the following correctly depicts the three-dimensional shape around each carbon in the structure below? 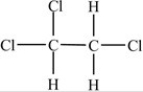
A)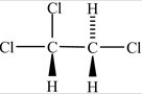
B)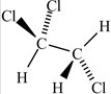
C)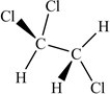
D)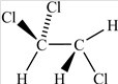

A)

B)

C)

D)


Unlock Deck
Unlock for access to all 123 flashcards in this deck.
Unlock Deck
k this deck
17
Which element is not a common heteroatom in organic compounds?
A)N
B)Na
C)O
D)Cl
E)F
A)N
B)Na
C)O
D)Cl
E)F

Unlock Deck
Unlock for access to all 123 flashcards in this deck.
Unlock Deck
k this deck
18
According to the common bonding patters for atoms in organic compounds,oxygen atoms typically form ________ bond(s).
A)4
B)3
C)2
D)1
A)4
B)3
C)2
D)1

Unlock Deck
Unlock for access to all 123 flashcards in this deck.
Unlock Deck
k this deck
19
What is the shape around the N (1)atom and around the C (2)atom in the structure of mepivacaine given below? 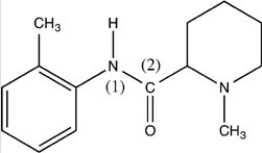
A)The shape around both N (1)and C (2)is tetrahedral.
B)The shape around both N (1)and C (2)is trigonal planar.
C)The shape around both N (1)and C (2)is trigonal pyramidal.
D)The shape around N(1)is trigonal pyramidal; the shape around C (2)is trigonal planar.
E)The shape around N(1)is trigonal planar; the shape around C (2)is trigonal pyramidal.

A)The shape around both N (1)and C (2)is tetrahedral.
B)The shape around both N (1)and C (2)is trigonal planar.
C)The shape around both N (1)and C (2)is trigonal pyramidal.
D)The shape around N(1)is trigonal pyramidal; the shape around C (2)is trigonal planar.
E)The shape around N(1)is trigonal planar; the shape around C (2)is trigonal pyramidal.

Unlock Deck
Unlock for access to all 123 flashcards in this deck.
Unlock Deck
k this deck
20
A carbon atom surrounded by ________ other atom(s)generally forms one double bond?
A)0
B)1
C)2
D)3
A)0
B)1
C)2
D)3

Unlock Deck
Unlock for access to all 123 flashcards in this deck.
Unlock Deck
k this deck
21
The functional group of which type of compound does not contain an oxygen atom?
A)Alcohol
B)Carboxylic acid
C)Ether
D)Alkyne
E)Ketone
A)Alcohol
B)Carboxylic acid
C)Ether
D)Alkyne
E)Ketone

Unlock Deck
Unlock for access to all 123 flashcards in this deck.
Unlock Deck
k this deck
22
How many hydrogen atoms are bonded to the labeled carbons in the structure below? 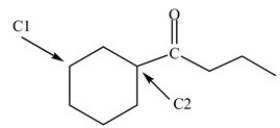
A)C1 has 2 hydrogens and C2 has 2 hydrogens.
B)C1 has 1 hydrogen and C2 has 0 hydrogens.
C)C1 has 2 hydrogens and C2 has 1 hydrogen.
D)C1 has 3 hydrogens and C2 has 1 hydrogen.

A)C1 has 2 hydrogens and C2 has 2 hydrogens.
B)C1 has 1 hydrogen and C2 has 0 hydrogens.
C)C1 has 2 hydrogens and C2 has 1 hydrogen.
D)C1 has 3 hydrogens and C2 has 1 hydrogen.

Unlock Deck
Unlock for access to all 123 flashcards in this deck.
Unlock Deck
k this deck
23
Which compound is an ether?
A)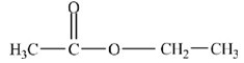
B)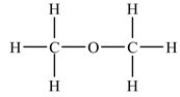
C)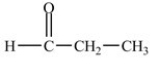
D)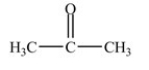
A)

B)

C)

D)


Unlock Deck
Unlock for access to all 123 flashcards in this deck.
Unlock Deck
k this deck
24
The molecule CH3SH is what type of compound?
A)Ether
B)Thiol
C)Amine
D)Alkyl Halide
E)Alcohol
A)Ether
B)Thiol
C)Amine
D)Alkyl Halide
E)Alcohol

Unlock Deck
Unlock for access to all 123 flashcards in this deck.
Unlock Deck
k this deck
25
Which skeletal structure represents the compound with the following condensed structure: (CH3)3CCH2OH?
A)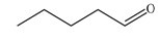
B)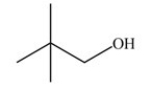
C)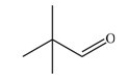
D)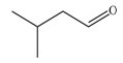
A)

B)

C)

D)


Unlock Deck
Unlock for access to all 123 flashcards in this deck.
Unlock Deck
k this deck
26
Which represents a skeletal structure for a five carbon hydrocarbon?
A)
B)
C)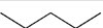
D)
A)

B)

C)

D)


Unlock Deck
Unlock for access to all 123 flashcards in this deck.
Unlock Deck
k this deck
27
How many hydrogen atoms are bonded to the labeled carbons in the structure below? 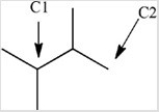
A)C1 has 2 hydrogens and C2 has 2 hydrogens.
B)C1 has 1 hydrogen and C2 has 3 hydrogens.
C)C1 has 2 hydrogens and C2 has 1 hydrogen.
D)C1 has 1 hydrogen and C2 has 1 hydrogen.

A)C1 has 2 hydrogens and C2 has 2 hydrogens.
B)C1 has 1 hydrogen and C2 has 3 hydrogens.
C)C1 has 2 hydrogens and C2 has 1 hydrogen.
D)C1 has 1 hydrogen and C2 has 1 hydrogen.

Unlock Deck
Unlock for access to all 123 flashcards in this deck.
Unlock Deck
k this deck
28
The functional group of which type of compound does not contain any multiple bonds?
A)Aldehyde
B)Alkene
C)Carboxylic acid
D)Alcohol
E)Aromatic
A)Aldehyde
B)Alkene
C)Carboxylic acid
D)Alcohol
E)Aromatic

Unlock Deck
Unlock for access to all 123 flashcards in this deck.
Unlock Deck
k this deck
29
Which structure is not possible?
A)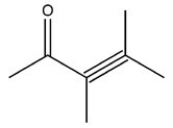
B)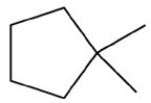
C)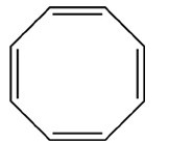
D)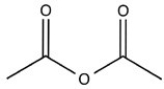
A)

B)

C)

D)


Unlock Deck
Unlock for access to all 123 flashcards in this deck.
Unlock Deck
k this deck
30
The compound below is classified as what type of compound? 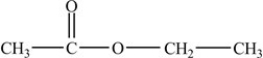
A)Aldehyde
B)Carboxylic acid
C)Ether
D)Ester
E)Ketone
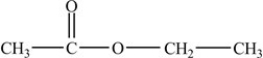
A)Aldehyde
B)Carboxylic acid
C)Ether
D)Ester
E)Ketone

Unlock Deck
Unlock for access to all 123 flashcards in this deck.
Unlock Deck
k this deck
31
How many total H atoms are present in the structure shown below? 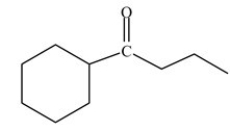
A)12 H atoms
B)18 H atoms
C)17 H atoms
D)19 H atoms

A)12 H atoms
B)18 H atoms
C)17 H atoms
D)19 H atoms

Unlock Deck
Unlock for access to all 123 flashcards in this deck.
Unlock Deck
k this deck
32
Which compound is an aldehyde?
A)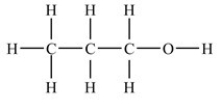
B)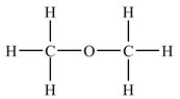
C)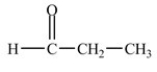
D)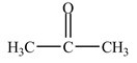
A)

B)

C)

D)


Unlock Deck
Unlock for access to all 123 flashcards in this deck.
Unlock Deck
k this deck
33
The compound below is classified as what type of compound? 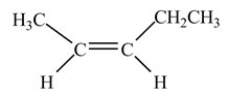
A)Alkyne
B)Alkane
C)Alkene
D)Aromatic hydrocarbon

A)Alkyne
B)Alkane
C)Alkene
D)Aromatic hydrocarbon

Unlock Deck
Unlock for access to all 123 flashcards in this deck.
Unlock Deck
k this deck
34
How many total H atoms are present in the structure shown below? 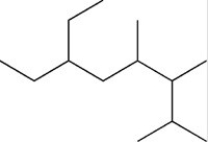
A)30 H atoms
B)13 H atoms
C)24 H atoms
D)28 H atoms

A)30 H atoms
B)13 H atoms
C)24 H atoms
D)28 H atoms

Unlock Deck
Unlock for access to all 123 flashcards in this deck.
Unlock Deck
k this deck
35
Which represents a condensed structure for a four carbon hydrocarbon?
A)CH3CH2CH2CH3
B)C4H10
C)
D)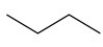
A)CH3CH2CH2CH3
B)C4H10
C)

D)


Unlock Deck
Unlock for access to all 123 flashcards in this deck.
Unlock Deck
k this deck
36
Which compound is an alcohol?
A)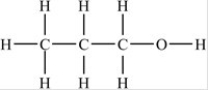
B)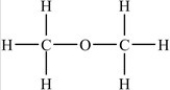
C)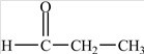
D)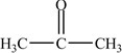
A)

B)

C)
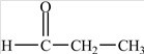
D)


Unlock Deck
Unlock for access to all 123 flashcards in this deck.
Unlock Deck
k this deck
37
Which structure is not possible?
A)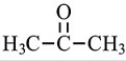
B)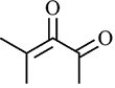
C)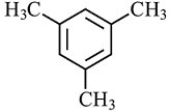
D)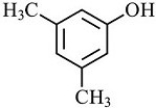
A)
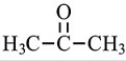
B)

C)

D)


Unlock Deck
Unlock for access to all 123 flashcards in this deck.
Unlock Deck
k this deck
38
Which compound is an amine?
A)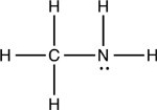
B)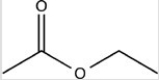
C)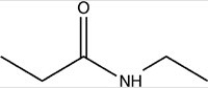
D)
A)

B)

C)

D)


Unlock Deck
Unlock for access to all 123 flashcards in this deck.
Unlock Deck
k this deck
39
The functional group of which type of compound does not contain a carbonyl group?
A)Aldehyde
B)Carboxylic acid
C)Ether
D)Ester
E)Ketone
A)Aldehyde
B)Carboxylic acid
C)Ether
D)Ester
E)Ketone

Unlock Deck
Unlock for access to all 123 flashcards in this deck.
Unlock Deck
k this deck
40
The compound below is classified as what type of compound? 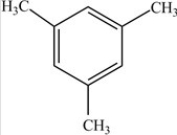
A)Alkyne
B)Alkane
C)Alkene
D)Aromatic hydrocarbon

A)Alkyne
B)Alkane
C)Alkene
D)Aromatic hydrocarbon

Unlock Deck
Unlock for access to all 123 flashcards in this deck.
Unlock Deck
k this deck
41
What is the shape about the O atoms in the environmental toxin dioxin,shown below? 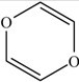
A)Tetrahedral
B)Bent
C)Trigonal planar
D)Trigonal pyramidal
E)Linear

A)Tetrahedral
B)Bent
C)Trigonal planar
D)Trigonal pyramidal
E)Linear

Unlock Deck
Unlock for access to all 123 flashcards in this deck.
Unlock Deck
k this deck
42
Which functional group is NOT present in the molecule below? 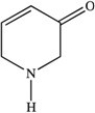
A)Carbonyl group
B)Amino group
C)Benzene ring
D)All of the functional groups mentioned appear in this molecule.

A)Carbonyl group
B)Amino group
C)Benzene ring
D)All of the functional groups mentioned appear in this molecule.

Unlock Deck
Unlock for access to all 123 flashcards in this deck.
Unlock Deck
k this deck
43
How many lone pairs of electrons are present but not shown in the molecule below? 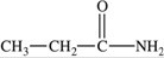
A)0
B)1
C)2
D)3
E)4

A)0
B)1
C)2
D)3
E)4

Unlock Deck
Unlock for access to all 123 flashcards in this deck.
Unlock Deck
k this deck
44
How many lone pairs of electrons are present but not shown in the molecule below? 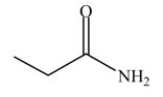
A)0
B)1
C)2
D)3
E)4

A)0
B)1
C)2
D)3
E)4

Unlock Deck
Unlock for access to all 123 flashcards in this deck.
Unlock Deck
k this deck
45
What is the condensed formula for the molecule shown below? 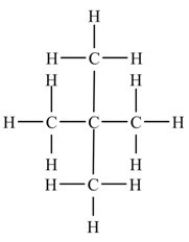
A)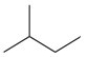
B)(CH3)4C
C)(CH3)2(CH2)2CH3
D)CH3CCH3CH3CH3

A)

B)(CH3)4C
C)(CH3)2(CH2)2CH3
D)CH3CCH3CH3CH3

Unlock Deck
Unlock for access to all 123 flashcards in this deck.
Unlock Deck
k this deck
46
Which compound is an amide?
A)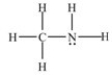
B)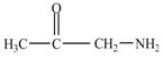
C)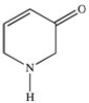
D)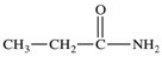
A)

B)

C)

D)
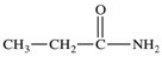

Unlock Deck
Unlock for access to all 123 flashcards in this deck.
Unlock Deck
k this deck
47
Which formula represents an inorganic compound?
A)CH3CO2CH2CH3
B)CH3NHCH2CH3
C)ClCCCl
D)CaCl2
A)CH3CO2CH2CH3
B)CH3NHCH2CH3
C)ClCCCl
D)CaCl2

Unlock Deck
Unlock for access to all 123 flashcards in this deck.
Unlock Deck
k this deck
48
The compound below is classified as what type of compound? 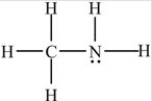
A)Ether
B)Amine
C)Amide
D)Aldehyde

A)Ether
B)Amine
C)Amide
D)Aldehyde

Unlock Deck
Unlock for access to all 123 flashcards in this deck.
Unlock Deck
k this deck
49
The functional group of which type of compound contains a carbonyl group?
A)Amide
B)Alcohol
C)Ether
D)Amine
A)Amide
B)Alcohol
C)Ether
D)Amine

Unlock Deck
Unlock for access to all 123 flashcards in this deck.
Unlock Deck
k this deck
50
How many covalent bonds does nitrogen typically form in organic compounds?
A)1
B)3
C)5
D)8
A)1
B)3
C)5
D)8

Unlock Deck
Unlock for access to all 123 flashcards in this deck.
Unlock Deck
k this deck
51
What is the correct molecular formula for the skeletal structure given below? 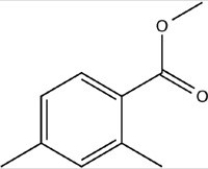
A)C11H16O2
B)C12H12O2
C)C10H12O2
D)C10H15O2

A)C11H16O2
B)C12H12O2
C)C10H12O2
D)C10H15O2

Unlock Deck
Unlock for access to all 123 flashcards in this deck.
Unlock Deck
k this deck
52
Which representation has the bond polarities properly shown?
A)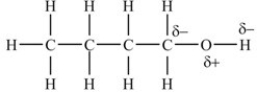
B)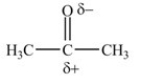
C)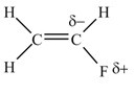
D)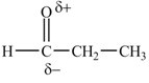
A)

B)

C)

D)


Unlock Deck
Unlock for access to all 123 flashcards in this deck.
Unlock Deck
k this deck
53
Which compound is most flammable?
A)HOCH2CH2OH
B)NaCl
C)CO2
D)HCl
A)HOCH2CH2OH
B)NaCl
C)CO2
D)HCl

Unlock Deck
Unlock for access to all 123 flashcards in this deck.
Unlock Deck
k this deck
54
What are the bond angles in the structure shown below? 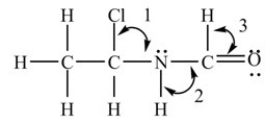
A)Angle 1 = 109.5°,Angle 2 = 109.5°,and Angle 3 = 109.5°
B)Angle 1 = 109.5°,Angle 2 = 120°,and Angle 3 = 120°
C)Angle 1 = 109.5°,Angle 2 = 90°,and Angle 3 = ~109.5°
D)Angle 1 = 109.5°,Angle 2 = ~109.5°,and Angle 3 = 120°

A)Angle 1 = 109.5°,Angle 2 = 109.5°,and Angle 3 = 109.5°
B)Angle 1 = 109.5°,Angle 2 = 120°,and Angle 3 = 120°
C)Angle 1 = 109.5°,Angle 2 = 90°,and Angle 3 = ~109.5°
D)Angle 1 = 109.5°,Angle 2 = ~109.5°,and Angle 3 = 120°

Unlock Deck
Unlock for access to all 123 flashcards in this deck.
Unlock Deck
k this deck
55
Which structure would have the molecular formula C6H10O2?
A)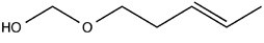
B)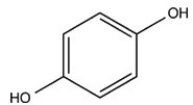
C)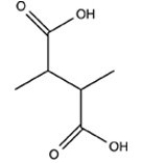
D)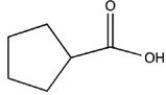
A)

B)

C)

D)


Unlock Deck
Unlock for access to all 123 flashcards in this deck.
Unlock Deck
k this deck
56
Which compound has the highest boiling point?
A)HOCH2CH2OH
B)CH3NHCH2CH3
C)CH3CO2CH2CH3
D)NaCH3COO
A)HOCH2CH2OH
B)CH3NHCH2CH3
C)CH3CO2CH2CH3
D)NaCH3COO

Unlock Deck
Unlock for access to all 123 flashcards in this deck.
Unlock Deck
k this deck
57
Which molecule is a polar molecule?
A)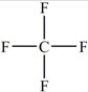
B)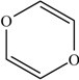
C)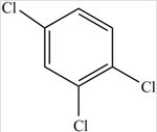
D)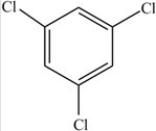
E)More than one of the molecules is a polar molecule.
A)

B)

C)

D)

E)More than one of the molecules is a polar molecule.

Unlock Deck
Unlock for access to all 123 flashcards in this deck.
Unlock Deck
k this deck
58
Three of the four structures below represent unstable organic compounds that are not likely to exist because they violate the octet rule. Which one of the four structures represents a stable organic compound that is likely to exist?
A)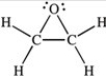
B)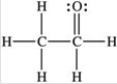
C)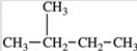
D)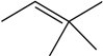
A)

B)

C)

D)


Unlock Deck
Unlock for access to all 123 flashcards in this deck.
Unlock Deck
k this deck
59
Which bonding pattern is NOT typical of carbon atoms in organic compounds?
A)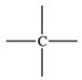
B)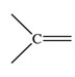
C)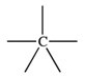
D)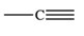
A)

B)

C)

D)
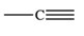

Unlock Deck
Unlock for access to all 123 flashcards in this deck.
Unlock Deck
k this deck
60
Based on the functional group present,the compound below is classified as what type of compound? 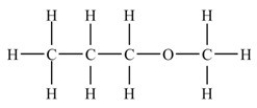
A)Aldehyde
B)Carboxylic acid
C)Ether
D)Ester
E)Ketone

A)Aldehyde
B)Carboxylic acid
C)Ether
D)Ester
E)Ketone

Unlock Deck
Unlock for access to all 123 flashcards in this deck.
Unlock Deck
k this deck
61
The molecule below is an example of an aromatic compound. 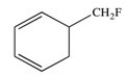


Unlock Deck
Unlock for access to all 123 flashcards in this deck.
Unlock Deck
k this deck
62
MTBE is soluble in both gasoline and in water.

Unlock Deck
Unlock for access to all 123 flashcards in this deck.
Unlock Deck
k this deck
63
Cholesterol is soluble in a nonpolar solvent,such as gasoline.

Unlock Deck
Unlock for access to all 123 flashcards in this deck.
Unlock Deck
k this deck
64
The compound represented by the skeletal structure below contains eleven (11)H atoms and two lone pairs of electrons that are not shown. 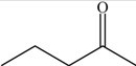


Unlock Deck
Unlock for access to all 123 flashcards in this deck.
Unlock Deck
k this deck
65
The two structures shown below represent the same compound. 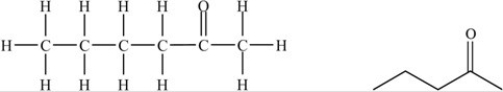


Unlock Deck
Unlock for access to all 123 flashcards in this deck.
Unlock Deck
k this deck
66
The molecule below is an example of a ketone. 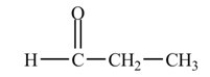
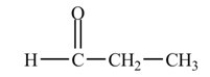

Unlock Deck
Unlock for access to all 123 flashcards in this deck.
Unlock Deck
k this deck
67
The condensed structure and skeletal structure shown below represent the same compound. 


Unlock Deck
Unlock for access to all 123 flashcards in this deck.
Unlock Deck
k this deck
68
The molecule with the condensed formula (CH3)2CHCH2CHO can be represented as the skeletal structure below. 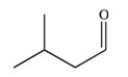


Unlock Deck
Unlock for access to all 123 flashcards in this deck.
Unlock Deck
k this deck
69
Polar organic compounds are water soluble only if they are small and contain a nitrogen or oxygen atom that can hydrogen bond with water.

Unlock Deck
Unlock for access to all 123 flashcards in this deck.
Unlock Deck
k this deck
70
The molecule below is an example of an aromatic compound. 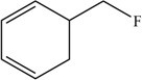


Unlock Deck
Unlock for access to all 123 flashcards in this deck.
Unlock Deck
k this deck
71
Vitamin C is a water-soluble vitamin. 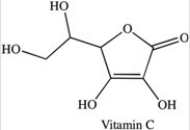


Unlock Deck
Unlock for access to all 123 flashcards in this deck.
Unlock Deck
k this deck
72
VSEPR theory is based on the concept that the most stable arrangement of atoms in a molecule keeps the atoms and lone pair electrons as far away from each other as possible.

Unlock Deck
Unlock for access to all 123 flashcards in this deck.
Unlock Deck
k this deck
73
The two structures shown below represent the same compound.
CH3CH2CH2CH2CO2CH3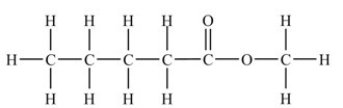
CH3CH2CH2CH2CO2CH3


Unlock Deck
Unlock for access to all 123 flashcards in this deck.
Unlock Deck
k this deck
74
The compound below is a nonpolar molecule. 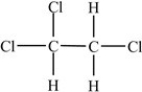


Unlock Deck
Unlock for access to all 123 flashcards in this deck.
Unlock Deck
k this deck
75
Hydrocarbons are nonpolar molecules.

Unlock Deck
Unlock for access to all 123 flashcards in this deck.
Unlock Deck
k this deck
76
The compound whose skeletal structure is shown below is used medicinally to treat fibromyalgia pain. Which statement is NOT a valid interpretation of its skeletal structure? 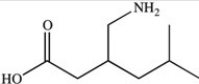
A)The compound contains 8 carbon atoms.
B)The compound contains 17 hydrogen atoms.
C)The compound contains 3 heteroatoms.
D)The compound contains 12 C-H bonds.

A)The compound contains 8 carbon atoms.
B)The compound contains 17 hydrogen atoms.
C)The compound contains 3 heteroatoms.
D)The compound contains 12 C-H bonds.

Unlock Deck
Unlock for access to all 123 flashcards in this deck.
Unlock Deck
k this deck
77
Vitamin B6,shown below,is a fat-soluble vitamin. 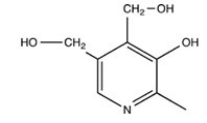


Unlock Deck
Unlock for access to all 123 flashcards in this deck.
Unlock Deck
k this deck
78
Which molecule is more water soluble?
A)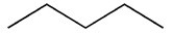
B)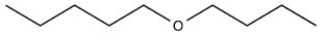
C)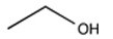
D)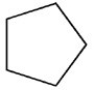
A)

B)

C)

D)


Unlock Deck
Unlock for access to all 123 flashcards in this deck.
Unlock Deck
k this deck
79
Which structure properly indicates the order of attachment of atoms as represented by the condensed structure CH3CHBrCH2CH(CH3)2?
A)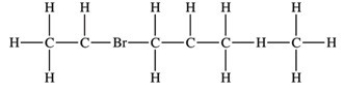
B)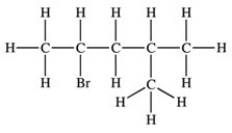
C)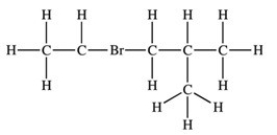
D)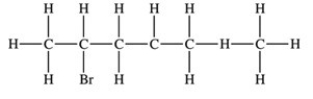
A)

B)

C)

D)


Unlock Deck
Unlock for access to all 123 flashcards in this deck.
Unlock Deck
k this deck
80
All molecules with polar bonds are polar molecules.

Unlock Deck
Unlock for access to all 123 flashcards in this deck.
Unlock Deck
k this deck


