Deck 12: The Carbonyl Containing Functional Groups
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/70
Play
Full screen (f)
Deck 12: The Carbonyl Containing Functional Groups
1
Which of the following molecules is an aldehyde? 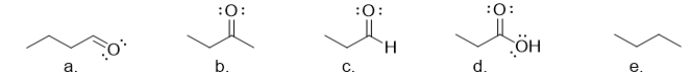
A) molecule a
B) molecule b
C) molecule c
D) molecule d
E) molecule e

A) molecule a
B) molecule b
C) molecule c
D) molecule d
E) molecule e
molecule a
2
Which structure is NOT an amide?
A) CH3CONHCH3
B) CH3CH2NHCHO
C) NH2CH2CHO
D) (CH3)2NOCCH3
E) CH3CH2NHCOCH2CH3
A) CH3CONHCH3
B) CH3CH2NHCHO
C) NH2CH2CHO
D) (CH3)2NOCCH3
E) CH3CH2NHCOCH2CH3
NH2CH2CHO
3
Because L-dopa is metabolized into an active drug in the body, L-dopa is a________.
A) metabolite
B) biomolecule
C) precursor
D) prodrug
E) generic drug
A) metabolite
B) biomolecule
C) precursor
D) prodrug
E) generic drug
prodrug
4
What do the following molecules have in common? 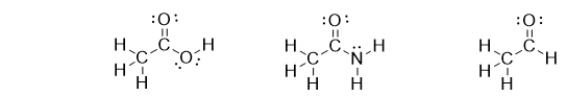
A) an ionic bond
B) They hydrogen bond with like molecules.
C) a carbon with a linear molecular geometry
D) an alcohol
E) a carbonyl-containing functional group

A) an ionic bond
B) They hydrogen bond with like molecules.
C) a carbon with a linear molecular geometry
D) an alcohol
E) a carbonyl-containing functional group

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
5
What is the role of dopamine in the body?
A) It is a neurotransmitter.
B) It is a protein.
C) It is the energy currency of the cell.
D) It is part of our genetic material.
E) It is part of the cell membrane.
A) It is a neurotransmitter.
B) It is a protein.
C) It is the energy currency of the cell.
D) It is part of our genetic material.
E) It is part of the cell membrane.

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
6
What is the name of the parent chain of the following molecule? 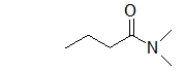
A) ethylamine
B) dimethylamine
C) propylamide
D) propylamine
E) butylamide

A) ethylamine
B) dimethylamine
C) propylamide
D) propylamine
E) butylamide

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
7
Does isopropyl alcohol have a higher or lower boiling point than acetone? 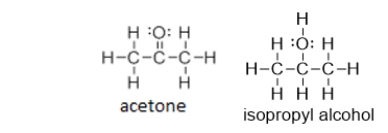
A) Higher, because isopropyl alcohol has hydrogen bonding.
B) Lower, because isopropyl alcohol has hydrogen bonding.
C) Higher, because isopropyl alcohol can form dispersion forces.
D) Lower, because isopropyl alcohol can form dispersion forces.
E) It is not possible to determine the answer based on the information provided.

A) Higher, because isopropyl alcohol has hydrogen bonding.
B) Lower, because isopropyl alcohol has hydrogen bonding.
C) Higher, because isopropyl alcohol can form dispersion forces.
D) Lower, because isopropyl alcohol can form dispersion forces.
E) It is not possible to determine the answer based on the information provided.

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
8
Below is a ball-and-stick model of acetone.Which of the following Lewis structures is acetone? 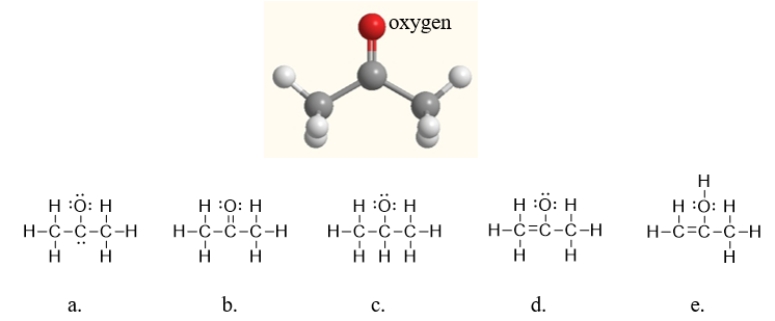
A) structure a
B) structure b
C) structure c
D) structure d
E) structure e

A) structure a
B) structure b
C) structure c
D) structure d
E) structure e

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
9
Below are several different molecules.Which ones contain a ketone? 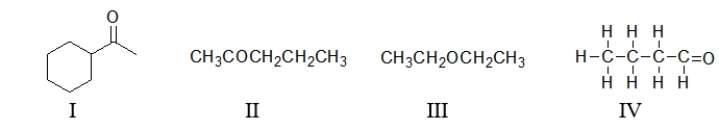
A) All of these molecules contain a ketone.
B) All of these molecules except III contain a ketone.
C) Only I and II are ketones.
D) Only I and IV are ketones.
E) Only I is a ketone.

A) All of these molecules contain a ketone.
B) All of these molecules except III contain a ketone.
C) Only I and II are ketones.
D) Only I and IV are ketones.
E) Only I is a ketone.

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
10
Which molecule is N-ethylpropanamide?
A) CH3CH2CH2NHCH2CH3
B) CH3CH2CONHCH2CH3
C) CH3CONHCH2CH2CH3
D) CH3CH2CH2CONHCH2CH3
E) CH3CH2CNH2CH2CH3
A) CH3CH2CH2NHCH2CH3
B) CH3CH2CONHCH2CH3
C) CH3CONHCH2CH2CH3
D) CH3CH2CH2CONHCH2CH3
E) CH3CH2CNH2CH2CH3

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
11
Provide the IUPAC name for the following carboxylic acid. 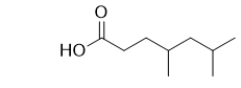
A) 4,6-dimethylheptanoic acid
B) 2,4-dimethyl-7-heptanoic acid
C) 4,6-dimethylheptanal
D) 2,4-dimethyl-7-heptanal
E) 2,4-dimethyl-7-heptanol

A) 4,6-dimethylheptanoic acid
B) 2,4-dimethyl-7-heptanoic acid
C) 4,6-dimethylheptanal
D) 2,4-dimethyl-7-heptanal
E) 2,4-dimethyl-7-heptanol

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
12
What is the IUPAC name of the following molecule? 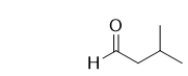
A) 2-methylbutanal
B) 2-carbonylpropane
C) 2-pentanal
D) 3-methylbutanone
E) 3-methylbutanal

A) 2-methylbutanal
B) 2-carbonylpropane
C) 2-pentanal
D) 3-methylbutanone
E) 3-methylbutanal

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
13
How many amides does the following peptide (a sequence of several amino acids)contain? 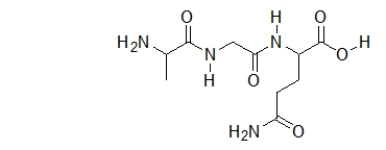
A) 0
B) 1
C) 2
D) 3
E) 4

A) 0
B) 1
C) 2
D) 3
E) 4

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
14
What is the name of the carbonyl-containing compound that has the following general structure: RCO2R?
A) aldehyde
B) amide
C) ether
D) ester
E) carboxylic acid
A) aldehyde
B) amide
C) ether
D) ester
E) carboxylic acid

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
15
Which functional group does NOT contain a carbonyl group?
A) amide
B) amine
C) ketone
D) carboxylic acid
E) ester
A) amide
B) amine
C) ketone
D) carboxylic acid
E) ester

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
16
Anandamide is a neurotransmitter that is involved in controlling mood and appetite.Which choice BEST describes the functional groups found in this molecule? 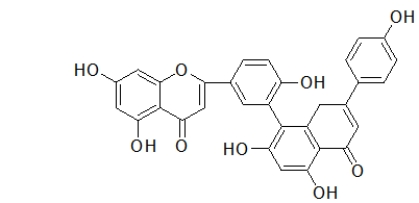
A) alcohols, esters, alkenes, and ketones
B) alcohols, ketones, and alkenes
C) alcohols, phenols, and ketones
D) phenols, ethers, alkenes, esters, and ketones
E) phenols, ethers, alkenes, and ketones

A) alcohols, esters, alkenes, and ketones
B) alcohols, ketones, and alkenes
C) alcohols, phenols, and ketones
D) phenols, ethers, alkenes, esters, and ketones
E) phenols, ethers, alkenes, and ketones

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
17
Which biomolecule contains three ester functional groups?
A)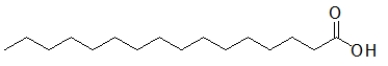
B)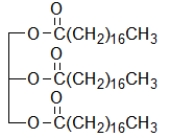
C)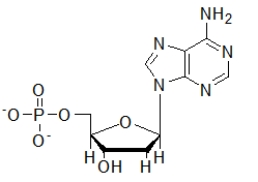
D)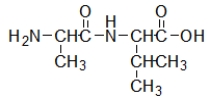
E)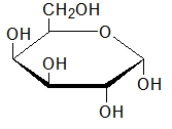
A)

B)
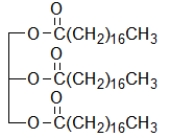
C)

D)

E)


Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
18
Which statement BEST describes the role of a neurotransmitter in the body?
A) Neurotransmitters facilitate the transfer of a signal from one neuron to the next, across the synaptic cleft.
B) Neurotransmitters facilitate the passage of an electrical impulse through a neuron.
C) Neurotransmitters facilitate the passage of an electrical impulse through an axon.
D) Neurotransmitters initiate an electrical response to a stimulus.
E) Neurotransmitters block all electrical impulses through neurons.
A) Neurotransmitters facilitate the transfer of a signal from one neuron to the next, across the synaptic cleft.
B) Neurotransmitters facilitate the passage of an electrical impulse through a neuron.
C) Neurotransmitters facilitate the passage of an electrical impulse through an axon.
D) Neurotransmitters initiate an electrical response to a stimulus.
E) Neurotransmitters block all electrical impulses through neurons.

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
19
A common misconception is that the following molecule contains two functional groups, an alcohol and a ketone, but this is not the case.What functional group does this molecule actually contain, and why is it important to differentiate between this functional group and an alcohol? 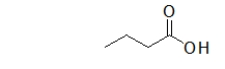
A) This molecule contains a carboxylic acid, which reacts very differently than an alcohol.
B) This molecule contains a carboxylic acid, which is an isomer of an alcohol.
C) This molecule contains a carbonyl, which hydrogen bonds much more than an alcohol.
D) This molecule contains a carbonyl, which reacts very differently than an alcohol.
E) This molecule contains a carboxylic acid, which is unreactive.

A) This molecule contains a carboxylic acid, which reacts very differently than an alcohol.
B) This molecule contains a carboxylic acid, which is an isomer of an alcohol.
C) This molecule contains a carbonyl, which hydrogen bonds much more than an alcohol.
D) This molecule contains a carbonyl, which reacts very differently than an alcohol.
E) This molecule contains a carboxylic acid, which is unreactive.

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
20
Which statement about amides is FALSE?
A) The C-N bond has some double bond character.
B) Amides are found in proteins.
C) Amides act as organic bases.
D) Amides do not form ions at physiological pH.
E) Amides are polar functional groups.
A) The C-N bond has some double bond character.
B) Amides are found in proteins.
C) Amides act as organic bases.
D) Amides do not form ions at physiological pH.
E) Amides are polar functional groups.

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
21
Isopropyl acetate is used to flavor banana candy.What functional group(s)is/are in a molecule of isopropyl acetate? 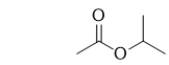
A) ketone and ether
B) aldehyde and alcohol
C) ketone and alcohol
D) carboxylic acid
E) ester

A) ketone and ether
B) aldehyde and alcohol
C) ketone and alcohol
D) carboxylic acid
E) ester

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
22
Soap has a polar head and nonpolar tail.Thus, soap is ________.
A) ambivalent
B) ambidextrous
C) amphoteric
D) amphibious
E) ambiguous
A) ambivalent
B) ambidextrous
C) amphoteric
D) amphibious
E) ambiguous

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
23
Which of the following molecules contains an aldehyde?
A) propanal
B) propanol
C) propanone
D) propanoic acid
E) propanamide
A) propanal
B) propanol
C) propanone
D) propanoic acid
E) propanamide

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
24
What functional groups are in aspartame, an artificial sweetener? 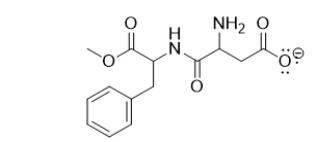
A) ether, three alkenes, ketone, amine, amide
B) ester, aromatic ring, amide, amine, carboxylate
C) ether, aromatic ring, two amines, two ketones, alcohol
D) ester, two amines, two ketones, carboxylate
E) ether, three alkenes, two amine, two ketones, alcohol

A) ether, three alkenes, ketone, amine, amide
B) ester, aromatic ring, amide, amine, carboxylate
C) ether, aromatic ring, two amines, two ketones, alcohol
D) ester, two amines, two ketones, carboxylate
E) ether, three alkenes, two amine, two ketones, alcohol

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
25
Tetracycline is a common antibiotic.Select the choice in which the two boxed functional groups are correctly identified. 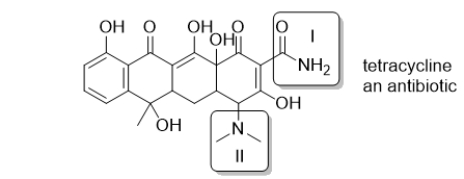
A) I: amine; II: amine
B) I: amide; II: amide
C) I: amine; II: amide
D) I: amide; II: amine
E) These functional groups are neither amines nor amides.

A) I: amine; II: amine
B) I: amide; II: amide
C) I: amine; II: amide
D) I: amide; II: amine
E) These functional groups are neither amines nor amides.

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
26
What is the name of the following molecule? 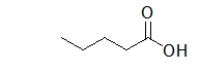
A) butanoic acid
B) 1-hydroxypentanal
C) 1-hydroxypentanone
D) pentanoic acid
E) 5-pentanoic acid

A) butanoic acid
B) 1-hydroxypentanal
C) 1-hydroxypentanone
D) pentanoic acid
E) 5-pentanoic acid

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
27
Which molecule contains an amide?
A)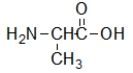
B)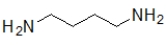
C)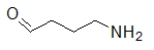
D)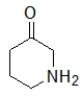
E)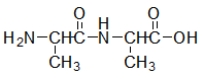
A)

B)

C)

D)

E)

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
28
What is the IUPAC name of the parent chain of the following molecule? 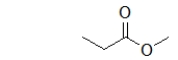
A) propanoate
B) ethanoate
C) pentanoate
D) methanoate
E) butanoate

A) propanoate
B) ethanoate
C) pentanoate
D) methanoate
E) butanoate

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
29
Vanillin is the molecule that gives vanilla extract its characteristic odor and flavor.Which functional group in vanillin is an aldehyde? 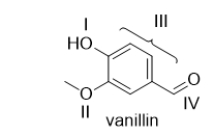
A) I
B) II
C) III
D) IV
E) Vanillin does not contain an aldehyde.

A) I
B) II
C) III
D) IV
E) Vanillin does not contain an aldehyde.

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
30
Which molecules contain a carboxylic acid?
A) CH3COOH
B)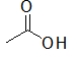
C) CH3CO2H
D)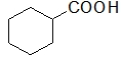
E) All of the above contain a carboxylic acid.
A) CH3COOH
B)

C) CH3CO2H
D)

E) All of the above contain a carboxylic acid.

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
31
Which compound is the MOST soluble in water?
A)
B)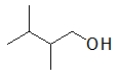
C)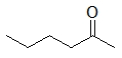
D)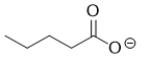
E)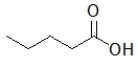
A)

B)

C)

D)

E)


Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
32
Which statement about carboxylic acids is FALSE?
A) Carboxylic acids are weak acids.
B) Carboxylic acids have the same properties as an alcohol and ketone.
C) Carboxylic acids hydrogen bond.
D) Carboxylic acids form carboxylates at physiological pH.
E) Carboxylic acids form dimers.
A) Carboxylic acids are weak acids.
B) Carboxylic acids have the same properties as an alcohol and ketone.
C) Carboxylic acids hydrogen bond.
D) Carboxylic acids form carboxylates at physiological pH.
E) Carboxylic acids form dimers.

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
33
The simplest aldehyde is formaldehyde, which is used as a preservative.Which of the following molecules is formaldehyde?
A) HCHO
B) CH3OH
C) CH3COCH3
D) CH3CH2OHCH3
E) CH3CHO
A) HCHO
B) CH3OH
C) CH3COCH3
D) CH3CH2OHCH3
E) CH3CHO

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
34
Do you expect a soap molecule to be more or less soluble than a carboxylic acid with the same number of carbons shown below? 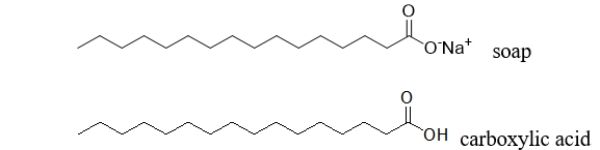
A) less soluble because the soap has a higher molecular weight
B) more soluble because the soap has a higher molecular weight
C) less soluble because the soap is more polar
D) less soluble because the soap is less polar
E) more soluble because the soap is negatively charged

A) less soluble because the soap has a higher molecular weight
B) more soluble because the soap has a higher molecular weight
C) less soluble because the soap is more polar
D) less soluble because the soap is less polar
E) more soluble because the soap is negatively charged

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
35
Which compound has the highest boiling point? 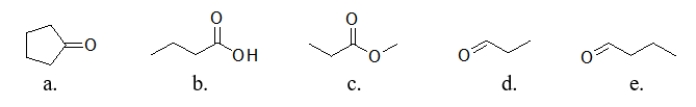
A) compound a
B) compound b
C) compound c
D) compound d
E) compound e

A) compound a
B) compound b
C) compound c
D) compound d
E) compound e

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
36
At physiological pH, what is the structure of stearic acid, a fatty acid? 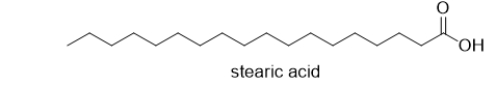
A)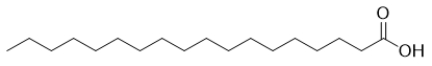
B)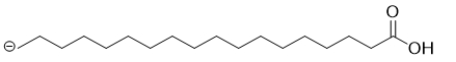
C)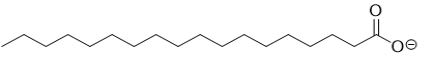
D)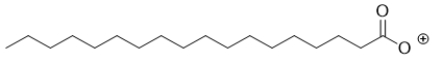
E)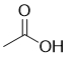

A)

B)

C)

D)

E)


Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
37
Which BEST describes the functional group(s)in this molecule? 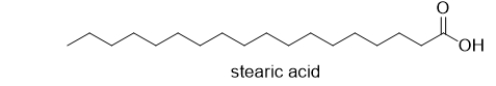
A) a carboxylic acid
B) a carboxylate
C) an alcohol and a ketone
D) an alcohol and an aldehyde
E) an alcohol, a sodium, and a carbonyl

A) a carboxylic acid
B) a carboxylate
C) an alcohol and a ketone
D) an alcohol and an aldehyde
E) an alcohol, a sodium, and a carbonyl

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
38
An aldehyde, ketone, and carboxylic acid have what in common?
A) a carbonyl group
B) a polar covalent bond
C) a permanent dipole
D) two single bonds to the carbon of the carbonyl
E) All of the above are correct.
A) a carbonyl group
B) a polar covalent bond
C) a permanent dipole
D) two single bonds to the carbon of the carbonyl
E) All of the above are correct.

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
39
Which molecule is acetic acid?
A) CH3COOH
B) CH3COO-
C) CH3CH2COOH
D) CH3CH2COO-
E) CH3CH2CH2COOH
A) CH3COOH
B) CH3COO-
C) CH3CH2COOH
D) CH3CH2COO-
E) CH3CH2CH2COOH

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
40
What is the strongest intermolecular force of attraction that esters form with like molecules?
A) ionic bonds
B) polar covalent bonds
C) dipole-dipole interactions
D) hydrogen bonding
E) dispersion forces
A) ionic bonds
B) polar covalent bonds
C) dipole-dipole interactions
D) hydrogen bonding
E) dispersion forces

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
41
What is the strongest intermolecular force of attraction that forms between molecules of butanone? 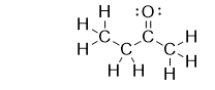
A) dipole-dipole interactions
B) dispersion forces
C) ionic bonds
D) hydrogen bonding
E) polar covalent bonds

A) dipole-dipole interactions
B) dispersion forces
C) ionic bonds
D) hydrogen bonding
E) polar covalent bonds

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
42
What type of carbonyl-containing compound has the following general structure? 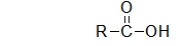
A) aldehyde
B) amide
C) ketone and alcohol
D) ester
E) carboxylic acid
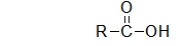
A) aldehyde
B) amide
C) ketone and alcohol
D) ester
E) carboxylic acid

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
43
Which molecule is cyclohexanone? 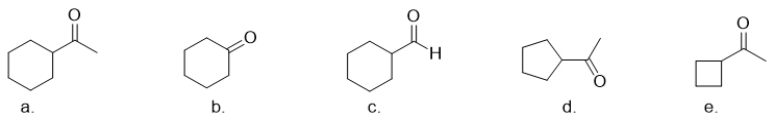
A) molecule a
B) molecule b
C) molecule c
D) molecule d
E) molecule e

A) molecule a
B) molecule b
C) molecule c
D) molecule d
E) molecule e

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
44
The following statements refer to the differences and similarities between an amide and an amine.Which statement is FALSE?
A) An amide has a carbonyl next to the nitrogen, and an amine does not.
B) An amide forms an ion, whereas an amine does not.
C) Both an amide and an amine contain a nitrogen.
D) Amides and amines have different chemical properties.
E) The nitrogen in both amines and amides can be attached to one, two, or three carbons.
A) An amide has a carbonyl next to the nitrogen, and an amine does not.
B) An amide forms an ion, whereas an amine does not.
C) Both an amide and an amine contain a nitrogen.
D) Amides and amines have different chemical properties.
E) The nitrogen in both amines and amides can be attached to one, two, or three carbons.

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
45
Which functional groups are in the male sex hormone, testosterone? 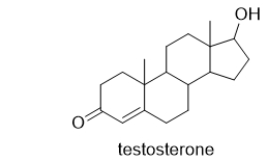
A) alkene, aldehyde, alcohol
B) alkyne, aldehyde, alcohol
C) alkene, ketone, aldehyde
D) alkene, ketone, alcohol
E) alkyne, ketone, aldehyde

A) alkene, aldehyde, alcohol
B) alkyne, aldehyde, alcohol
C) alkene, ketone, aldehyde
D) alkene, ketone, alcohol
E) alkyne, ketone, aldehyde

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
46
D-Fructose is a simple sugar.What is the functional group indicated by the arrow? 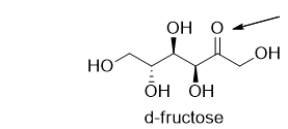
A) ketone
B) carboxylic acid
C) alcohol
D) aldehyde
E) polyol

A) ketone
B) carboxylic acid
C) alcohol
D) aldehyde
E) polyol

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
47
Which molecule is ethyl acetate?
A) CH3COOCH2CH3
B) CH3CH2COOCH3
C) HCOOCH2CH3
D) CH3COCH2CH3
E) CH3CH2COCH3
A) CH3COOCH2CH3
B) CH3CH2COOCH3
C) HCOOCH2CH3
D) CH3COCH2CH3
E) CH3CH2COCH3

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
48
Which of the following compounds is the most soluble in water? 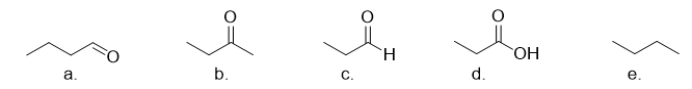
A) compound a
B) compound b
C) compound c
D) compound d
E) compound e

A) compound a
B) compound b
C) compound c
D) compound d
E) compound e

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
49
Which of the following molecules contains an aldehyde?
A) CH3CH2OH
B) CH3COCH3
C) CH3CHO
D) CH3OCH3
E) All of the above molecules contain aldehydes.
A) CH3CH2OH
B) CH3COCH3
C) CH3CHO
D) CH3OCH3
E) All of the above molecules contain aldehydes.

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
50
What is the name of this ester? 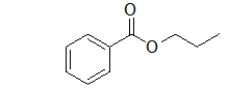
A) benzyl propanoate
B) phenol propanoate
C) propyl benzoate
D) propyl phenoate
E) benzene propyl ester

A) benzyl propanoate
B) phenol propanoate
C) propyl benzoate
D) propyl phenoate
E) benzene propyl ester

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
51
In order for many drugs to be active, they must fit into cell receptors.In order for the drug to fit into the cell receptor, the drug must
A) be a complementary shape to the receptor.
B) be able to form intermolecular forces with the receptor.
C) have functional groups in the correct position.
D) have the correct polarity.
E) All of the above are correct.
A) be a complementary shape to the receptor.
B) be able to form intermolecular forces with the receptor.
C) have functional groups in the correct position.
D) have the correct polarity.
E) All of the above are correct.

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
52
What is the name of the following molecule? 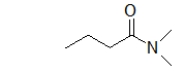
A) N-methylbutanamide
B) N-methylhexanamide
C) N-dimethylbutanamide
D) N,N-dimethylbutanamide
E) N,N-dimethylhexanamide

A) N-methylbutanamide
B) N-methylhexanamide
C) N-dimethylbutanamide
D) N,N-dimethylbutanamide
E) N,N-dimethylhexanamide

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
53
Which functional groups are in the following molecule of aspirin? 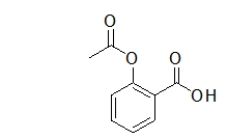
A) a benzene ring, an ester, a ketone, and an alcohol
B) a benzene ring, two ketones, an ether, and an alcohol
C) a benzene ring, a carboxylic acid, an ether, and a ketone
D) a benzene ring, a carboxylic acid, and an ester
E) a phenol, two ketones, and an alcohol

A) a benzene ring, an ester, a ketone, and an alcohol
B) a benzene ring, two ketones, an ether, and an alcohol
C) a benzene ring, a carboxylic acid, an ether, and a ketone
D) a benzene ring, a carboxylic acid, and an ester
E) a phenol, two ketones, and an alcohol

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
54
What is the name of the following ester? 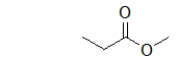
A) ethyl acetate
B) methyl propanoate
C) propyl acetate
D) propyl ethanoate
E) ethyl propanoate

A) ethyl acetate
B) methyl propanoate
C) propyl acetate
D) propyl ethanoate
E) ethyl propanoate

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
55
Which of the following molecules contains at least one ketone?
A) testosterone
B) acetone
C) hydrocodone
D) prednisone
E) All of the above are examples
A) testosterone
B) acetone
C) hydrocodone
D) prednisone
E) All of the above are examples

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
56
Methadone, a drug used to aid in the withdrawal from heroin, contains several functional groups.Which functional group is NOT found in methadone? 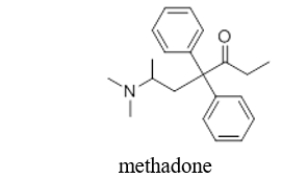
A) two benzene rings
B) a tertiary amine
C) a ketone
D) an aldehyde
E) Methadone does not contain any functional groups.

A) two benzene rings
B) a tertiary amine
C) a ketone
D) an aldehyde
E) Methadone does not contain any functional groups.

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
57
The portion of the molecule labeled I is 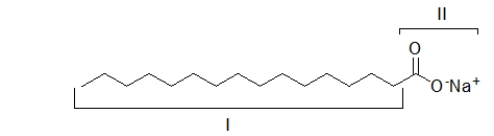
A) hydrophobic.It dissolves grease.
B) hydrophilic.It dissolves grease.
C) hydrophobic.It dissolves in water.
D) hydrophilic.It dissolves in water.
E) both hydrophobic and hydrophilic.It dissolves in both water and grease.

A) hydrophobic.It dissolves grease.
B) hydrophilic.It dissolves grease.
C) hydrophobic.It dissolves in water.
D) hydrophilic.It dissolves in water.
E) both hydrophobic and hydrophilic.It dissolves in both water and grease.

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
58
What type of biomolecule is shown below? 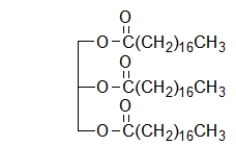
A) a fat
B) a nucleic acid
C) an amino acid
D) a steroid
E) a sugar

A) a fat
B) a nucleic acid
C) an amino acid
D) a steroid
E) a sugar

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
59
Which of the following molecules has the highest boiling point?
A)
B)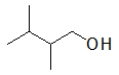
C)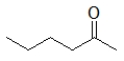
D)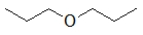
E)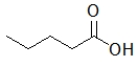
A)

B)

C)

D)

E)


Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
60
What type of biomolecule is shown below? 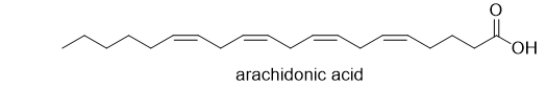
A) a polyunsaturated fatty acid
B) a saturated fatty acid
C) a nucleic acid
D) an amino acid
E) a sugar

A) a polyunsaturated fatty acid
B) a saturated fatty acid
C) a nucleic acid
D) an amino acid
E) a sugar

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
61
What is the name of the following molecule? 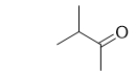
A) pentan-2-one
B) 2-methylbutan-3-al
C) 2-methylbutan-3-one
D) 3-methylbutan-2-al
E) 3-methylbutan-2-one

A) pentan-2-one
B) 2-methylbutan-3-al
C) 2-methylbutan-3-one
D) 3-methylbutan-2-al
E) 3-methylbutan-2-one

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
62
Which condensed structure is the same molecule as the skeletal line structure shown below? 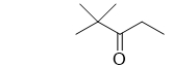
A) (CH3)3CCOCH2CH3
B) (CH3)3COCH2CH3
C) (CH3)3CCH2OCH2CH3
D) (CH3)3CCH2COCH3
E) (CH3)3CCH2OCH3

A) (CH3)3CCOCH2CH3
B) (CH3)3COCH2CH3
C) (CH3)3CCH2OCH2CH3
D) (CH3)3CCH2COCH3
E) (CH3)3CCH2OCH3

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
63
Which BEST describes the functional groups in this molecule of soap? 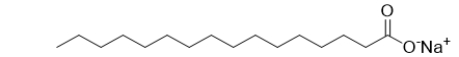
A) a carboxylic acid
B) a carboxylate group
C) an alcohol and a ketone
D) an alcohol and an aldehyde
E) an alcohol, a sodium, and a carbonyl

A) a carboxylic acid
B) a carboxylate group
C) an alcohol and a ketone
D) an alcohol and an aldehyde
E) an alcohol, a sodium, and a carbonyl

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
64
Glucose, the most common sugar, contains which of the following carbonyl-containing functional groups? 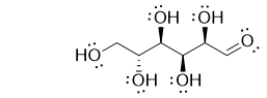
A) carboxylic acid
B) amide
C) aldehyde
D) ketone
E) ester

A) carboxylic acid
B) amide
C) aldehyde
D) ketone
E) ester

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
65
Which molecules contain a carboxylic acid?
A) HOCH2CHO
B) CH3CHOHCH2COCH3
C) CH3CO2H
D) CHOOCH3
E) All of the above contain a carboxylic acid.
A) HOCH2CHO
B) CH3CHOHCH2COCH3
C) CH3CO2H
D) CHOOCH3
E) All of the above contain a carboxylic acid.

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
66
What is the product when a carboxylic acid donates a proton ion (H+)to water? 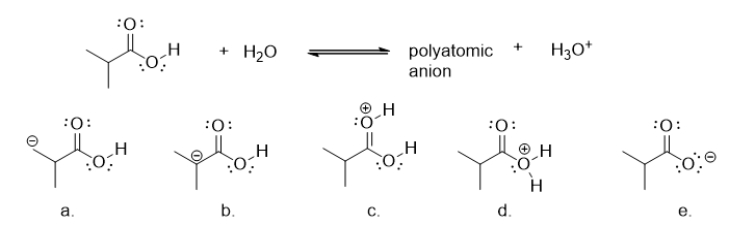
A) molecule a
B) molecule b
C) molecule c
D) molecule d
E) molecule e

A) molecule a
B) molecule b
C) molecule c
D) molecule d
E) molecule e

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
67
Which of the following compounds has the highest boiling point? 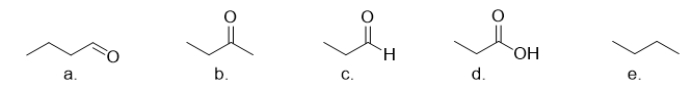
A) compound a
B) compound b
C) compound c
D) compound d
E) compound e

A) compound a
B) compound b
C) compound c
D) compound d
E) compound e

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
68
At physiological pH, which statement about the following reaction is TRUE? 
A) Equilibrium favors the reactants.
B) The reaction does not occur at all.
C) Equilibrium favors the products.
D) Different products form.
E) The carboxylic acid acts as a base.

A) Equilibrium favors the reactants.
B) The reaction does not occur at all.
C) Equilibrium favors the products.
D) Different products form.
E) The carboxylic acid acts as a base.

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
69
Which molecule is butanal? 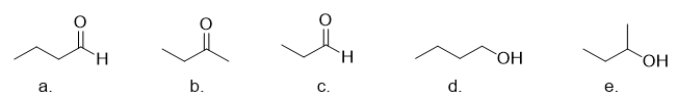
A) molecule a
B) molecule b
C) molecule c
D) molecule d
E) molecule e

A) molecule a
B) molecule b
C) molecule c
D) molecule d
E) molecule e

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
70
Which molecule is ethyl butanoate?
A)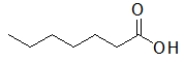
B)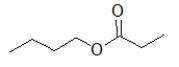
C)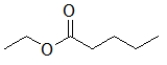
D)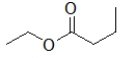
E)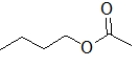
A)

B)

C)

D)

E)


Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck


