Deck 10: Introduction to Organic Chemistry:
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/111
Play
Full screen (f)
Deck 10: Introduction to Organic Chemistry:
1
A molecule has the following IUPAC name: 1-hexyne.What does this name mean?
A) The molecule is an unbranched six-carbon alkane.
B) The molecule is a branched six-carbon alkane.
C) The molecule is an unbranched six-carbon alkene.
D) The molecule is a branched six-carbon alkyne.
E) The molecule is an unbranched six-carbon alkyne.
A) The molecule is an unbranched six-carbon alkane.
B) The molecule is a branched six-carbon alkane.
C) The molecule is an unbranched six-carbon alkene.
D) The molecule is a branched six-carbon alkyne.
E) The molecule is an unbranched six-carbon alkyne.
The molecule is an unbranched six-carbon alkyne.
2
Benzene is not classified as an alkene.Why?
A) It does not have 120° bond angles.
B) It actually contains three double bonds, not just one.
C) It is flat.
D) It is more stable and unreactive than an alkene.
E) Actually, benzene is classified as an alkene.
A) It does not have 120° bond angles.
B) It actually contains three double bonds, not just one.
C) It is flat.
D) It is more stable and unreactive than an alkene.
E) Actually, benzene is classified as an alkene.
It is more stable and unreactive than an alkene.
3
Which pair(s)of molecules below are neither isomers nor conformers? 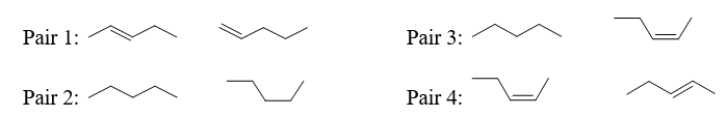
A) pair 1
B) pair 2
C) pair 3
D) pair 4
E) These are all either isomers or conformers.
A) pair 1
B) pair 2
C) pair 3
D) pair 4
E) These are all either isomers or conformers.
pair 3
4
Which skeletal line structure BEST represents the Lewis structure below? 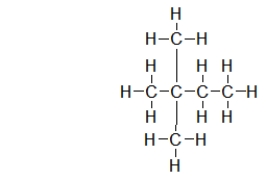
A)
B)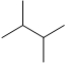
C)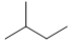
D)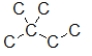
E)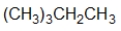

A)

B)

C)

D)
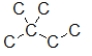
E)
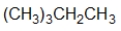

Unlock Deck
Unlock for access to all 111 flashcards in this deck.
Unlock Deck
k this deck
5
Which food should be avoided in order to limit the consumption of saturated fat?
A) whole milk
B) cheese
C) ice cream
D) red meat
E) All of the above should be avoided.
A) whole milk
B) cheese
C) ice cream
D) red meat
E) All of the above should be avoided.

Unlock Deck
Unlock for access to all 111 flashcards in this deck.
Unlock Deck
k this deck
6
Which structure is the BEST condensed structural formula for the Lewis structure given below? 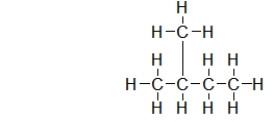
A) CH3CH3CHCH2CH3
B) C-C-C-C-C
C)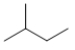
D)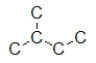
E)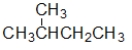

A) CH3CH3CHCH2CH3
B) C-C-C-C-C
C)

D)
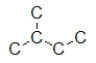
E)
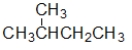

Unlock Deck
Unlock for access to all 111 flashcards in this deck.
Unlock Deck
k this deck
7
A cyclohexane molecule and a benzene molecule are illustrated below.Which statement describing the relationship between benzene and cyclohexane is true? 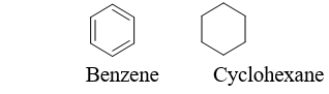 I.Benzene and cyclohexane have different molecular formulas. II.Benzene and cyclohexane are both flat.
I.Benzene and cyclohexane have different molecular formulas. II.Benzene and cyclohexane are both flat.
III)Benzene and cyclohexane have different C-C bond lengths.
IV)Benzene is more flexible than cyclohexane
A) Only I is true.
B) I and III are true.
C) I, II, and III are true.
D) I and IV are true.
E) All of the statements are true.
 I.Benzene and cyclohexane have different molecular formulas. II.Benzene and cyclohexane are both flat.
I.Benzene and cyclohexane have different molecular formulas. II.Benzene and cyclohexane are both flat.III)Benzene and cyclohexane have different C-C bond lengths.
IV)Benzene is more flexible than cyclohexane
A) Only I is true.
B) I and III are true.
C) I, II, and III are true.
D) I and IV are true.
E) All of the statements are true.

Unlock Deck
Unlock for access to all 111 flashcards in this deck.
Unlock Deck
k this deck
8
Organic chemistry is the study of compounds that
A) are biologically important.
B) contain oxygen.
C) make up living things.
D) are not harmful to the environment.
E) contain carbon.
A) are biologically important.
B) contain oxygen.
C) make up living things.
D) are not harmful to the environment.
E) contain carbon.

Unlock Deck
Unlock for access to all 111 flashcards in this deck.
Unlock Deck
k this deck
9
Which pair of compounds are geometric isomers?
A)
B) HC≡CCH3 CH3C≡CH
C)
D)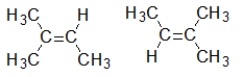
E)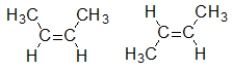
A)

B) HC≡CCH3 CH3C≡CH
C)

D)
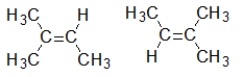
E)
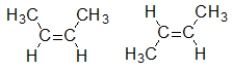

Unlock Deck
Unlock for access to all 111 flashcards in this deck.
Unlock Deck
k this deck
10
Which terms from this list BEST describe the following compound? I.Alkane
II.Alkene
III.Alkyne
IV.Aromatic
V.Saturated hydrocarbon
VI.Unsaturated hydrocarbon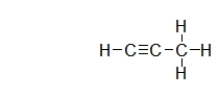
A) I and V
B) II and VI
C) III and VI
D) II and V
E) III and V
II.Alkene
III.Alkyne
IV.Aromatic
V.Saturated hydrocarbon
VI.Unsaturated hydrocarbon
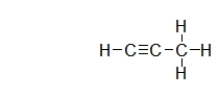
A) I and V
B) II and VI
C) III and VI
D) II and V
E) III and V

Unlock Deck
Unlock for access to all 111 flashcards in this deck.
Unlock Deck
k this deck
11
Which is a saturated hydrocarbon?
A) C3H6
B) C6H6
C) C4H6
D) C4H10
E) C2H4
A) C3H6
B) C6H6
C) C4H6
D) C4H10
E) C2H4

Unlock Deck
Unlock for access to all 111 flashcards in this deck.
Unlock Deck
k this deck
12
Which compound has the lowest boiling point?
A) CH4
B) H2O
C) NH3
D) CH2O
E) CH3OH
A) CH4
B) H2O
C) NH3
D) CH2O
E) CH3OH

Unlock Deck
Unlock for access to all 111 flashcards in this deck.
Unlock Deck
k this deck
13
Which of the following compounds is a valid Lewis structure of a hydrocarbon? 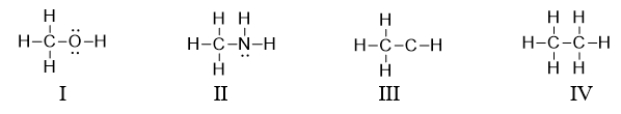
A) All of these compounds are valid structures of hydrocarbons.
B) I, II, and IV are valid structures of hydrocarbons.
C) III and IV are valid structures of hydrocarbons.
D) Only III is a valid structure of hydrocarbons.
E) Only IV is a valid structure of hydrocarbons.
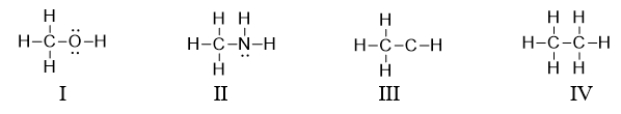
A) All of these compounds are valid structures of hydrocarbons.
B) I, II, and IV are valid structures of hydrocarbons.
C) III and IV are valid structures of hydrocarbons.
D) Only III is a valid structure of hydrocarbons.
E) Only IV is a valid structure of hydrocarbons.

Unlock Deck
Unlock for access to all 111 flashcards in this deck.
Unlock Deck
k this deck
14
What is the IUPAC name of this hydrocarbon? 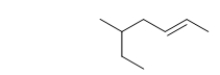
A) trans-5-methyl-2-heptene
B) cis-5-ethyl-2-hexene
C) trans-3-methyl-5-heptene
D) cis-2-ethyl-5-hexene
E) trans-1-ethyl-1-methyl-2-butene

A) trans-5-methyl-2-heptene
B) cis-5-ethyl-2-hexene
C) trans-3-methyl-5-heptene
D) cis-2-ethyl-5-hexene
E) trans-1-ethyl-1-methyl-2-butene

Unlock Deck
Unlock for access to all 111 flashcards in this deck.
Unlock Deck
k this deck
15
What is the IUPAC name of the following branched-chain hydrocarbon? 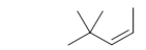
A) trans-4-methyl-4-methyl-2-pentene
B) cis-2-methyl-2-methyl-3-pentene
C) cis-4,4-dimethyl-2-pentene
D) trans-2,2-dimethyl-3-pentene
E) cis-4,4,4-trimethyl-2-propene

A) trans-4-methyl-4-methyl-2-pentene
B) cis-2-methyl-2-methyl-3-pentene
C) cis-4,4-dimethyl-2-pentene
D) trans-2,2-dimethyl-3-pentene
E) cis-4,4,4-trimethyl-2-propene

Unlock Deck
Unlock for access to all 111 flashcards in this deck.
Unlock Deck
k this deck
16
What is the name of the following cycloalkane? 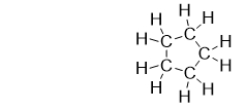
A) pentane
B) cyclopentane
C) butane
D) cyclobutane
E) cycloalkane

A) pentane
B) cyclopentane
C) butane
D) cyclobutane
E) cycloalkane

Unlock Deck
Unlock for access to all 111 flashcards in this deck.
Unlock Deck
k this deck
17
Why do saturated fats have higher melting points than unsaturated fats?
A) Saturated fats stack better than unsaturated fats and therefore have more dispersion forces between them.
B) Saturated fats stack better than unsaturated fats and therefore have fewer dispersion forces between them.
C) Unsaturated fats stack better than saturated fats and therefore have more dispersion forces between them.
D) Unsaturated fats stack better than saturated fats and therefore have fewer dispersion forces between them.
E) Actually, saturated fats have lower melting points than unsaturated fats.
A) Saturated fats stack better than unsaturated fats and therefore have more dispersion forces between them.
B) Saturated fats stack better than unsaturated fats and therefore have fewer dispersion forces between them.
C) Unsaturated fats stack better than saturated fats and therefore have more dispersion forces between them.
D) Unsaturated fats stack better than saturated fats and therefore have fewer dispersion forces between them.
E) Actually, saturated fats have lower melting points than unsaturated fats.

Unlock Deck
Unlock for access to all 111 flashcards in this deck.
Unlock Deck
k this deck
18
Which of the following structure(s)is represented by the following IUPAC name 4-ethyl-2-methylheptane? 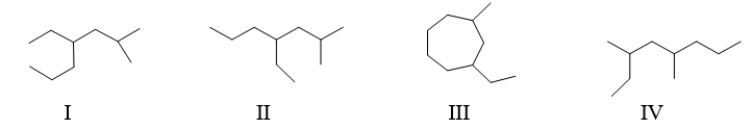
A) II only
B) I and II
C) I, II, and IV
D) II and III
E) All of these are 4-ethyl-2-methylheptane.

A) II only
B) I and II
C) I, II, and IV
D) II and III
E) All of these are 4-ethyl-2-methylheptane.

Unlock Deck
Unlock for access to all 111 flashcards in this deck.
Unlock Deck
k this deck
19
What is the IUPAC name of the following molecule? 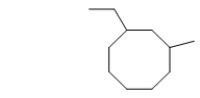
A) 2,4-cyclopentylhexane
B) 1-ethyl-6-methylcyclooctane
C) ethylmethylcyclooctane
D) 1,3-ethylmethylcyclohexane
E) 1-ethyl-3-methylcyclooctane

A) 2,4-cyclopentylhexane
B) 1-ethyl-6-methylcyclooctane
C) ethylmethylcyclooctane
D) 1,3-ethylmethylcyclohexane
E) 1-ethyl-3-methylcyclooctane

Unlock Deck
Unlock for access to all 111 flashcards in this deck.
Unlock Deck
k this deck
20
Which pair of molecules are geometric isomers?
A)
B)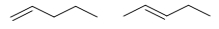
C)
D)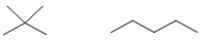
E)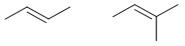
A)

B)
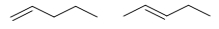
C)

D)

E)
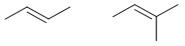

Unlock Deck
Unlock for access to all 111 flashcards in this deck.
Unlock Deck
k this deck
21
Aspirin contains a benzene ring substituted on the first and second carbon.Which molecule is aspirin?
A)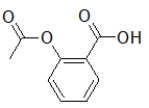
B)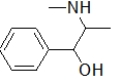
C)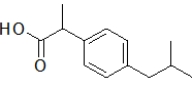
D)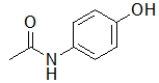
E) All of the above are aspirin molecules.
A)

B)

C)

D)

E) All of the above are aspirin molecules.

Unlock Deck
Unlock for access to all 111 flashcards in this deck.
Unlock Deck
k this deck
22
Which molecule is a polyene?
A)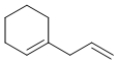
B)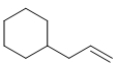
C)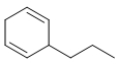
D)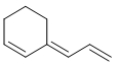
E) All of these molecules are polyenes.
A)

B)

C)

D)

E) All of these molecules are polyenes.

Unlock Deck
Unlock for access to all 111 flashcards in this deck.
Unlock Deck
k this deck
23
How many hydrogen atoms are attached to the carbons indicated below? 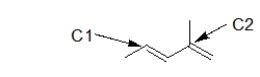
A) C1: 2 C2: 0
B) C1: 0 C2: 2
C) C1: 1 C2: 1
D) C1: 0 C2: 1
E) C1: 1 C2: 0

A) C1: 2 C2: 0
B) C1: 0 C2: 2
C) C1: 1 C2: 1
D) C1: 0 C2: 1
E) C1: 1 C2: 0

Unlock Deck
Unlock for access to all 111 flashcards in this deck.
Unlock Deck
k this deck
24
Which statement about organic molecules is FALSE?
A) Most medications are organic compounds.
B) Organic compounds contain carbon and hydrogen atoms.
C) Many organic compounds contain heteroatoms.
D) Organic compounds can only be produced by living organisms.
E) Lipids, nucleic acids, carbohydrates, and proteins are organic compounds.
A) Most medications are organic compounds.
B) Organic compounds contain carbon and hydrogen atoms.
C) Many organic compounds contain heteroatoms.
D) Organic compounds can only be produced by living organisms.
E) Lipids, nucleic acids, carbohydrates, and proteins are organic compounds.

Unlock Deck
Unlock for access to all 111 flashcards in this deck.
Unlock Deck
k this deck
25
What is the IUPAC name of the following molecule? 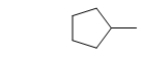
A) methylcyclopentane
B) 2-cyclohexane
C) cyclohexane
D) ethylcyclobutane
E) 1-ethylcyclobutane

A) methylcyclopentane
B) 2-cyclohexane
C) cyclohexane
D) ethylcyclobutane
E) 1-ethylcyclobutane

Unlock Deck
Unlock for access to all 111 flashcards in this deck.
Unlock Deck
k this deck
26
Ethynyl estradiol, a synthetic hormone, is a common component of birth control pills.What is the angle of the C-C-O bond indicated with the arrow? 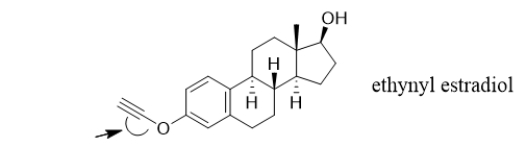
A) 45°
B) 90°
C) 109.5°
D) 120°
E) 180°

A) 45°
B) 90°
C) 109.5°
D) 120°
E) 180°

Unlock Deck
Unlock for access to all 111 flashcards in this deck.
Unlock Deck
k this deck
27
Which skeletal line structure has the molecular formula C5H8?
A)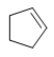
B)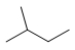
C)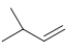
D)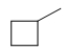
E) None of these structures has the molecular formula C5H8.
A)

B)

C)

D)

E) None of these structures has the molecular formula C5H8.

Unlock Deck
Unlock for access to all 111 flashcards in this deck.
Unlock Deck
k this deck
28
An alkene has _______ a saturated hydrocarbon with the same number of carbon atom.
A) two more hydrogen atoms than
B) one more hydrogen atom than
C) the same number of hydrogen atoms as
D) one fewer hydrogen atom than
E) two fewer hydrogen atoms than
A) two more hydrogen atoms than
B) one more hydrogen atom than
C) the same number of hydrogen atoms as
D) one fewer hydrogen atom than
E) two fewer hydrogen atoms than

Unlock Deck
Unlock for access to all 111 flashcards in this deck.
Unlock Deck
k this deck
29
Which compound is an inorganic carbon compound?
A) CH4O
B) CH4
C) CO2
D) NH3
E) CH2O
A) CH4O
B) CH4
C) CO2
D) NH3
E) CH2O

Unlock Deck
Unlock for access to all 111 flashcards in this deck.
Unlock Deck
k this deck
30
In which of the following statements is the hydrocarbon correctly described by its bonding to carbon?
A) alkyne: three single bonds and one triple bond
B) alkane: three, four or five single bonds
C) alkene: one double bond and two single bonds
D) alkyne: one single bond and three triple bonds
E) aromatic: two single bonds and two double bonds
A) alkyne: three single bonds and one triple bond
B) alkane: three, four or five single bonds
C) alkene: one double bond and two single bonds
D) alkyne: one single bond and three triple bonds
E) aromatic: two single bonds and two double bonds

Unlock Deck
Unlock for access to all 111 flashcards in this deck.
Unlock Deck
k this deck
31
What is the IUPAC name of the following hydrocarbon? 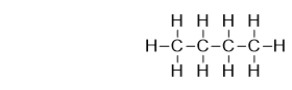
A) propane
B) pentane
C) butane
D) hexane
E) ethane

A) propane
B) pentane
C) butane
D) hexane
E) ethane

Unlock Deck
Unlock for access to all 111 flashcards in this deck.
Unlock Deck
k this deck
32
A molecule has the molecular formula C5H10.This molecule could be a(n)______ or a(n)_____.
A) alkane; alkyne
B) alkane; cycloalkane
C) alkene; alkyne
D) alkene; cycloalkane
E) alkene; alkane
A) alkane; alkyne
B) alkane; cycloalkane
C) alkene; alkyne
D) alkene; cycloalkane
E) alkene; alkane

Unlock Deck
Unlock for access to all 111 flashcards in this deck.
Unlock Deck
k this deck
33
Which of the following condensed structures are branched and which are straight chains? 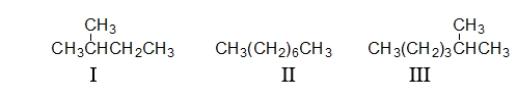
A) branched: II straight chain: I and III
B) branched: I and III straight chain: II
C) branched: II and III straight chain: I
D) branched: III straight chain: I and II
E) branched: I and II straight chain: III
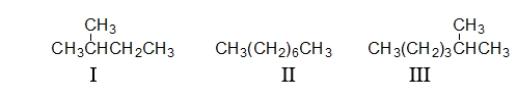
A) branched: II straight chain: I and III
B) branched: I and III straight chain: II
C) branched: II and III straight chain: I
D) branched: III straight chain: I and II
E) branched: I and II straight chain: III

Unlock Deck
Unlock for access to all 111 flashcards in this deck.
Unlock Deck
k this deck
34
The following structure is a poor representation of benzene.What is wrong with it? 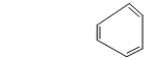
A) Benzene does not have six sides.
B) Benzene is not flat.
C) Benzene is never represented with three double bonds and three single bonds.
D) Benzene does not have the molecular formula C6H6.
E) The bonds in benzene should all be the same length.

A) Benzene does not have six sides.
B) Benzene is not flat.
C) Benzene is never represented with three double bonds and three single bonds.
D) Benzene does not have the molecular formula C6H6.
E) The bonds in benzene should all be the same length.

Unlock Deck
Unlock for access to all 111 flashcards in this deck.
Unlock Deck
k this deck
35
What is the IUPAC name of this hydrocarbon? 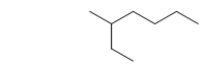
A) 5-methylheptane
B) 3-methylheptane
C) 2-ethylhexane
D) 5-ethylhexane
E) 1-ethyl-1-methyl-2-butane

A) 5-methylheptane
B) 3-methylheptane
C) 2-ethylhexane
D) 5-ethylhexane
E) 1-ethyl-1-methyl-2-butane

Unlock Deck
Unlock for access to all 111 flashcards in this deck.
Unlock Deck
k this deck
36
What type of change is illustrated in the reaction of rhodopsin in the presence of light shown below? 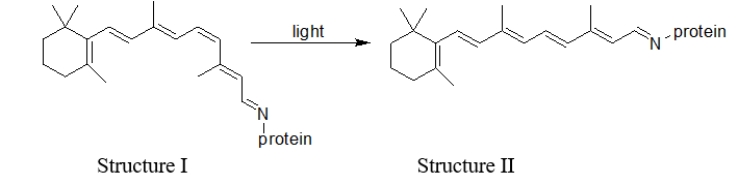
A) a physical change
B) a conformational change
C) an isomerization reaction
D) rotation about a bond as a result of free bond rotation
E) None of these are illustrated.

A) a physical change
B) a conformational change
C) an isomerization reaction
D) rotation about a bond as a result of free bond rotation
E) None of these are illustrated.

Unlock Deck
Unlock for access to all 111 flashcards in this deck.
Unlock Deck
k this deck
37
Which of the following structures represent benzene? 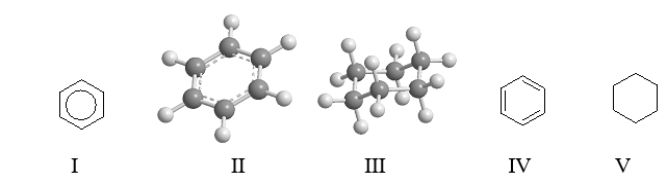
A) II and IV
B) I, II, and IV
C) III and V
D) all of them except V
E) All of them represent benzene.

A) II and IV
B) I, II, and IV
C) III and V
D) all of them except V
E) All of them represent benzene.

Unlock Deck
Unlock for access to all 111 flashcards in this deck.
Unlock Deck
k this deck
38
Which of the following definition BEST describes structural isomers?
A) They are molecules with different atom connectivity.
B) They are molecules with the same molecular formula and the atom connectivity.
C) They are molecules with the same physical properties.
D) They are molecules that have the same molecular formula but different atom connectivity.
E) They are molecules that differ by carbon-carbon bond rotation.
A) They are molecules with different atom connectivity.
B) They are molecules with the same molecular formula and the atom connectivity.
C) They are molecules with the same physical properties.
D) They are molecules that have the same molecular formula but different atom connectivity.
E) They are molecules that differ by carbon-carbon bond rotation.

Unlock Deck
Unlock for access to all 111 flashcards in this deck.
Unlock Deck
k this deck
39
Which pair(s)of molecules below are conformers? 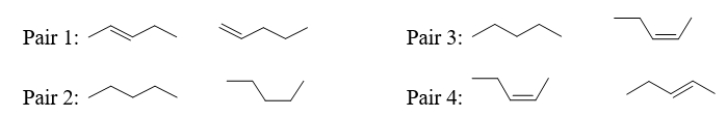
A) pairs 1, 3, and 4
B) pairs 2 and 4
C) pairs 2 and 3
D) pair 2 only
E) pair 4 only
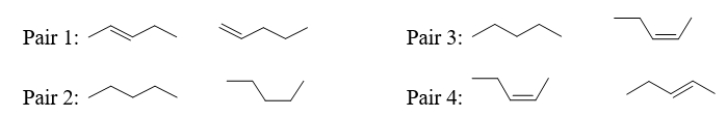
A) pairs 1, 3, and 4
B) pairs 2 and 4
C) pairs 2 and 3
D) pair 2 only
E) pair 4 only

Unlock Deck
Unlock for access to all 111 flashcards in this deck.
Unlock Deck
k this deck
40
Which statement about aromatic rings is FALSE?
A) Aromatic rings are very common.
B) Aromatic rings are found in many pharmaceuticals.
C) Aromatic rings are highly reactive.
D) Aromatic rings are flat (planar).
E) Aromatic rings are stable.
A) Aromatic rings are very common.
B) Aromatic rings are found in many pharmaceuticals.
C) Aromatic rings are highly reactive.
D) Aromatic rings are flat (planar).
E) Aromatic rings are stable.

Unlock Deck
Unlock for access to all 111 flashcards in this deck.
Unlock Deck
k this deck
41
What is the name of the following branched-chain hydrocarbon? 
A) 1,2,3-trimethyl-4-hexyne
B) 4,5-dimethyl-2-heptyne
C) 3,4-dimethyl-5-heptyne
D) 5-ethyl-4-methyl-2-hexyne
E) 2-propynyl-3-methylpentane

A) 1,2,3-trimethyl-4-hexyne
B) 4,5-dimethyl-2-heptyne
C) 3,4-dimethyl-5-heptyne
D) 5-ethyl-4-methyl-2-hexyne
E) 2-propynyl-3-methylpentane

Unlock Deck
Unlock for access to all 111 flashcards in this deck.
Unlock Deck
k this deck
42
What is the IUPAC name of the following hydrocarbon? 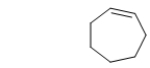
A) cycloheptene
B) heptane
C) hexyne
D) cyclohexene
E) cycloheptyne

A) cycloheptene
B) heptane
C) hexyne
D) cyclohexene
E) cycloheptyne

Unlock Deck
Unlock for access to all 111 flashcards in this deck.
Unlock Deck
k this deck
43
Which statement BEST describes the difference(s)between structures I and II in this reaction of rhodopsin in the presence of light? 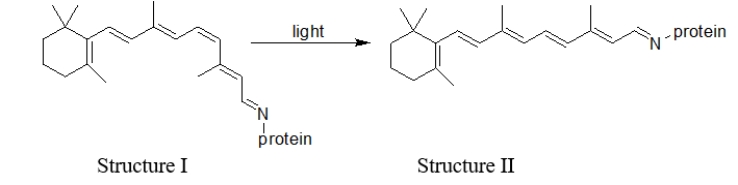 I.Structure II is more linear than structure I. II.Structure I is more linear than structure II.
I.Structure II is more linear than structure I. II.Structure I is more linear than structure II.
III)A cis double bond in structure I has been converted to a trans double bond in structure II.
IV)A trans double bond in structure I has been converted to a cis double bond in structure II.
A) All of these statements are true.
B) I and IV are true.
C) II and IV are true.
D) I and III are true.
E) II and III are true.
 I.Structure II is more linear than structure I. II.Structure I is more linear than structure II.
I.Structure II is more linear than structure I. II.Structure I is more linear than structure II.III)A cis double bond in structure I has been converted to a trans double bond in structure II.
IV)A trans double bond in structure I has been converted to a cis double bond in structure II.
A) All of these statements are true.
B) I and IV are true.
C) II and IV are true.
D) I and III are true.
E) II and III are true.

Unlock Deck
Unlock for access to all 111 flashcards in this deck.
Unlock Deck
k this deck
44
Which of these structures below are conformers? 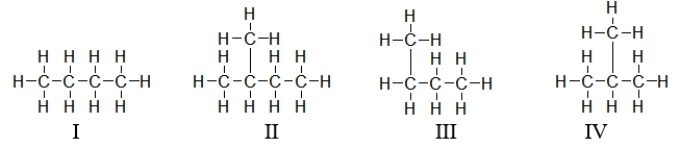
A) I and II
B) I and IV
C) I, III, and IV
D) I and III
E) All of these are conformers.

A) I and II
B) I and IV
C) I, III, and IV
D) I and III
E) All of these are conformers.

Unlock Deck
Unlock for access to all 111 flashcards in this deck.
Unlock Deck
k this deck
45
What are the locator number(s)and substituent name(s)for this branched-chain hydrocarbon? 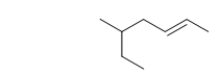
A) 3-methyl
B) 2-ethyl
C) 1-ethyl-1-methyl
D) 5-ethyl
E) 5-methyl

A) 3-methyl
B) 2-ethyl
C) 1-ethyl-1-methyl
D) 5-ethyl
E) 5-methyl

Unlock Deck
Unlock for access to all 111 flashcards in this deck.
Unlock Deck
k this deck
46
Which of the molecules below are branched? 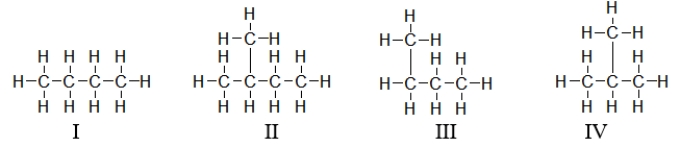
A) I only
B) II and IV
C) II, III, and IV
D) I and III
E) All of these molecules are branched.

A) I only
B) II and IV
C) II, III, and IV
D) I and III
E) All of these molecules are branched.

Unlock Deck
Unlock for access to all 111 flashcards in this deck.
Unlock Deck
k this deck
47
Which of the following hydrocarbons are branched and which are straight chains? 
A) All of these hydrocarbons are branched.
B) All of these hydrocarbons are branched except II.
C) All of these hydrocarbons are straight chains except III.
D) I and III are branched, and II and IV are straight chains.
E) II, III, and IV are straight chains and I is branched.

A) All of these hydrocarbons are branched.
B) All of these hydrocarbons are branched except II.
C) All of these hydrocarbons are straight chains except III.
D) I and III are branched, and II and IV are straight chains.
E) II, III, and IV are straight chains and I is branched.

Unlock Deck
Unlock for access to all 111 flashcards in this deck.
Unlock Deck
k this deck
48
What is the name of the parent chain of the hydrocarbon below? 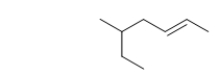
A) butene
B) hexane
C) hexene
D) heptane
E) heptene

A) butene
B) hexane
C) hexene
D) heptane
E) heptene

Unlock Deck
Unlock for access to all 111 flashcards in this deck.
Unlock Deck
k this deck
49
What is the IUPAC name of the following branched-chain hydrocarbon? 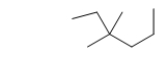
A) trans-2-ethyl-2-propylpropane
B) 3,3-dimethylhexane
C) 3-ethyl-3,3-dimethylpropane
D) 2-ethyl-2-methylpentane
E) 2-propyl-2-methylbutane

A) trans-2-ethyl-2-propylpropane
B) 3,3-dimethylhexane
C) 3-ethyl-3,3-dimethylpropane
D) 2-ethyl-2-methylpentane
E) 2-propyl-2-methylbutane

Unlock Deck
Unlock for access to all 111 flashcards in this deck.
Unlock Deck
k this deck
50
What happens in the retina as a result of the change illustrated in the reaction of rhodopsin in the presence of light? 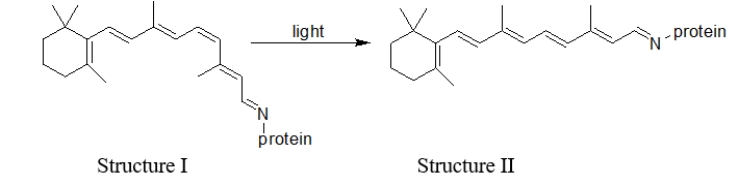
A) Nothing happens.
B) The retina is damaged.
C) A signal is sent to the brain that light is detected.
D) A signal is sent to the spinal cord to react to the light.
E) The retina makes more protein.

A) Nothing happens.
B) The retina is damaged.
C) A signal is sent to the brain that light is detected.
D) A signal is sent to the spinal cord to react to the light.
E) The retina makes more protein.

Unlock Deck
Unlock for access to all 111 flashcards in this deck.
Unlock Deck
k this deck
51
Which compound is an inorganic compound?
A) CH4O
B) CH4
C) CO2
D) NH3
E) CH2O
A) CH4O
B) CH4
C) CO2
D) NH3
E) CH2O

Unlock Deck
Unlock for access to all 111 flashcards in this deck.
Unlock Deck
k this deck
52
What is the molecular shape around the carbon indicated with an arrow? 
A) linear
B) trigonal pyramidal
C) tetrahedral
D) bent
E) trigonal planar

A) linear
B) trigonal pyramidal
C) tetrahedral
D) bent
E) trigonal planar

Unlock Deck
Unlock for access to all 111 flashcards in this deck.
Unlock Deck
k this deck
53
What is the parent chain name of the hydrocarbon below? 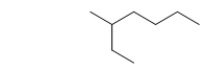
A) butane
B) hexane
C) methane
D) ethane
E) heptane

A) butane
B) hexane
C) methane
D) ethane
E) heptane

Unlock Deck
Unlock for access to all 111 flashcards in this deck.
Unlock Deck
k this deck
54
Which statement BEST describes the relationship between the following two structures? 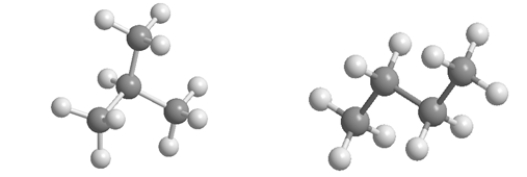
A) They are identical molecules in the same conformation.
B) They have different molecular formulas.
C) They are isomers.
D) They are different conformations of the same molecule.
E) One is saturated, and the other is unsaturated.

A) They are identical molecules in the same conformation.
B) They have different molecular formulas.
C) They are isomers.
D) They are different conformations of the same molecule.
E) One is saturated, and the other is unsaturated.

Unlock Deck
Unlock for access to all 111 flashcards in this deck.
Unlock Deck
k this deck
55
How many hydrogens are bonded to the carbons with the arrows pointing to them? 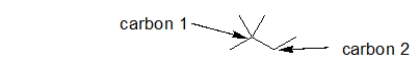
A) carbon 1: 0 carbon 2: 0
B) carbon 1: 0 carbon 2: 2
C) carbon 1: 1 carbon 2: 2
D) carbon 1: 4 carbon 2: 4
E) It is not possible to tell given the skeletal line structure.

A) carbon 1: 0 carbon 2: 0
B) carbon 1: 0 carbon 2: 2
C) carbon 1: 1 carbon 2: 2
D) carbon 1: 4 carbon 2: 4
E) It is not possible to tell given the skeletal line structure.

Unlock Deck
Unlock for access to all 111 flashcards in this deck.
Unlock Deck
k this deck
56
What is the C-C-C bond angle indicated by the arrow in limonene, one of the components of oil of lemon? 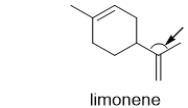
A) 180°
B) 120°
C) 109.5°
D) 90°
E) 45°

A) 180°
B) 120°
C) 109.5°
D) 90°
E) 45°

Unlock Deck
Unlock for access to all 111 flashcards in this deck.
Unlock Deck
k this deck
57
A compound with the molecular formula CnH2n+2 is called a(n)______or a ______.
A) alkane; saturated hydrocarbon
B) alkane; unsaturated hydrocarbon
C) alkene; saturated hydrocarbon
D) alkene; unsaturated hydrocarbon
E) alkene; alkyne
A) alkane; saturated hydrocarbon
B) alkane; unsaturated hydrocarbon
C) alkene; saturated hydrocarbon
D) alkene; unsaturated hydrocarbon
E) alkene; alkyne

Unlock Deck
Unlock for access to all 111 flashcards in this deck.
Unlock Deck
k this deck
58
Which statement BEST describes the relationship between the following two structures? 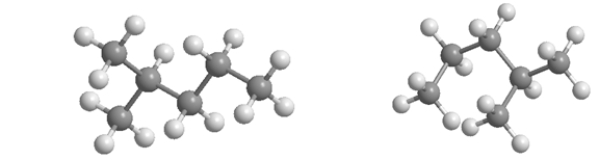
A) They are identical molecules in the same conformation.
B) They have different molecular formulas.
C) They are isomers.
D) They are different conformations of the same molecule.
E) One is saturated, and the other is unsaturated.

A) They are identical molecules in the same conformation.
B) They have different molecular formulas.
C) They are isomers.
D) They are different conformations of the same molecule.
E) One is saturated, and the other is unsaturated.

Unlock Deck
Unlock for access to all 111 flashcards in this deck.
Unlock Deck
k this deck
59
Arrange the molecules from lowest to highest boiling point. 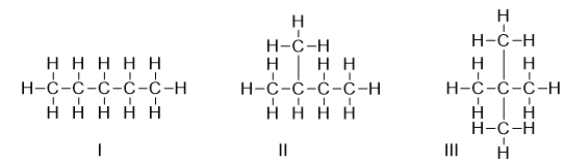
A) I < II < III
B) II < III < I
C) III < II < I
D) II < I < III
E) III < I < II

A) I < II < III
B) II < III < I
C) III < II < I
D) II < I < III
E) III < I < II

Unlock Deck
Unlock for access to all 111 flashcards in this deck.
Unlock Deck
k this deck
60
For naming purposes, on which carbon is the double bond located in this branched-chain hydrocarbon? 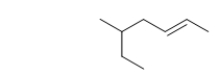
A) 1
B) 2
C) 3
D) 4
E) 5

A) 1
B) 2
C) 3
D) 4
E) 5

Unlock Deck
Unlock for access to all 111 flashcards in this deck.
Unlock Deck
k this deck
61
Which structure is the condensed structural formula of 1-hexene?
A) CH3CH=CHCH2CH2CH3
B) CH2=CHCH2CH2CH2CH3
C) CH3=CH2CH2CH2CH2CH3
D) CH3CH2=CH2CH2CH2CH3
E) CH3CH2CH=CHCH2CH3
A) CH3CH=CHCH2CH2CH3
B) CH2=CHCH2CH2CH2CH3
C) CH3=CH2CH2CH2CH2CH3
D) CH3CH2=CH2CH2CH2CH3
E) CH3CH2CH=CHCH2CH3

Unlock Deck
Unlock for access to all 111 flashcards in this deck.
Unlock Deck
k this deck
62
What is the molecular formula of hexane?
A) C2H6
B) C3H8
C) C4H10
D) C5H12
E) C6H14
A) C2H6
B) C3H8
C) C4H10
D) C5H12
E) C6H14

Unlock Deck
Unlock for access to all 111 flashcards in this deck.
Unlock Deck
k this deck
63
Which skeletal line structure best represents 2-butyne?
A)
B)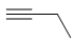
C)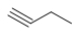
D)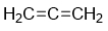
E)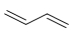
A)

B)

C)

D)
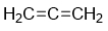
E)


Unlock Deck
Unlock for access to all 111 flashcards in this deck.
Unlock Deck
k this deck
64
Which structure is nonane?
A)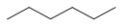
B)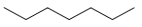
C)
D)
E)
A)

B)

C)

D)

E)


Unlock Deck
Unlock for access to all 111 flashcards in this deck.
Unlock Deck
k this deck
65
Which of the following structures is a branched-chain hydrocarbon? 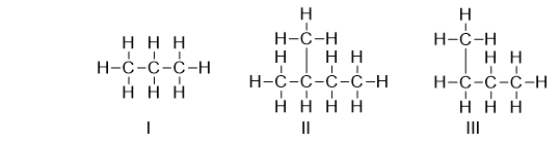
A) I only
B) II only
C) III only
D) I and II
E) II and III

A) I only
B) II only
C) III only
D) I and II
E) II and III

Unlock Deck
Unlock for access to all 111 flashcards in this deck.
Unlock Deck
k this deck
66
Which condensed structure BEST represents the skeletal line structure below? 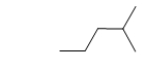
A)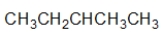
B)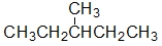
C)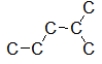
D)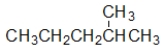
E)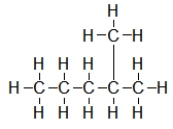

A)
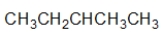
B)
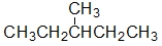
C)
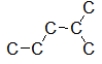
D)
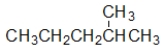
E)


Unlock Deck
Unlock for access to all 111 flashcards in this deck.
Unlock Deck
k this deck
67
Which of the following compounds contains a heteroatom? 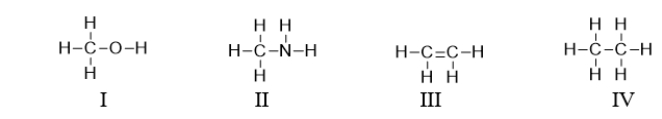
A) All of these compounds contain a heteroatom.
B) I and II contain a heteroatom.
C) III and IV contain a heteroatom.
D) Only III contains a heteroatom.
E) I, II, and III contain a heteroatom.
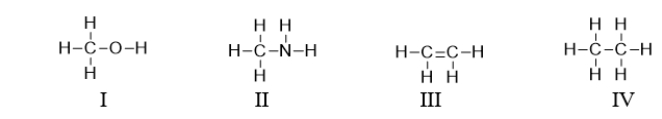
A) All of these compounds contain a heteroatom.
B) I and II contain a heteroatom.
C) III and IV contain a heteroatom.
D) Only III contains a heteroatom.
E) I, II, and III contain a heteroatom.

Unlock Deck
Unlock for access to all 111 flashcards in this deck.
Unlock Deck
k this deck
68
Ethynyl estradiol, a synthetic hormone, is a common component of birth control pills.Which bond in ethynyl estradiol is an alkyne? 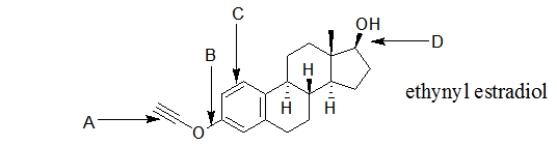
A) bond A
B) bond B
C) bond C
D) bond D
E) bonds A and C

A) bond A
B) bond B
C) bond C
D) bond D
E) bonds A and C

Unlock Deck
Unlock for access to all 111 flashcards in this deck.
Unlock Deck
k this deck
69
Are the following two molecules structural isomers? 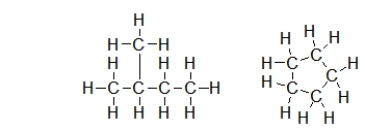
A) No, because they do not have the same molecular formula.
B) Yes, because they have the same molecular formula but different structures.
C) Yes, because they have the same number of carbons.
D) Yes, because they do not have the same molecular formula.
E) No, because only one is branched.

A) No, because they do not have the same molecular formula.
B) Yes, because they have the same molecular formula but different structures.
C) Yes, because they have the same number of carbons.
D) Yes, because they do not have the same molecular formula.
E) No, because only one is branched.

Unlock Deck
Unlock for access to all 111 flashcards in this deck.
Unlock Deck
k this deck
70
Which of the following statements describes why "good fats" are considered good and "bad fats" are considered bad? I.A diet high in saturated fat can result in heart disease.
II)A diet high in unsaturated fat can result in type 2 diabetes.
III)A diet high is trans fats can result in a decreased risk of cancer.
IV)A diet high in unsaturated fats can result in a decreased risk of cancer.
A) All of these statements are true.
B) I and II are true.
C) I, III, and IV are true.
D) I and IV are true.
E) II and III are true.
II)A diet high in unsaturated fat can result in type 2 diabetes.
III)A diet high is trans fats can result in a decreased risk of cancer.
IV)A diet high in unsaturated fats can result in a decreased risk of cancer.
A) All of these statements are true.
B) I and II are true.
C) I, III, and IV are true.
D) I and IV are true.
E) II and III are true.

Unlock Deck
Unlock for access to all 111 flashcards in this deck.
Unlock Deck
k this deck
71
Which of these structures below have the same physical properties? 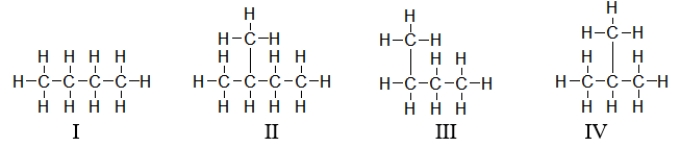
A) I and II
B) I and IV
C) I, III, and IV
D) I and III
E) All of these have the same physical properties.

A) I and II
B) I and IV
C) I, III, and IV
D) I and III
E) All of these have the same physical properties.

Unlock Deck
Unlock for access to all 111 flashcards in this deck.
Unlock Deck
k this deck
72
Which of the following skeletal line structures represent the same molecule? 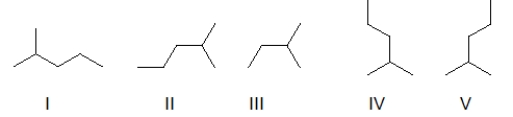
A) I and II
B) II and III
C) I, II, IV, and V
D) IV and V
E) II, IV, and V

A) I and II
B) II and III
C) I, II, IV, and V
D) IV and V
E) II, IV, and V

Unlock Deck
Unlock for access to all 111 flashcards in this deck.
Unlock Deck
k this deck
73
Which skeletal line structure is the same molecule as the ball-and-stick model below? 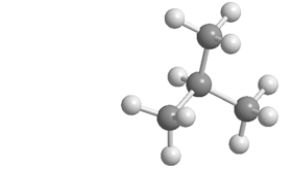
A)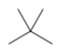
B)
C)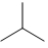
D)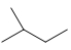
E)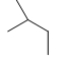

A)

B)

C)

D)

E)


Unlock Deck
Unlock for access to all 111 flashcards in this deck.
Unlock Deck
k this deck
74
In which of the following choices are the alkenes correctly labeled?
A)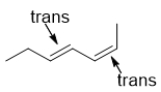
B)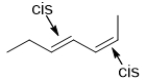
C)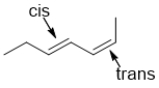
D)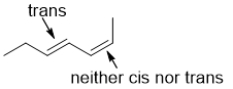
E)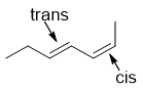
A)

B)

C)

D)

E)


Unlock Deck
Unlock for access to all 111 flashcards in this deck.
Unlock Deck
k this deck
75
Which element is NOT a heteroatom?
A) carbon
B) sulfur
C) nitrogen
D) oxygen
E) phosphorous
A) carbon
B) sulfur
C) nitrogen
D) oxygen
E) phosphorous

Unlock Deck
Unlock for access to all 111 flashcards in this deck.
Unlock Deck
k this deck
76
Below is an illustrated a molecule of butane.Which of the given choices is another molecule of butane in a different conformation? Assume that all atoms in each are visible. 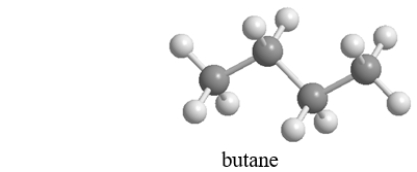
A)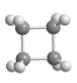
B)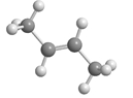
C)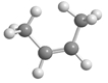
D)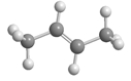
E)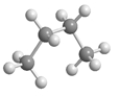

A)

B)

C)

D)

E)


Unlock Deck
Unlock for access to all 111 flashcards in this deck.
Unlock Deck
k this deck
77
What is the molecular formula of cyclobutane?
A) C3H6
B) C3H8
C) C4H10
D) C4H8
E) C6H14
A) C3H6
B) C3H8
C) C4H10
D) C4H8
E) C6H14

Unlock Deck
Unlock for access to all 111 flashcards in this deck.
Unlock Deck
k this deck
78
Identify the substituents and the parent chain of the following molecule. 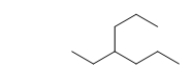
A) substituent: ethyl and propyl parent chain: hexane
B) substituent: ethyl and propyl parent chain: heptane
C) substituent: propyl parent chain: hexane
D) substituent: ethyl parent chain: heptane
E) substituent: ethyl parent chain: hexane

A) substituent: ethyl and propyl parent chain: hexane
B) substituent: ethyl and propyl parent chain: heptane
C) substituent: propyl parent chain: hexane
D) substituent: ethyl parent chain: heptane
E) substituent: ethyl parent chain: hexane

Unlock Deck
Unlock for access to all 111 flashcards in this deck.
Unlock Deck
k this deck
79
Which of the following compounds is a saturated hydrocarbon? 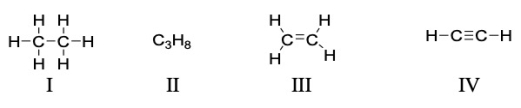
A) I only
B) I and II
C) IV only
D) III and IV
E) All of these are saturated hydrocarbons.
A) I only
B) I and II
C) IV only
D) III and IV
E) All of these are saturated hydrocarbons.

Unlock Deck
Unlock for access to all 111 flashcards in this deck.
Unlock Deck
k this deck
80
How do IUPAC names differ from the brand names of pharmaceuticals?
A) IUPAC names are systematic, and brand names are not.
B) Brand names are systematic, and IUPAC names are not.
C) IUPAC names are usually shorter than brand names.
D) Brand names are not used as frequently as IUPAC names.
E) IUPAC names do not follow a set of rules like brand names do.
A) IUPAC names are systematic, and brand names are not.
B) Brand names are systematic, and IUPAC names are not.
C) IUPAC names are usually shorter than brand names.
D) Brand names are not used as frequently as IUPAC names.
E) IUPAC names do not follow a set of rules like brand names do.

Unlock Deck
Unlock for access to all 111 flashcards in this deck.
Unlock Deck
k this deck


