Deck 11: Alcohols, Phenols, Thiols, Ethers, and Amines
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/69
Play
Full screen (f)
Deck 11: Alcohols, Phenols, Thiols, Ethers, and Amines
1
Select the compound containing the alkyne functional group. 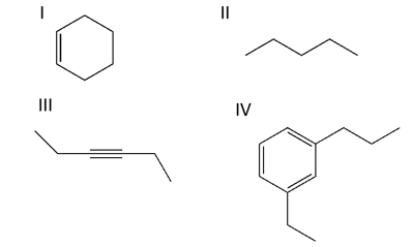
A) compound I
B) compound II
C) compound III
D) compound IV
E) compounds II and IV

A) compound I
B) compound II
C) compound III
D) compound IV
E) compounds II and IV
compound III
2
Which choice BEST describes the functional groups in adrenaline, the neurotransmitter responsible for the "fight or flight" response? 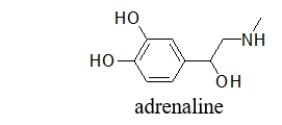
A) phenol, secondary alcohol, secondary amine
B) phenol, secondary alcohol, primary amine
C) phenol, primary alcohol, secondary amine
D) phenol, secondary alcohol, secondary amide
E) phenol, secondary alcohol, primary amide

A) phenol, secondary alcohol, secondary amine
B) phenol, secondary alcohol, primary amine
C) phenol, primary alcohol, secondary amine
D) phenol, secondary alcohol, secondary amide
E) phenol, secondary alcohol, primary amide
phenol, secondary alcohol, secondary amine
3
Which of these molecules has the lowest boiling point? 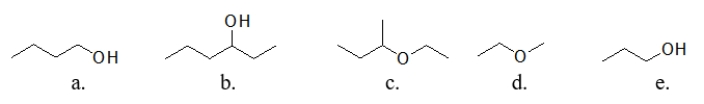
A) molecule a
B) molecule b
C) molecule c
D) molecule d
E) molecule e

A) molecule a
B) molecule b
C) molecule c
D) molecule d
E) molecule e
molecule d
4
Select the correct structure for phenol.
A)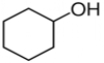
B)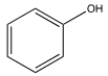
C)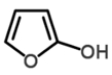
D)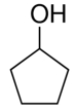
E) None of these
A)

B)

C)

D)

E) None of these

Unlock Deck
Unlock for access to all 69 flashcards in this deck.
Unlock Deck
k this deck
5
What is the name of the parent chain of the following molecule? 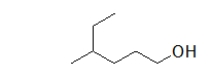
A) 1-hexanol
B) 1-pentanol
C) 5-pentanol
D) 6-hexanol
E) 2-hexanol

A) 1-hexanol
B) 1-pentanol
C) 5-pentanol
D) 6-hexanol
E) 2-hexanol

Unlock Deck
Unlock for access to all 69 flashcards in this deck.
Unlock Deck
k this deck
6
Another name for a thiol is
A) sulfur alcohol.
B) sulfhydryl.
C) sulfanol.
D) sulfoxide.
E) sulfone.
A) sulfur alcohol.
B) sulfhydryl.
C) sulfanol.
D) sulfoxide.
E) sulfone.

Unlock Deck
Unlock for access to all 69 flashcards in this deck.
Unlock Deck
k this deck
7
Examples of thiols in nature include
A) skunk spray.
B) onions.
C) garlics.
D) cheddar cheese.
E) All of these are examples of thiols.
A) skunk spray.
B) onions.
C) garlics.
D) cheddar cheese.
E) All of these are examples of thiols.

Unlock Deck
Unlock for access to all 69 flashcards in this deck.
Unlock Deck
k this deck
8
Can adrenaline form hydrogen bonds, and if so, which functional groups are involved? 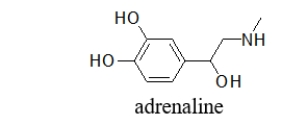
A) No, adrenaline is too stable to form hydrogen bonds.
B) No, adrenaline has an aromatic ring that prevents it from forming hydrogen bonds.
C) Yes, the amine hydrogen bonds.
D) Yes, the alcohol hydrogen bonds.
E) Yes, both the alcohol and amine hydrogen bond.

A) No, adrenaline is too stable to form hydrogen bonds.
B) No, adrenaline has an aromatic ring that prevents it from forming hydrogen bonds.
C) Yes, the amine hydrogen bonds.
D) Yes, the alcohol hydrogen bonds.
E) Yes, both the alcohol and amine hydrogen bond.

Unlock Deck
Unlock for access to all 69 flashcards in this deck.
Unlock Deck
k this deck
9
Which functional group is called a thiol?
A) R-OH
B) R-S-S-R
C) R-C=O
D) R-SH
E) R-C=S
A) R-OH
B) R-S-S-R
C) R-C=O
D) R-SH
E) R-C=S

Unlock Deck
Unlock for access to all 69 flashcards in this deck.
Unlock Deck
k this deck
10
As the number of hydroxyl groups in a molecule __________, the solubility of the molecule increases.As the number of carbons in a molecule ___________, the solubility decreases.
A) increases; increases
B) decreases; decreases
C) decreases, increases
D) increases, decreases
E) Actually, the number of hydroxyl groups and carbons in a molecule do not affect a molecule's solubility.
A) increases; increases
B) decreases; decreases
C) decreases, increases
D) increases, decreases
E) Actually, the number of hydroxyl groups and carbons in a molecule do not affect a molecule's solubility.

Unlock Deck
Unlock for access to all 69 flashcards in this deck.
Unlock Deck
k this deck
11
Select the correct statement regarding phenols.
A) Phenols are in the same functional group as alcohols.
B) Phenols are in the same functional group as aromatic hydrocarbons.
C) Phenols are a separate functional group from alcohols or aromatic hydrocarbons.
D) Both a and b are correct.
E) None of these is correct.
A) Phenols are in the same functional group as alcohols.
B) Phenols are in the same functional group as aromatic hydrocarbons.
C) Phenols are a separate functional group from alcohols or aromatic hydrocarbons.
D) Both a and b are correct.
E) None of these is correct.

Unlock Deck
Unlock for access to all 69 flashcards in this deck.
Unlock Deck
k this deck
12
Which statement BEST describes the following molecule? 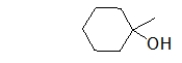
A) This molecule is a primary ether.
B) This molecule is a tertiary ether.
C) This molecule is a primary alcohol.
D) This molecule is a secondary alcohol.
E) This molecule is a tertiary alcohol.

A) This molecule is a primary ether.
B) This molecule is a tertiary ether.
C) This molecule is a primary alcohol.
D) This molecule is a secondary alcohol.
E) This molecule is a tertiary alcohol.

Unlock Deck
Unlock for access to all 69 flashcards in this deck.
Unlock Deck
k this deck
13
Which of the following compounds contains a heteroatom? 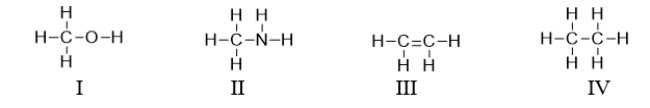
A) All of these compounds contain a heteroatom.
B) I and II contain a heteroatom.
C) III and IV contain a heteroatom.
D) Only III contains a heteroatom.
E) I, II, and III contain a heteroatom.
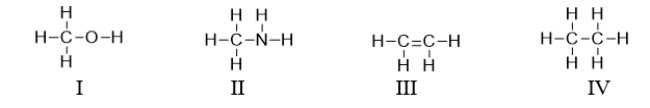
A) All of these compounds contain a heteroatom.
B) I and II contain a heteroatom.
C) III and IV contain a heteroatom.
D) Only III contains a heteroatom.
E) I, II, and III contain a heteroatom.

Unlock Deck
Unlock for access to all 69 flashcards in this deck.
Unlock Deck
k this deck
14
Which of the following molecules is the MOST water soluble?
A)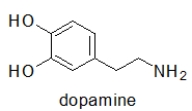
B)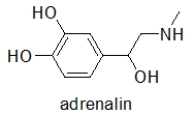
C)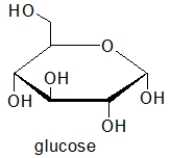
D)
E)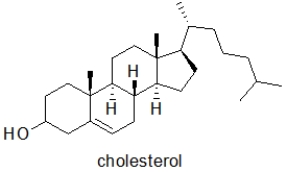
A)

B)

C)

D)

E)


Unlock Deck
Unlock for access to all 69 flashcards in this deck.
Unlock Deck
k this deck
15
Which of the following molecules contain(s)a polar functional group? 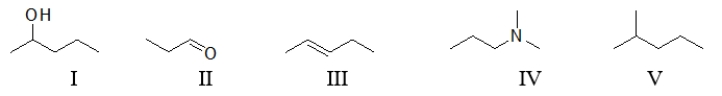
A) All of these molecules contain polar functional groups.
B) All of these molecules except V contain a polar functional group.
C) All of these molecules except III and V contain a polar functional group.
D) Only I and II contain a polar functional group.
E) Only III contains a polar functional group.

A) All of these molecules contain polar functional groups.
B) All of these molecules except V contain a polar functional group.
C) All of these molecules except III and V contain a polar functional group.
D) Only I and II contain a polar functional group.
E) Only III contains a polar functional group.

Unlock Deck
Unlock for access to all 69 flashcards in this deck.
Unlock Deck
k this deck
16
What is the IUPAC name of this compound? 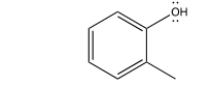
A) phenol
B) methylphenol
C) 2-methylphenol
D) methyl-5-phenol
E) 5-methylphenol

A) phenol
B) methylphenol
C) 2-methylphenol
D) methyl-5-phenol
E) 5-methylphenol

Unlock Deck
Unlock for access to all 69 flashcards in this deck.
Unlock Deck
k this deck
17
What is the BEST description of this alcohol? 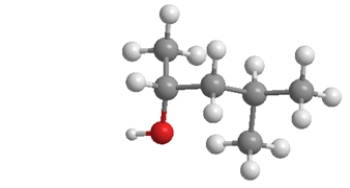
A) It is a 1° alcohol.
B) It is a 2° alcohol.
C) It is a 3° alcohol.
D) It is a 4° alcohol.
E) It is both a 1° and a 2° alcohol.

A) It is a 1° alcohol.
B) It is a 2° alcohol.
C) It is a 3° alcohol.
D) It is a 4° alcohol.
E) It is both a 1° and a 2° alcohol.

Unlock Deck
Unlock for access to all 69 flashcards in this deck.
Unlock Deck
k this deck
18
Which of the following molecules is a secondary amine? 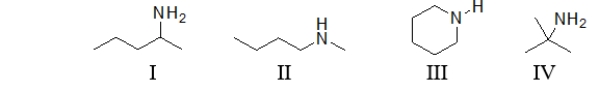
A) All of these molecules are secondary amines.
B) I is the only secondary amine.
C) II is the only secondary amine.
D) I and II are both secondary amines.
E) II and III are both secondary amines.

A) All of these molecules are secondary amines.
B) I is the only secondary amine.
C) II is the only secondary amine.
D) I and II are both secondary amines.
E) II and III are both secondary amines.

Unlock Deck
Unlock for access to all 69 flashcards in this deck.
Unlock Deck
k this deck
19
Which of these molecules is the MOST soluble in water? 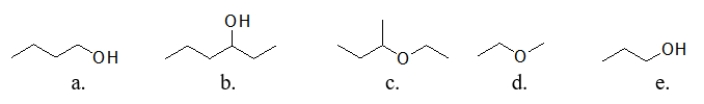
A) molecule a
B) molecule b
C) molecule c
D) molecule d
E) molecule e

A) molecule a
B) molecule b
C) molecule c
D) molecule d
E) molecule e

Unlock Deck
Unlock for access to all 69 flashcards in this deck.
Unlock Deck
k this deck
20
Which diagram BEST illustrates the interaction of dimethyl ether with water? Note that dashed lines indicate intermolecular forces of attraction. 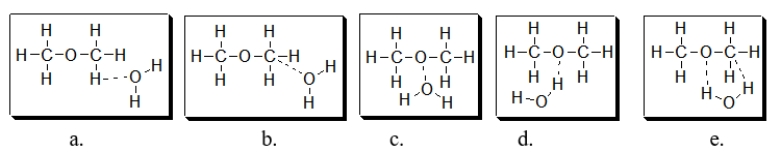
A) diagram a
B) diagram b
C) diagram c
D) diagram d
E) diagram e

A) diagram a
B) diagram b
C) diagram c
D) diagram d
E) diagram e

Unlock Deck
Unlock for access to all 69 flashcards in this deck.
Unlock Deck
k this deck
21
Which of these molecules contain(s)a secondary alcohol? 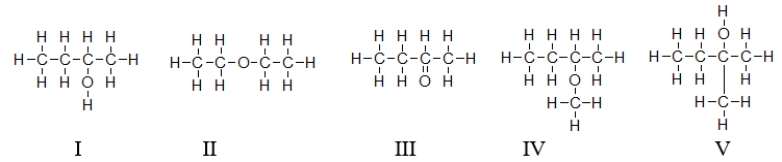
A) Molecules I, III, and IV contain a secondary alcohol.
B) Only molecule V contains a secondary alcohol.
C) Molecules II and IV contain a secondary alcohol.
D) Only molecule I contains a secondary alcohol.
E) All molecules except molecule II contain a secondary alcohol.
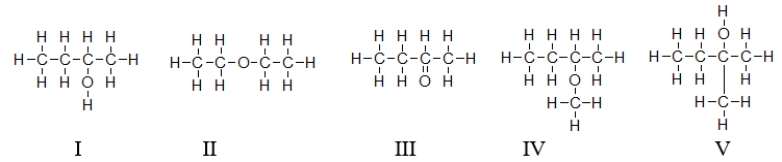
A) Molecules I, III, and IV contain a secondary alcohol.
B) Only molecule V contains a secondary alcohol.
C) Molecules II and IV contain a secondary alcohol.
D) Only molecule I contains a secondary alcohol.
E) All molecules except molecule II contain a secondary alcohol.

Unlock Deck
Unlock for access to all 69 flashcards in this deck.
Unlock Deck
k this deck
22
How do molecules with polar functional groups behave differently than molecules with nonpolar functional groups?
A) Molecules with polar functional groups have higher boiling points.
B) Molecules with polar functional groups can be soluble in water.
C) Molecules with polar functional groups have higher melting points.
D) All of the above are correct.
A) Molecules with polar functional groups have higher boiling points.
B) Molecules with polar functional groups can be soluble in water.
C) Molecules with polar functional groups have higher melting points.
D) All of the above are correct.

Unlock Deck
Unlock for access to all 69 flashcards in this deck.
Unlock Deck
k this deck
23
Glucose is an example of a(n) 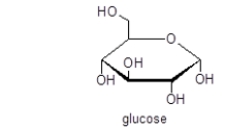
A) diol.
B) triol.
C) polyol.
D) ether.
E) phenol.

A) diol.
B) triol.
C) polyol.
D) ether.
E) phenol.

Unlock Deck
Unlock for access to all 69 flashcards in this deck.
Unlock Deck
k this deck
24
Which statement BEST describes the relationship between the following two molecules? 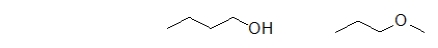
A) They react in the same way.
B) They have the same boiling point.
C) They have the same solubility.
D) They are isomers.
E) All of the above describe the relationship.

A) They react in the same way.
B) They have the same boiling point.
C) They have the same solubility.
D) They are isomers.
E) All of the above describe the relationship.

Unlock Deck
Unlock for access to all 69 flashcards in this deck.
Unlock Deck
k this deck
25
Phenols can be powerful antioxidants.Which statement describes antioxidants?
A) Antioxidants denature proteins and destroy bacteria.
B) Antioxidants capture free radicals, which are involved in certain disease processes.
C) Most vitamins are antioxidants.
D) Antioxidants are highly water soluble.
E) All of these describe antioxidants.
A) Antioxidants denature proteins and destroy bacteria.
B) Antioxidants capture free radicals, which are involved in certain disease processes.
C) Most vitamins are antioxidants.
D) Antioxidants are highly water soluble.
E) All of these describe antioxidants.

Unlock Deck
Unlock for access to all 69 flashcards in this deck.
Unlock Deck
k this deck
26
What is the structure of the conjugate acid formed when methylamine accepts a hydrogen ion (H+)from water? 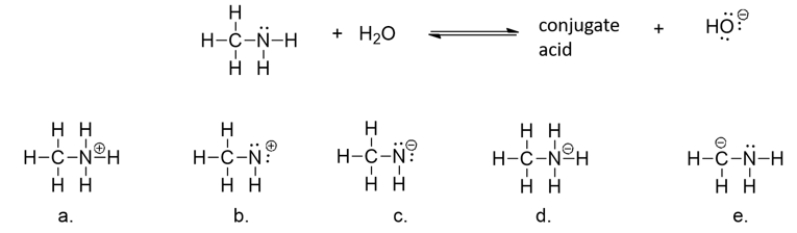
A) structure a
B) structure b
C) structure c
D) structure d
E) structure e

A) structure a
B) structure b
C) structure c
D) structure d
E) structure e

Unlock Deck
Unlock for access to all 69 flashcards in this deck.
Unlock Deck
k this deck
27
Predict the order of boiling points, low to high, for these three amines. I.(CH3)3N II.CH3CH2NHCH3 III.CH3CH2CH2NH2
A) I < II < III
B) III < II < I
C) I < III < II
D) II < III < I
E) II < I < III
A) I < II < III
B) III < II < I
C) I < III < II
D) II < III < I
E) II < I < III

Unlock Deck
Unlock for access to all 69 flashcards in this deck.
Unlock Deck
k this deck
28
When administered as a treatment for anaphylaxis, adrenaline (structure shown below)is administered as a positively charged ion.What is the structure of this positively charged ion? 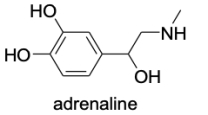
A)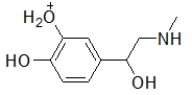
B)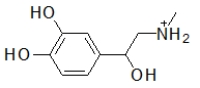
C)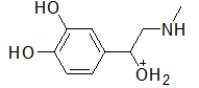
D)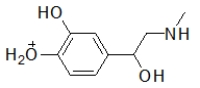
E)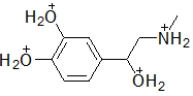

A)

B)

C)

D)

E)


Unlock Deck
Unlock for access to all 69 flashcards in this deck.
Unlock Deck
k this deck
29
Which of the following molecules is 2-methyl-2-butanol? 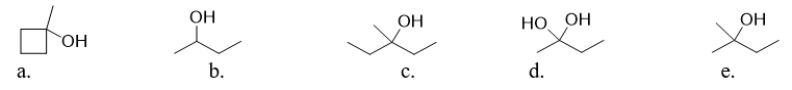
A) molecule a
B) molecule b
C) molecule c
D) molecule d
E) molecule e

A) molecule a
B) molecule b
C) molecule c
D) molecule d
E) molecule e

Unlock Deck
Unlock for access to all 69 flashcards in this deck.
Unlock Deck
k this deck
30
Which of these molecules has the highest boiling point? 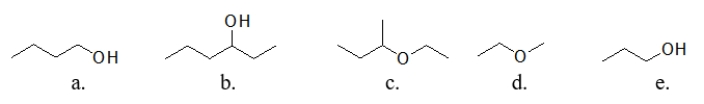
A) molecule a
B) molecule b
C) molecule c
D) molecule d
E) molecule e

A) molecule a
B) molecule b
C) molecule c
D) molecule d
E) molecule e

Unlock Deck
Unlock for access to all 69 flashcards in this deck.
Unlock Deck
k this deck
31
Molecules containing two hydroxyl groups are referred to as
A) diols.
B) triols.
C) polyols.
D) ethers.
E) phenols.
A) diols.
B) triols.
C) polyols.
D) ethers.
E) phenols.

Unlock Deck
Unlock for access to all 69 flashcards in this deck.
Unlock Deck
k this deck
32
Which of the following molecules contain(s)a thiol functional group? 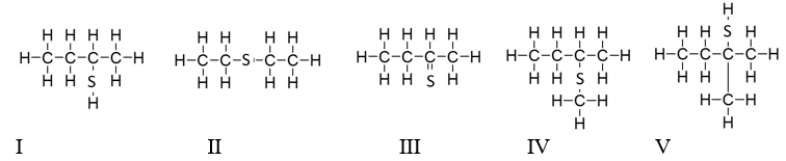
A) All of these molecules are thiols.
B) Only II is a thiol.
C) Molecules II and IV are thiols.
D) Molecules I and V are thiols.
E) All the molecules except molecule III are thiols.

A) All of these molecules are thiols.
B) Only II is a thiol.
C) Molecules II and IV are thiols.
D) Molecules I and V are thiols.
E) All the molecules except molecule III are thiols.

Unlock Deck
Unlock for access to all 69 flashcards in this deck.
Unlock Deck
k this deck
33
How are schizophrenia and Parkinson's disease similar?
A) They have similar effects on the body.
B) Both can be easily cured with drugs.
C) The same drugs are used to cure both of them.
D) Both are diseases that in some way involve dopamine.
E) Both diseases are well understood.
A) They have similar effects on the body.
B) Both can be easily cured with drugs.
C) The same drugs are used to cure both of them.
D) Both are diseases that in some way involve dopamine.
E) Both diseases are well understood.

Unlock Deck
Unlock for access to all 69 flashcards in this deck.
Unlock Deck
k this deck
34
An arrow is pointing to the oxygen of the alcohol.What is the C-O-H bond angle? 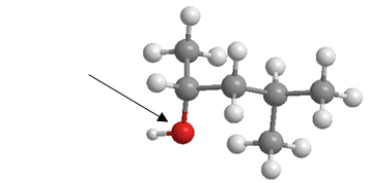
A) 90°
B) 109.5°
C) 120°
D) 180°
E) 270°

A) 90°
B) 109.5°
C) 120°
D) 180°
E) 270°

Unlock Deck
Unlock for access to all 69 flashcards in this deck.
Unlock Deck
k this deck
35
What is the molecular shape around the oxygen atom? 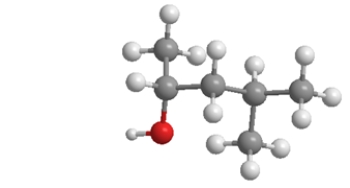
A) bent
B) trigonal planar
C) trigonal pyramidal
D) tetrahedral
E) linear

A) bent
B) trigonal planar
C) trigonal pyramidal
D) tetrahedral
E) linear

Unlock Deck
Unlock for access to all 69 flashcards in this deck.
Unlock Deck
k this deck
36
What are the names of the functional groups in the following molecules? 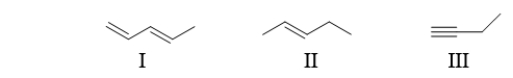
A) I: diene II: alkyne III: alkene
B) I: alkene II: alkane III: alkyne
C) I: alkyne II: alkene III: alkyne
D) I: alkene II: diene III: alkyne
E) I: diene II: alkene III: alkyne

A) I: diene II: alkyne III: alkene
B) I: alkene II: alkane III: alkyne
C) I: alkyne II: alkene III: alkyne
D) I: alkene II: diene III: alkyne
E) I: diene II: alkene III: alkyne

Unlock Deck
Unlock for access to all 69 flashcards in this deck.
Unlock Deck
k this deck
37
Alcohols are classified as primary, secondary, or tertiary alcohols.In order to determine how to classify an alcohol, the number of carbon groups attached to a particular atom in the molecule is counted.Which atom in the molecule below is it? 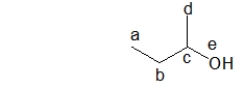
A) atom a
B) atom b
C) atom c
D) atom d
E) atom e

A) atom a
B) atom b
C) atom c
D) atom d
E) atom e

Unlock Deck
Unlock for access to all 69 flashcards in this deck.
Unlock Deck
k this deck
38
Which of these molecules is the LEAST soluble in water? 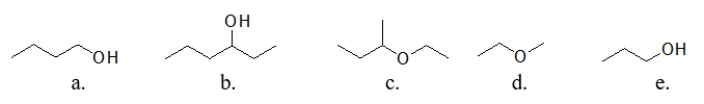
A) molecule a
B) molecule b
C) molecule c
D) molecule d
E) molecule e

A) molecule a
B) molecule b
C) molecule c
D) molecule d
E) molecule e

Unlock Deck
Unlock for access to all 69 flashcards in this deck.
Unlock Deck
k this deck
39
Which of the following statements does NOT describe functional groups?
A) A functional group must contain a heteroatom.
B) Functional groups are groups of atoms that have characteristic reactivity.
C) Functional groups are groups of atoms that have characteristic physical properties.
D) Functional groups are characteristic arrangements of atoms and bonds.
E) Carbon-carbon single bonds are not normally considered functional groups.
A) A functional group must contain a heteroatom.
B) Functional groups are groups of atoms that have characteristic reactivity.
C) Functional groups are groups of atoms that have characteristic physical properties.
D) Functional groups are characteristic arrangements of atoms and bonds.
E) Carbon-carbon single bonds are not normally considered functional groups.

Unlock Deck
Unlock for access to all 69 flashcards in this deck.
Unlock Deck
k this deck
40
Which statement BEST describes why ethers have lower boiling points than alcohols of the same molecular weight?
A) Ethers cannot hydrogen bond to one another.
B) Alcohols cannot hydrogen bond to one another.
C) Ethers can hydrogen bond with water.
D) Alcohols can hydrogen bond with water.
E) Ethers cannot hydrogen bond with water.
A) Ethers cannot hydrogen bond to one another.
B) Alcohols cannot hydrogen bond to one another.
C) Ethers can hydrogen bond with water.
D) Alcohols can hydrogen bond with water.
E) Ethers cannot hydrogen bond with water.

Unlock Deck
Unlock for access to all 69 flashcards in this deck.
Unlock Deck
k this deck
41
Which of the following statements does NOT describe how functional groups are used in organic chemistry?
A) Functional groups are used to organize and classify organic molecules.
B) Functional groups are used to predict reactivity of molecules.
C) Functional groups are used to predict physical properties of molecules.
D) Functional groups are used in naming organic molecules.
E) Functional groups are used to determine the natural abundance of a molecule.
A) Functional groups are used to organize and classify organic molecules.
B) Functional groups are used to predict reactivity of molecules.
C) Functional groups are used to predict physical properties of molecules.
D) Functional groups are used in naming organic molecules.
E) Functional groups are used to determine the natural abundance of a molecule.

Unlock Deck
Unlock for access to all 69 flashcards in this deck.
Unlock Deck
k this deck
42
Select the molecule containing a thiol functional group. 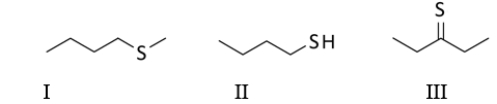
A) All of these molecules contain a thiol.
B) I only
C) II only
D) III only
E) Both I and II contain a thiol.

A) All of these molecules contain a thiol.
B) I only
C) II only
D) III only
E) Both I and II contain a thiol.

Unlock Deck
Unlock for access to all 69 flashcards in this deck.
Unlock Deck
k this deck
43
Which of the following alcohols are found in alcoholic beverages such as wine and beer?
A) methanol
B) ethanol
C) propanol
D) isopropanol
E) tert-butanol
A) methanol
B) ethanol
C) propanol
D) isopropanol
E) tert-butanol

Unlock Deck
Unlock for access to all 69 flashcards in this deck.
Unlock Deck
k this deck
44
Which of the following molecules contain(s)a nonpolar functional group? 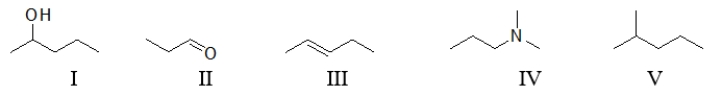
A) All of these molecules contain nonpolar functional groups.
B) All of these molecules except V contain a nonpolar functional group.
C) All of these molecules except III and V contain a polar functional group.
D) Only III and V contain a nonpolar functional group.
E) Only III contains a nonpolar functional group.

A) All of these molecules contain nonpolar functional groups.
B) All of these molecules except V contain a nonpolar functional group.
C) All of these molecules except III and V contain a polar functional group.
D) Only III and V contain a nonpolar functional group.
E) Only III contains a nonpolar functional group.

Unlock Deck
Unlock for access to all 69 flashcards in this deck.
Unlock Deck
k this deck
45
Which of the molecules contain(s)a hydroxyl group? 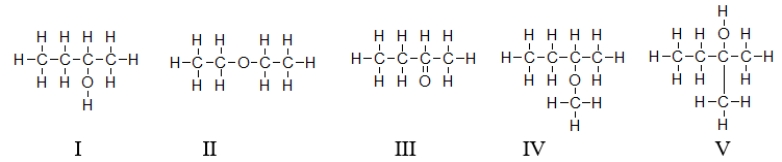
A) All of these molecules contain a hydroxyl group.
B) Only molecule I contains a hydroxyl group.
C) Molecules II and IV contain a hydroxyl group.
D) Molecules I and V contain a hydroxyl group.
E) All molecules except molecule III contain hydroxyl groups.
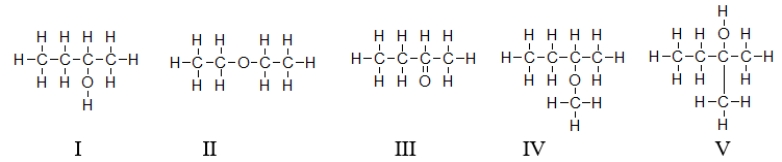
A) All of these molecules contain a hydroxyl group.
B) Only molecule I contains a hydroxyl group.
C) Molecules II and IV contain a hydroxyl group.
D) Molecules I and V contain a hydroxyl group.
E) All molecules except molecule III contain hydroxyl groups.

Unlock Deck
Unlock for access to all 69 flashcards in this deck.
Unlock Deck
k this deck
46
Thiol groups are ____________ and therefore ___________ in water.
A) polar; soluble
B) polar; insoluble
C) nonpolar; soluble
D) nonpolar; insoluble
E) ionic; soluble
A) polar; soluble
B) polar; insoluble
C) nonpolar; soluble
D) nonpolar; insoluble
E) ionic; soluble

Unlock Deck
Unlock for access to all 69 flashcards in this deck.
Unlock Deck
k this deck
47
Which of the molecules contain(s)an ether functional group? 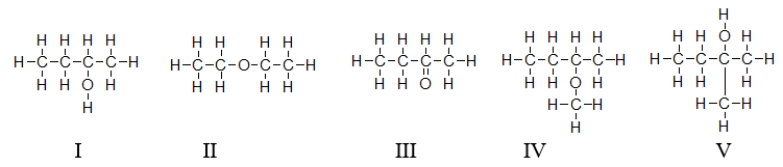
A) All of these molecules are ethers.
B) Only II is an ether.
C) Molecules II and IV are ethers.
D) Molecules I and V are ethers.
E) All molecules except molecule III are ethers.
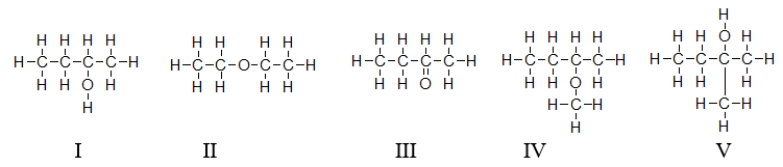
A) All of these molecules are ethers.
B) Only II is an ether.
C) Molecules II and IV are ethers.
D) Molecules I and V are ethers.
E) All molecules except molecule III are ethers.

Unlock Deck
Unlock for access to all 69 flashcards in this deck.
Unlock Deck
k this deck
48
Which of the following molecules contain(s)an alcohol? 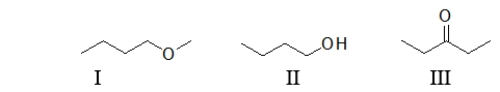
A) All of these molecules contain an alcohol.
B) I only
C) II only
D) III only
E) Both I and II contain an alcohol.

A) All of these molecules contain an alcohol.
B) I only
C) II only
D) III only
E) Both I and II contain an alcohol.

Unlock Deck
Unlock for access to all 69 flashcards in this deck.
Unlock Deck
k this deck
49
Which of the following compounds contains a heteroatom? 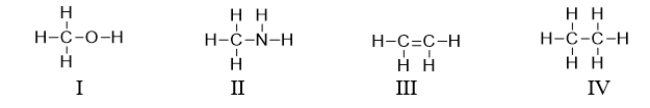
A) All of these compounds contain a heteroatom.
B) I and II contain a heteroatom.
C) III and IV contain a heteroatom.
D) Only III contains a heteroatom.
E) I, II, and III contain a heteroatom.
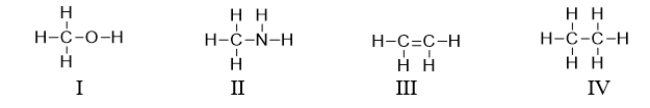
A) All of these compounds contain a heteroatom.
B) I and II contain a heteroatom.
C) III and IV contain a heteroatom.
D) Only III contains a heteroatom.
E) I, II, and III contain a heteroatom.

Unlock Deck
Unlock for access to all 69 flashcards in this deck.
Unlock Deck
k this deck
50
Amines are _____________ because they react with water to ___________ a proton.
A) acids; donate
B) bases; donate
C) acids; accept
D) bases; accept
E) None of the above is correct.
A) acids; donate
B) bases; donate
C) acids; accept
D) bases; accept
E) None of the above is correct.

Unlock Deck
Unlock for access to all 69 flashcards in this deck.
Unlock Deck
k this deck
51
Adrenaline is responsible for the body's "fight or flight" response.Taking amphetamines results in a similar response.Which statement BEST explains this observation? 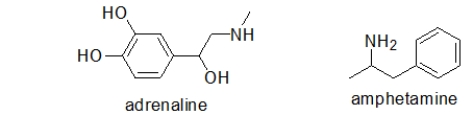
A) Adrenaline and amphetamine are both metabolized in the same way because they have similar structures.
B) Adrenaline and amphetamine both bind to the same receptor because they have similar structures.
C) Amphetamine replaces adrenaline in important biosynthetic pathways.
D) There are two different pathways for the "fight or flight" response.Adrenaline activates one, and amphetamine activates the other.
E) No one understands why this occurs.

A) Adrenaline and amphetamine are both metabolized in the same way because they have similar structures.
B) Adrenaline and amphetamine both bind to the same receptor because they have similar structures.
C) Amphetamine replaces adrenaline in important biosynthetic pathways.
D) There are two different pathways for the "fight or flight" response.Adrenaline activates one, and amphetamine activates the other.
E) No one understands why this occurs.

Unlock Deck
Unlock for access to all 69 flashcards in this deck.
Unlock Deck
k this deck
52
Which of the following molecules contain(s)a primary alcohol? 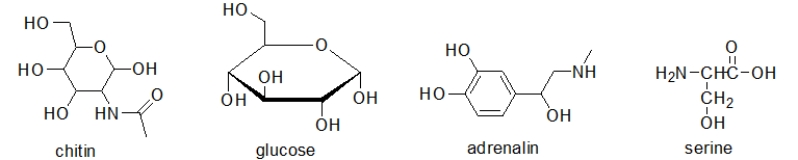
A) chitin and glucose
B) glucose and adrenalin
C) adrenalin and serine
D) chitin, glucose, and serine
E) All of these molecules contain a primary alcohol.

A) chitin and glucose
B) glucose and adrenalin
C) adrenalin and serine
D) chitin, glucose, and serine
E) All of these molecules contain a primary alcohol.

Unlock Deck
Unlock for access to all 69 flashcards in this deck.
Unlock Deck
k this deck
53
What characteristics of an alcohol must be considered to determine whether or not the alcohol is soluble in water?
A) the molecular weight of the alcohol
B) the number of carbons in the alcohol
C) the number of hydroxyl groups in the molecule
D) the degree of branching of the molecule
E) both the number of carbons and the number of hydroxyl groups in the molecule
A) the molecular weight of the alcohol
B) the number of carbons in the alcohol
C) the number of hydroxyl groups in the molecule
D) the degree of branching of the molecule
E) both the number of carbons and the number of hydroxyl groups in the molecule

Unlock Deck
Unlock for access to all 69 flashcards in this deck.
Unlock Deck
k this deck
54
Which of the following are examples of phenols?
A) vanilla
B) antioxidants
C) disinfectants
D) vitamin E
E) All of the above are examples of phenols.
A) vanilla
B) antioxidants
C) disinfectants
D) vitamin E
E) All of the above are examples of phenols.

Unlock Deck
Unlock for access to all 69 flashcards in this deck.
Unlock Deck
k this deck
55
What is the IUPAC name for this compound? 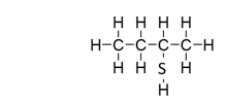
A) butanethiol
B) 2-thiobutane
C) 2-butanethiol
D) 2-propanethiol
E) 2-pentanethiol

A) butanethiol
B) 2-thiobutane
C) 2-butanethiol
D) 2-propanethiol
E) 2-pentanethiol

Unlock Deck
Unlock for access to all 69 flashcards in this deck.
Unlock Deck
k this deck
56
Physiologically active nitrogen compounds that are derived from plants are called
A) antacids.
B) acids.
C) amides.
D) alkaloids.
E) steroids.
A) antacids.
B) acids.
C) amides.
D) alkaloids.
E) steroids.

Unlock Deck
Unlock for access to all 69 flashcards in this deck.
Unlock Deck
k this deck
57
What is the IUPAC name for this compound? 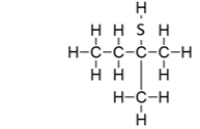
A) methylbutanethiol
B) methyl2-thiobutane
C) 2-methylbutanethiol
D) 2-methyl-2-butanethiol
E) methyl-2-pentanethiol

A) methylbutanethiol
B) methyl2-thiobutane
C) 2-methylbutanethiol
D) 2-methyl-2-butanethiol
E) methyl-2-pentanethiol

Unlock Deck
Unlock for access to all 69 flashcards in this deck.
Unlock Deck
k this deck
58
What is the IUPAC name of the following molecule? 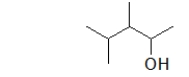
A) 1,2,3,3-tetramethyl-1-propanol
B) 1,2,3-trimethyl-1-butanol
C) 2,3,4-trimethyl-1-butanol
D) 3,4-dimethyl-2-pentanol
E) 2,3-dimethyl-2-penanol

A) 1,2,3,3-tetramethyl-1-propanol
B) 1,2,3-trimethyl-1-butanol
C) 2,3,4-trimethyl-1-butanol
D) 3,4-dimethyl-2-pentanol
E) 2,3-dimethyl-2-penanol

Unlock Deck
Unlock for access to all 69 flashcards in this deck.
Unlock Deck
k this deck
59
When drawing skeletal line structures of compounds involving heteroatoms, you must
A) show the heteroatoms and bonds between heteroatoms and H.
B) show heteroatoms but omit between heteroatoms and H.
C) show all atoms within a functional group.
D) just show the heteroatom.
E) None of the above is correct.
A) show the heteroatoms and bonds between heteroatoms and H.
B) show heteroatoms but omit between heteroatoms and H.
C) show all atoms within a functional group.
D) just show the heteroatom.
E) None of the above is correct.

Unlock Deck
Unlock for access to all 69 flashcards in this deck.
Unlock Deck
k this deck
60
What is the molecular shape of the amine nitrogen in adrenaline? 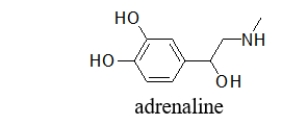
A) linear
B) bent
C) trigonal planar
D) trigonal pyramidal
E) tetrahedral

A) linear
B) bent
C) trigonal planar
D) trigonal pyramidal
E) tetrahedral

Unlock Deck
Unlock for access to all 69 flashcards in this deck.
Unlock Deck
k this deck
61
Which of the following molecules contain(s)an ether functional group? 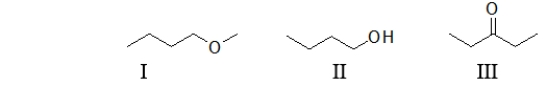
A) All of these molecules contain an ether.
B) I only
C) II only
D) III only
E) Both I and II contain an ether.

A) All of these molecules contain an ether.
B) I only
C) II only
D) III only
E) Both I and II contain an ether.

Unlock Deck
Unlock for access to all 69 flashcards in this deck.
Unlock Deck
k this deck
62
Which of the following elements is NOT a heteroatom?
A) carbon
B) sulfur
C) nitrogen
D) oxygen
E) phosphorous
A) carbon
B) sulfur
C) nitrogen
D) oxygen
E) phosphorous

Unlock Deck
Unlock for access to all 69 flashcards in this deck.
Unlock Deck
k this deck
63
What is the role of dopamine in the body?
A) It is a neurotransmitter.
B) It is a protein.
C) It is the energy currency of the cell.
D) It is part of our genetic material.
E) It is part of the cell membrane.
A) It is a neurotransmitter.
B) It is a protein.
C) It is the energy currency of the cell.
D) It is part of our genetic material.
E) It is part of the cell membrane.

Unlock Deck
Unlock for access to all 69 flashcards in this deck.
Unlock Deck
k this deck
64
Which of the following structures is 3-methylphenol? 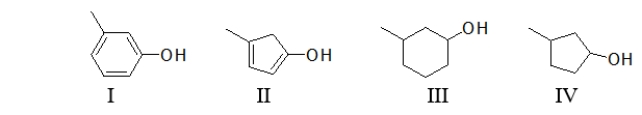
A) All of these structures are 3-methylphenol.
B) Both I and III are 3-methylphenol.
C) Both II and IV are 3-methylphenol.
D) Only I is 3-methylphenol.
E) Only III is 3-methylphenol.

A) All of these structures are 3-methylphenol.
B) Both I and III are 3-methylphenol.
C) Both II and IV are 3-methylphenol.
D) Only I is 3-methylphenol.
E) Only III is 3-methylphenol.

Unlock Deck
Unlock for access to all 69 flashcards in this deck.
Unlock Deck
k this deck
65
Which of the following organic molecules contains at least one functional group? 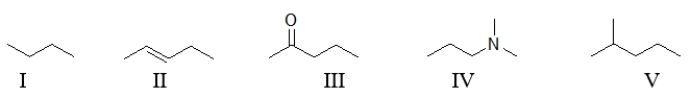
A) All of these molecules contain at least one functional group.
B) All of these molecules except I contain at least one functional group.
C) Molecules II, III, and IV contain at least one functional group.
D) Only molecules III and IV contain a functional group.
E) None of these molecules contain a functional group.

A) All of these molecules contain at least one functional group.
B) All of these molecules except I contain at least one functional group.
C) Molecules II, III, and IV contain at least one functional group.
D) Only molecules III and IV contain a functional group.
E) None of these molecules contain a functional group.

Unlock Deck
Unlock for access to all 69 flashcards in this deck.
Unlock Deck
k this deck
66
What is the IUPAC name of this molecule? 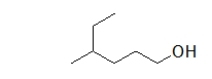
A) 2-ethyl-5-pentanol
B) 4-ethyl-1-pentanol
C) 4-methyl-1-hexanol
D) 3-methyl-6-hexanol
E) 5-methyl-1-hexanol

A) 2-ethyl-5-pentanol
B) 4-ethyl-1-pentanol
C) 4-methyl-1-hexanol
D) 3-methyl-6-hexanol
E) 5-methyl-1-hexanol

Unlock Deck
Unlock for access to all 69 flashcards in this deck.
Unlock Deck
k this deck
67
The following molecule is a _____ amine. 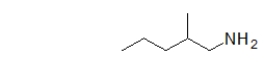
A) primary
B) secondary
C) tertiary
D) quaternary
E) This molecule is not an amine.

A) primary
B) secondary
C) tertiary
D) quaternary
E) This molecule is not an amine.

Unlock Deck
Unlock for access to all 69 flashcards in this deck.
Unlock Deck
k this deck
68
Which illustration BEST describes the polarity of methylamine? 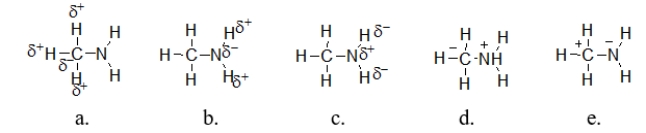
A) illustration a
B) illustration b
C) illustration c
D) illustration d
E) illustration e
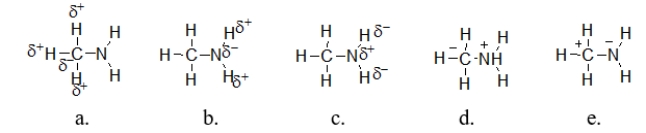
A) illustration a
B) illustration b
C) illustration c
D) illustration d
E) illustration e

Unlock Deck
Unlock for access to all 69 flashcards in this deck.
Unlock Deck
k this deck
69
Which statement BEST describes the role of a neurotransmitter in the body?
A) Neurotransmitters facilitate the transfer of a signal from one neuron to the next, across the synaptic cleft.
B) Neurotransmitters facilitate the passage of an electrical impulse through a neuron.
C) Neurotransmitters facilitate the passage of an electrical impulse through an axon.
D) Neurotransmitters initiate an electrical response to a stimulus.
E) Neurotransmitters block all electrical impulses through neurons.
A) Neurotransmitters facilitate the transfer of a signal from one neuron to the next, across the synaptic cleft.
B) Neurotransmitters facilitate the passage of an electrical impulse through a neuron.
C) Neurotransmitters facilitate the passage of an electrical impulse through an axon.
D) Neurotransmitters initiate an electrical response to a stimulus.
E) Neurotransmitters block all electrical impulses through neurons.

Unlock Deck
Unlock for access to all 69 flashcards in this deck.
Unlock Deck
k this deck


