Deck 20: The Micromacro Connection
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/41
Play
Full screen (f)
Deck 20: The Micromacro Connection
1
A container is filled with a mixture of helium (light molecules) and oxygen (heavy molecules) gases. A thermometer in the container reads 22°C. Which gas molecules have the greater average kinetic energy?
A) It is the same for both of the gases because the temperatures are the same.
B) The oxygen molecules do because they are diatomic.
C) The oxygen molecules do because they are more massive.
D) The helium molecules do because they are less massive.
E) The helium molecules do because they are monatomic.
A) It is the same for both of the gases because the temperatures are the same.
B) The oxygen molecules do because they are diatomic.
C) The oxygen molecules do because they are more massive.
D) The helium molecules do because they are less massive.
E) The helium molecules do because they are monatomic.
It is the same for both of the gases because the temperatures are the same.
2
As a result of any natural process, the total entropy of any system plus that of its environment
A) never decreases.
B) sometimes decreases.
C) never increases.
D) always stays the same.
A) never decreases.
B) sometimes decreases.
C) never increases.
D) always stays the same.
never decreases.
3
A sample of an ideal gas is slowly compressed to one-half its original volume with no change in temperature. What happens to the average speed of the molecules in the sample?
A) It does not change.
B) It becomes 4 times as great.
C) It becomes 2 times as great.
D) It becomes 1/2 as great.
E) It becomes 1/4 as great.
A) It does not change.
B) It becomes 4 times as great.
C) It becomes 2 times as great.
D) It becomes 1/2 as great.
E) It becomes 1/4 as great.
It does not change.
4
A sample of an ideal gas is slowly compressed to one-half its original volume with no change in pressure. If the original root-mean-square speed (thermal speed) of the gas molecules was V, the new speed is
A) V.
B) 2V.
C)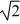 V.
V.
D) V/2.
E) V/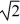
A) V.
B) 2V.
C)
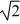 V.
V.D) V/2.
E) V/
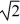

Unlock Deck
Unlock for access to all 41 flashcards in this deck.
Unlock Deck
k this deck
5
The entropy of an isolated system must be conserved, so it never changes.
A) True
B) False
A) True
B) False

Unlock Deck
Unlock for access to all 41 flashcards in this deck.
Unlock Deck
k this deck
6
If the temperature of a fixed amount of an ideal gas is increased, it NECESSARILY follows that
A) the pressure of the gas will increase.
B) the volume of the gas will increase.
C) the speed of the gas molecules will increase.
D) All of the above statements are correct.
A) the pressure of the gas will increase.
B) the volume of the gas will increase.
C) the speed of the gas molecules will increase.
D) All of the above statements are correct.

Unlock Deck
Unlock for access to all 41 flashcards in this deck.
Unlock Deck
k this deck
7
A container is filled with a mixture of helium (light molecules) and oxygen (heavy molecules) gases. A thermometer in the container reads 22°C. Which gas molecules have the greater average speed?
A) It is the same for both of the gases because the temperatures are the same.
B) The oxygen molecules do because they are diatomic.
C) The oxygen molecules do because they are more massive.
D) The helium molecules do because they are less massive.
E) The helium molecules do because they are monatomic.
A) It is the same for both of the gases because the temperatures are the same.
B) The oxygen molecules do because they are diatomic.
C) The oxygen molecules do because they are more massive.
D) The helium molecules do because they are less massive.
E) The helium molecules do because they are monatomic.

Unlock Deck
Unlock for access to all 41 flashcards in this deck.
Unlock Deck
k this deck
8
An ice cube at 0°C is placed in a very large bathtub filled with water at 30°C and allowed to melt, causing no appreciable change in the temperature of the bath water. Which one of the following statements is true?
A) The entropy gained by the ice cube is equal to the entropy lost by the water.
B) The entropy lost by the ice cube is equal to the entropy gained by the water.
C) The net entropy change of the system (ice plus water) is zero because no heat was added to the system.
D) The entropy of the system (ice plus water) increases because the process is irreversible.
E) The entropy of the water does not change because its temperature did not change.
A) The entropy gained by the ice cube is equal to the entropy lost by the water.
B) The entropy lost by the ice cube is equal to the entropy gained by the water.
C) The net entropy change of the system (ice plus water) is zero because no heat was added to the system.
D) The entropy of the system (ice plus water) increases because the process is irreversible.
E) The entropy of the water does not change because its temperature did not change.

Unlock Deck
Unlock for access to all 41 flashcards in this deck.
Unlock Deck
k this deck
9
The root-mean-square speed (thermal speed) for a certain gas at 100°C is 0.500 km/s. If the temperature of the gas is now increased to 200°C, the root-mean-square (thermal) speed will be closest to
A) 563 m/s.
B) 634 m/s.
C) 707 m/s.
D) 804 m/s.
E) 1000 m/s.
A) 563 m/s.
B) 634 m/s.
C) 707 m/s.
D) 804 m/s.
E) 1000 m/s.

Unlock Deck
Unlock for access to all 41 flashcards in this deck.
Unlock Deck
k this deck
10
A 5.0-liter gas tank holds 1.4 moles of helium (He) and 0.70 moles of oxygen (O2), at a temperature of 260 K. The atomic masses of helium and oxygen are 4.0 g/mol and 16.0 g/mol, respectively. Avogadro's number is 6.022 × 1023 molecules/mol and the Boltzmann constant is 1.38 × 10-23 J/K. The total random translational kinetic energy of the gas in the tank is closest to
A) 6.8 kJ.
B) 6.1 kJ.
C) 7.6 kJ.
D) 8.3 kJ.
E) 9.1 kJ.
A) 6.8 kJ.
B) 6.1 kJ.
C) 7.6 kJ.
D) 8.3 kJ.
E) 9.1 kJ.

Unlock Deck
Unlock for access to all 41 flashcards in this deck.
Unlock Deck
k this deck
11
According to the second law of thermodynamics, the entropy of any system always increases.
A) True
B) False
A) True
B) False

Unlock Deck
Unlock for access to all 41 flashcards in this deck.
Unlock Deck
k this deck
12
A 5.0-liter gas tank holds 1.7 moles of monatomic helium (He) and 1.10 mole of diatomic oxygen (O2), at a temperature of 260 K. The ATOMIC masses of helium and oxygen are 4.0 g/mol and 16.0 g/mol, respectively. What is the ratio of the root-mean-square (thermal) speed of helium to that of oxygen?
A) 1.4
B) 2.0
C) 2.8
D) 4.0
E) 5.6
A) 1.4
B) 2.0
C) 2.8
D) 4.0
E) 5.6

Unlock Deck
Unlock for access to all 41 flashcards in this deck.
Unlock Deck
k this deck
13
A hot piece of iron is thrown into the ocean and its temperature eventually stabilizes. Which of the following statements concerning this process is correct? (There may be more than one correct choice.)
A) The entropy lost by the iron is equal to the entropy gained by the ocean.
B) The entropy gained by the iron is equal to the entropy lost by the ocean.
C) The change in the entropy of the iron-ocean system is zero.
D) The ocean gains more entropy than the iron loses.
E) The ocean gains less entropy than the iron loses.
A) The entropy lost by the iron is equal to the entropy gained by the ocean.
B) The entropy gained by the iron is equal to the entropy lost by the ocean.
C) The change in the entropy of the iron-ocean system is zero.
D) The ocean gains more entropy than the iron loses.
E) The ocean gains less entropy than the iron loses.

Unlock Deck
Unlock for access to all 41 flashcards in this deck.
Unlock Deck
k this deck
14
What is the total translational kinetic energy in a test chamber filled with nitrogen (N2) at 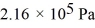 and
and 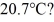 The dimensions of the chamber are
The dimensions of the chamber are 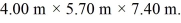 The ATOMIC weight of nitrogen is 28.0 g/mol, Avogadro's number is 6.022 × 1023 molecules/mol and the Boltzmann constant is 1.38 × 10-23 J/K.
The ATOMIC weight of nitrogen is 28.0 g/mol, Avogadro's number is 6.022 × 1023 molecules/mol and the Boltzmann constant is 1.38 × 10-23 J/K.
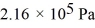 and
and 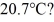 The dimensions of the chamber are
The dimensions of the chamber are 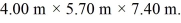 The ATOMIC weight of nitrogen is 28.0 g/mol, Avogadro's number is 6.022 × 1023 molecules/mol and the Boltzmann constant is 1.38 × 10-23 J/K.
The ATOMIC weight of nitrogen is 28.0 g/mol, Avogadro's number is 6.022 × 1023 molecules/mol and the Boltzmann constant is 1.38 × 10-23 J/K.
Unlock Deck
Unlock for access to all 41 flashcards in this deck.
Unlock Deck
k this deck
15
An ideal gas is kept in a rigid container. When its temperature is 100 K, the mean-free-path of the gas molecules is λ. What will be the mean-free-path of the molecules at 400 K?
A) 4λ
B) 2λ
C) λ
D) λ/2
E) λ/4
A) 4λ
B) 2λ
C) λ
D) λ/2
E) λ/4

Unlock Deck
Unlock for access to all 41 flashcards in this deck.
Unlock Deck
k this deck
16
An ideal gas is kept in a rigid container that expands negligibly when heated. The gas starts at a temperature of 20.0°C, and heat is added to increase its temperature. At what temperature will its root-mean-square speed (thermal speed) be double its value at 20.0°C?
A) 40.0°C
B) 141°C
C) 313°C
D) 400°C
E) 899°C
A) 40.0°C
B) 141°C
C) 313°C
D) 400°C
E) 899°C

Unlock Deck
Unlock for access to all 41 flashcards in this deck.
Unlock Deck
k this deck
17
The second law of thermodynamics leads us to conclude that
A) the total energy of the universe is constant.
B) disorder in the universe is increasing with the passage of time.
C) it is theoretically impossible to convert work into heat with 100% efficiency.
D) the total energy in the universe is increasing with time.
E) the total energy in the universe is decreasing with time.
A) the total energy of the universe is constant.
B) disorder in the universe is increasing with the passage of time.
C) it is theoretically impossible to convert work into heat with 100% efficiency.
D) the total energy in the universe is increasing with time.
E) the total energy in the universe is decreasing with time.

Unlock Deck
Unlock for access to all 41 flashcards in this deck.
Unlock Deck
k this deck
18
A mole of oxygen (O2) molecules and a mole of carbon dioxide (CO2) molecules at the same temperature and pressure have
A) the same average molecular speeds.
B) the same number of atoms.
C) different average kinetic energy per molecule.
D) the same number of molecules.
E) different volumes.
A) the same average molecular speeds.
B) the same number of atoms.
C) different average kinetic energy per molecule.
D) the same number of molecules.
E) different volumes.

Unlock Deck
Unlock for access to all 41 flashcards in this deck.
Unlock Deck
k this deck
19
If we double the root-mean-square speed (thermal speed) of the molecules of a gas, then
A) its temperature must increase by a factor of 4.
B) its temperature must increase by a factor of 2.
C) its temperature must increase by a factor of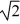 .
.
D) its pressure must increase by a factor of 2.
E) its pressure must increase by a factor of 4.
A) its temperature must increase by a factor of 4.
B) its temperature must increase by a factor of 2.
C) its temperature must increase by a factor of
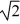 .
.D) its pressure must increase by a factor of 2.
E) its pressure must increase by a factor of 4.

Unlock Deck
Unlock for access to all 41 flashcards in this deck.
Unlock Deck
k this deck
20
The average molecular kinetic energy of a gas can be determined by knowing
A) only the number of molecules in the gas.
B) only the volume of the gas.
C) only the pressure of the gas.
D) only the temperature of the gas.
E) All of the above quantities must be known to determine the average molecular kinetic energy.
A) only the number of molecules in the gas.
B) only the volume of the gas.
C) only the pressure of the gas.
D) only the temperature of the gas.
E) All of the above quantities must be known to determine the average molecular kinetic energy.

Unlock Deck
Unlock for access to all 41 flashcards in this deck.
Unlock Deck
k this deck
21
At what temperature would the root-mean-square speed (thermal speed) of oxygen molecules be 13.0 m/s? Assume that oxygen approximates an ideal gas. The mass of one O2 molecule is 5.312 × 10-26 kg. The Boltzmann constant is 1.38 × 10-23 J/K.
A) 0.217 K
B) 1800 K
C) 5410 K
D) 0.0666 K
A) 0.217 K
B) 1800 K
C) 5410 K
D) 0.0666 K

Unlock Deck
Unlock for access to all 41 flashcards in this deck.
Unlock Deck
k this deck
22
An oxygen molecule falls in a vacuum. From what height must it fall so that its kinetic energy at the bottom equals the average energy of an oxygen molecule at 800 K? (The Boltzmann constant is 1.38 × 10-23 J/K, the molecular weight of oxygen is 32.0 g/mol, and Avogadro's number is 6.022 × 1023 molecules/mol.)
A) 31.8 km
B) 10.6 km
C) 21.1 km
D) 42.3 km
A) 31.8 km
B) 10.6 km
C) 21.1 km
D) 42.3 km

Unlock Deck
Unlock for access to all 41 flashcards in this deck.
Unlock Deck
k this deck
23
Eleven molecules have speeds 16, 17, 18, . . . , 26 m/s. Calculate the root-mean-square of this group of molecules.
A) 21.2 m/s
B) 21.0 m/s
C) 21.5 m/s
D) 21.7 m/s
A) 21.2 m/s
B) 21.0 m/s
C) 21.5 m/s
D) 21.7 m/s

Unlock Deck
Unlock for access to all 41 flashcards in this deck.
Unlock Deck
k this deck
24
The root-mean-square speed (thermal speed) of the molecules of a gas is 200 m/s at a temperature 23.0°C. What is the mass of the individual molecules? The Boltzmann constant is 1.38 × 10-23 J/K.
A) 2.13 × 10-25 kg
B) 2.45 × 10-25 kg
C) 5.66 × 10-25 kg
D) 1.78 × 10-25 kg
E) 3.11 × 10-25 kg
A) 2.13 × 10-25 kg
B) 2.45 × 10-25 kg
C) 5.66 × 10-25 kg
D) 1.78 × 10-25 kg
E) 3.11 × 10-25 kg

Unlock Deck
Unlock for access to all 41 flashcards in this deck.
Unlock Deck
k this deck
25
What is the average kinetic energy of an ideal gas molecule at 569°C? (The Boltzmann constant is 1.38 × 10-23 J/K.)
A) 1.74 × 10-20 J
B) 5.81 × 10-21 J
C) 1.18 × 10-17 J
D) 3.93 × 10-19 J
A) 1.74 × 10-20 J
B) 5.81 × 10-21 J
C) 1.18 × 10-17 J
D) 3.93 × 10-19 J

Unlock Deck
Unlock for access to all 41 flashcards in this deck.
Unlock Deck
k this deck
26
Assuming the radius of diatomic molecules is approximately 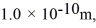 for what pressure will the mean free path in room-temperature (20°C) nitrogen be 4.6 m? The Boltzmann constant is 1.38 × 10-23 J/K, Avogadro's number is 6.02 × 1023 molecules/mole, and the ideal gas constant is
for what pressure will the mean free path in room-temperature (20°C) nitrogen be 4.6 m? The Boltzmann constant is 1.38 × 10-23 J/K, Avogadro's number is 6.02 × 1023 molecules/mole, and the ideal gas constant is 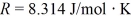 = 0.0821 L ∙ atm/mol ∙ K.
= 0.0821 L ∙ atm/mol ∙ K.
A) 4.9 × 10-8 atm
B) 6.9 × 10-8 atm
C) 1.5 × 10-7 atm
D) 2.2 × 10-7 atm
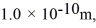 for what pressure will the mean free path in room-temperature (20°C) nitrogen be 4.6 m? The Boltzmann constant is 1.38 × 10-23 J/K, Avogadro's number is 6.02 × 1023 molecules/mole, and the ideal gas constant is
for what pressure will the mean free path in room-temperature (20°C) nitrogen be 4.6 m? The Boltzmann constant is 1.38 × 10-23 J/K, Avogadro's number is 6.02 × 1023 molecules/mole, and the ideal gas constant is 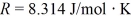 = 0.0821 L ∙ atm/mol ∙ K.
= 0.0821 L ∙ atm/mol ∙ K.A) 4.9 × 10-8 atm
B) 6.9 × 10-8 atm
C) 1.5 × 10-7 atm
D) 2.2 × 10-7 atm

Unlock Deck
Unlock for access to all 41 flashcards in this deck.
Unlock Deck
k this deck
27
Dust particles are pulverized rock, which has density 2500 kg/m3. They are approximately spheres 20 μm in diameter. Treating dust as an ideal gas, what is the root-mean-square speed (thermal speed) of a dust particle at 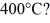 (The Boltzmann constant is 1.38 × 10-23 J/K.)
(The Boltzmann constant is 1.38 × 10-23 J/K.)
A) 5.2 × 10-5 m/s
B) 1.7 × 10-5 m/s
C) 3.0 × 10-5 m/s
D) 7.3 × 10-5 m/s
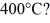 (The Boltzmann constant is 1.38 × 10-23 J/K.)
(The Boltzmann constant is 1.38 × 10-23 J/K.)A) 5.2 × 10-5 m/s
B) 1.7 × 10-5 m/s
C) 3.0 × 10-5 m/s
D) 7.3 × 10-5 m/s

Unlock Deck
Unlock for access to all 41 flashcards in this deck.
Unlock Deck
k this deck
28
A 0.10- 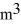 gas tank holds 5.0 moles of nitrogen gas (N2), at a temperature of
gas tank holds 5.0 moles of nitrogen gas (N2), at a temperature of 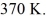 The atomic mass of nitrogen is 14 g/mol, the molecular radius is 3.0 × 10-10 m, and the Boltzmann constant is 1.38 × 10-23 J/K. The root-mean-square speed (thermal speed) of the molecules is closest to
The atomic mass of nitrogen is 14 g/mol, the molecular radius is 3.0 × 10-10 m, and the Boltzmann constant is 1.38 × 10-23 J/K. The root-mean-square speed (thermal speed) of the molecules is closest to
A) 570 m/s.
B) 810 m/s.
C) 410 m/s.
D) 22 m/s.
E) 99 m/s.
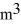 gas tank holds 5.0 moles of nitrogen gas (N2), at a temperature of
gas tank holds 5.0 moles of nitrogen gas (N2), at a temperature of 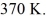 The atomic mass of nitrogen is 14 g/mol, the molecular radius is 3.0 × 10-10 m, and the Boltzmann constant is 1.38 × 10-23 J/K. The root-mean-square speed (thermal speed) of the molecules is closest to
The atomic mass of nitrogen is 14 g/mol, the molecular radius is 3.0 × 10-10 m, and the Boltzmann constant is 1.38 × 10-23 J/K. The root-mean-square speed (thermal speed) of the molecules is closest toA) 570 m/s.
B) 810 m/s.
C) 410 m/s.
D) 22 m/s.
E) 99 m/s.

Unlock Deck
Unlock for access to all 41 flashcards in this deck.
Unlock Deck
k this deck
29
The mean free path of an oxygen molecule is 2.0 × 10-5 m, when the gas is at a pressure of 120 Pa and a temperature of 275 K. The atomic mass of oxygen is 16.0 g/mol, the Boltzmann constant is 1.38 × 10-23 J/K, Avogadro's number is 6.02 × 1023 molecules/mole, and the ideal gas constant is J/mol ∙ K = 0.0821 L ∙ atm/mol ∙ K. The radius of an oxygen molecule is closest to
A) 0.22 nm.
B) 0.24 nm.
C) 0.26 nm.
D) 0.28 nm.
E) 0.30 nm.
A) 0.22 nm.
B) 0.24 nm.
C) 0.26 nm.
D) 0.28 nm.
E) 0.30 nm.

Unlock Deck
Unlock for access to all 41 flashcards in this deck.
Unlock Deck
k this deck
30
A sealed container holds 0.020 moles of nitrogen (N2) gas at a pressure of 1.5 atmospheres and a temperature of 290 K. The atomic mass of nitrogen is 14 g/mol. The Boltzmann constant is 1.38 × 10-23 J/K and the ideal gas constant is R = 8.314 J/mol ∙ K = 0.0821 L ∙ atm/mol ∙ K. The average translational kinetic energy of a nitrogen molecule is closest to
A) 4.0 × 10-21 J.
B) 6.0 × 10-21 J.
C) 8.0 × 10-21 J.
D) 10 × 10-21 J.
E) 12 × 10-21 J.
A) 4.0 × 10-21 J.
B) 6.0 × 10-21 J.
C) 8.0 × 10-21 J.
D) 10 × 10-21 J.
E) 12 × 10-21 J.

Unlock Deck
Unlock for access to all 41 flashcards in this deck.
Unlock Deck
k this deck
31
At 50.0°C, the average translational kinetic energy of a gas molecule is K. If the temperature is now increased to 100.0°C, the average translational kinetic energy of a molecule will be closest to
A) 1.07K.
B) 1.15K.
C) 1.41K.
D) 2.00K.
E) 4.00K.
A) 1.07K.
B) 1.15K.
C) 1.41K.
D) 2.00K.
E) 4.00K.

Unlock Deck
Unlock for access to all 41 flashcards in this deck.
Unlock Deck
k this deck
32
The root-mean-square speed (thermal speed) of the molecules of a gas is 200 m/s at 23.0°C. At 227°C the root-mean-square speed (thermal speed) of the molecules will be closest to
A) 160 m/s.
B) 330 m/s.
C) 260 m/s.
D) 630 m/s.
E) 2000 m/s.
A) 160 m/s.
B) 330 m/s.
C) 260 m/s.
D) 630 m/s.
E) 2000 m/s.

Unlock Deck
Unlock for access to all 41 flashcards in this deck.
Unlock Deck
k this deck
33
What is the average translational kinetic energy per molecule of an ideal gas at a temperature of 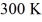 ? The Boltzmann constant is 1.38 × 10-23 J/K.
? The Boltzmann constant is 1.38 × 10-23 J/K.
A) 1.7 × 10-21 J
B) 8.3 × 10-21 J
C) 6.2 × 10-21 J
D) 2.1 × 10-21 J
E) 4.1 × 10-21 J
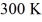 ? The Boltzmann constant is 1.38 × 10-23 J/K.
? The Boltzmann constant is 1.38 × 10-23 J/K.A) 1.7 × 10-21 J
B) 8.3 × 10-21 J
C) 6.2 × 10-21 J
D) 2.1 × 10-21 J
E) 4.1 × 10-21 J

Unlock Deck
Unlock for access to all 41 flashcards in this deck.
Unlock Deck
k this deck
34
What is the mean free path for the molecules in an ideal gas when the pressure is 100 kPa and the temperature is 300 K given that the collision cross-section for the molecules of that gas is 2.0 × 10-20 m2? The Boltzmann constant is 1.38 × 10-23 J/K, Avogadro's number is 6.02 × 1023 molecules/mole, and the ideal gas constant is R = 8.314 J/mol ∙ K =
0)0821 L ∙ atm/mol ∙ K.
A) 1.1 × 10-6 m
B) 2.1 × 10-6 m
C) 1.7 × 10-6 m
D) 5.3 × 10-6 m
E) 1.5 × 10-6 m
0)0821 L ∙ atm/mol ∙ K.
A) 1.1 × 10-6 m
B) 2.1 × 10-6 m
C) 1.7 × 10-6 m
D) 5.3 × 10-6 m
E) 1.5 × 10-6 m

Unlock Deck
Unlock for access to all 41 flashcards in this deck.
Unlock Deck
k this deck
35
On a hot summer day, the temperature is 40.0°C and the pressure is 1.01 × 105 Pa. Let us model the air as all nitrogen of molecular mass 28.0 g/mol having molecules of diameter
0.500 nm that are moving at their root-mean-square speed. Avogadro's number is
6.02 × 1023 molecules/mol, the ideal gas constant is 8.31 J/mol ∙ K, and the Boltzmann constant is 1.38 × 10-23 J/K. Calculate reasonable estimates for
(a) the root-mean-square speed of the nitrogen molecules.
(b) the average distance a typical molecule travels between collisions.
(c) the average time a molecule travels between collisions, assuming that the molecules are moving at their root-mean-square speeds.
(d) the number of collisions an average molecule undergoes per second.
0.500 nm that are moving at their root-mean-square speed. Avogadro's number is
6.02 × 1023 molecules/mol, the ideal gas constant is 8.31 J/mol ∙ K, and the Boltzmann constant is 1.38 × 10-23 J/K. Calculate reasonable estimates for
(a) the root-mean-square speed of the nitrogen molecules.
(b) the average distance a typical molecule travels between collisions.
(c) the average time a molecule travels between collisions, assuming that the molecules are moving at their root-mean-square speeds.
(d) the number of collisions an average molecule undergoes per second.

Unlock Deck
Unlock for access to all 41 flashcards in this deck.
Unlock Deck
k this deck
36
A cubic box with sides of 20.0 cm contains 2.00 × 1023 molecules of helium with a root-mean-square speed (thermal speed) of 200 m/s. The mass of a helium molecule is 3.40 × 10-27 kg. What is the average pressure exerted by the molecules on the walls of the container? The Boltzmann constant is 1.38 × 10-23 J/K and the ideal gas constant is
R = 8.314 J/mol ∙ K = 0.0821 L ∙ atm/mol ∙ K.
A) 3.39 kPa
B) 1.13 kPa
C) 570 Pa
D) 2.26 kPa
E) 9.10 Pa
R = 8.314 J/mol ∙ K = 0.0821 L ∙ atm/mol ∙ K.
A) 3.39 kPa
B) 1.13 kPa
C) 570 Pa
D) 2.26 kPa
E) 9.10 Pa

Unlock Deck
Unlock for access to all 41 flashcards in this deck.
Unlock Deck
k this deck
37
The mean free path of an oxygen molecule is 2.0 × 10-5 m, when the gas is at a pressure of 120 Pa and a temperature of 275 K. The molecular mass of oxygen is 32.0 g/mol and the Boltzmann constant is 1.38 × 10-23 J/K, Avogadro's number is 6.02 × 1023 molecules/mole, and the ideal gas constant is R = 8.314 J/mol ∙ K = 0.0821 L ∙ atm/mol ∙ K. Assuming that the molecules are moving at their root-mean-square speeds, the average time interval between collisions of an oxygen molecule is closest to
A) 20 ns.
B) 43 ns.
C) 95 ns.
D) 200 ns.
E) 420 ns.
A) 20 ns.
B) 43 ns.
C) 95 ns.
D) 200 ns.
E) 420 ns.

Unlock Deck
Unlock for access to all 41 flashcards in this deck.
Unlock Deck
k this deck
38
What is the root-mean-square value of the following velocity components: 2.0 m/s, -3.0 m/s, and 4.0 m/s?
A) 5.4 m/s
B) 1.9 m/s
C) 3.1 m/s
D) 3.3 m/s
E) 1.0 m/s
A) 5.4 m/s
B) 1.9 m/s
C) 3.1 m/s
D) 3.3 m/s
E) 1.0 m/s

Unlock Deck
Unlock for access to all 41 flashcards in this deck.
Unlock Deck
k this deck
39
The root-mean-square speed (thermal speed) of a certain sample of carbon dioxide molecules, with a molecular weight of 44 g/mol, is 396 m/s. What is the root-mean-square speed (thermal speed) of water vapor molecules, with a molecular weight of 18 g/mol, at the same temperature?
A) 253 m/s
B) 396 m/s
C) 421 m/s
D) 506 m/s
E) 619 m/s
A) 253 m/s
B) 396 m/s
C) 421 m/s
D) 506 m/s
E) 619 m/s

Unlock Deck
Unlock for access to all 41 flashcards in this deck.
Unlock Deck
k this deck
40
What is the mean free path of a gas of randomly-moving hard spheres of radius 2.00 × 10-9 m when the density of spheres is 3.00 × 1019 per cubic meter?
A) 1.17 × 10-4 m
B) 4.00 × 10-9 m
C) 9.76 × 10-5 m
D) 1.88 × 10-3 m
E) 4.69 × 10-4 m
A) 1.17 × 10-4 m
B) 4.00 × 10-9 m
C) 9.76 × 10-5 m
D) 1.88 × 10-3 m
E) 4.69 × 10-4 m

Unlock Deck
Unlock for access to all 41 flashcards in this deck.
Unlock Deck
k this deck
41
What is the mean free path of molecules in an ideal gas in which the mean collision time is 3.00 × 10-10 s, the temperature is 300 K, and the mass of the molecules is 6.00 × 10-25 kg? Assume that the molecules are moving at their root-mean-square speeds. The Boltzmann constant is 1.38 × 10-23 J/K.
A) 7.22 × 10-8 m
B) 4.32 × 10-8 m
C) 9.19 × 10-8 m
D) 1.39 × 10-8 m
E) 6.71 × 10-8 m
A) 7.22 × 10-8 m
B) 4.32 × 10-8 m
C) 9.19 × 10-8 m
D) 1.39 × 10-8 m
E) 6.71 × 10-8 m

Unlock Deck
Unlock for access to all 41 flashcards in this deck.
Unlock Deck
k this deck


