Deck 3: Atoms
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/30
Play
Full screen (f)
Deck 3: Atoms
1
Rutherford's gold foil experiment showed that:
A) atoms are neither created nor destroyed.
B) atoms are mostly empty space with a very dense nucleus.
C) atoms contain electrons.
D) atoms can combine to form compounds.
A) atoms are neither created nor destroyed.
B) atoms are mostly empty space with a very dense nucleus.
C) atoms contain electrons.
D) atoms can combine to form compounds.
atoms are mostly empty space with a very dense nucleus.
2
The symbol for iron, as shown on the periodic table, is given here. What is the atomic number of iron? 
A) Fe
B) 26
C) 55.845
D) 19.9845

A) Fe
B) 26
C) 55.845
D) 19.9845
26
3
The law of conservation of _____ tells us that matter is not created or destroyed in a chemical reaction.
A) energy
B) mass
C) atoms
D) compounds
A) energy
B) mass
C) atoms
D) compounds
mass
4
Which subatomic particle has the GREATEST mass?
A) a proton
B) a neutron
C) an electron
D) All of these subatomic particles have equal masses.
A) a proton
B) a neutron
C) an electron
D) All of these subatomic particles have equal masses.

Unlock Deck
Unlock for access to all 30 flashcards in this deck.
Unlock Deck
k this deck
5
Which element is NOT a metal?
A) Li
B) N
C) Sn
D) Co
A) Li
B) N
C) Sn
D) Co

Unlock Deck
Unlock for access to all 30 flashcards in this deck.
Unlock Deck
k this deck
6
Rust has the formula Fe2O3, and it's made from iron metal reacting with oxygen over time. Assume that you had a 2.0-g block of pure iron metal that fully oxidized to rust and the mass of that product was 6.5 g. What must have been the mass of oxygen that initially reacted with pure iron?
A) 2.0 g
B) 6.5 g
C) 4.5 g
D) 4.0 g
A) 2.0 g
B) 6.5 g
C) 4.5 g
D) 4.0 g

Unlock Deck
Unlock for access to all 30 flashcards in this deck.
Unlock Deck
k this deck
7
_____ allows scientists to construct images showing how atoms combine to form molecules.
A) X-ray crystallography
B) Scanning tunneling microscopy
C) Cathode ray tubes
D) Atomic theory
A) X-ray crystallography
B) Scanning tunneling microscopy
C) Cathode ray tubes
D) Atomic theory

Unlock Deck
Unlock for access to all 30 flashcards in this deck.
Unlock Deck
k this deck
8
Which scientist developed the periodic table?
A) Democritus
B) Dalton
C) Thomson
D) Mendeleev
A) Democritus
B) Dalton
C) Thomson
D) Mendeleev

Unlock Deck
Unlock for access to all 30 flashcards in this deck.
Unlock Deck
k this deck
9
F, Cl, and Br all belong to what family?
A) alkali metals
B) transition metals
C) halogens
D) noble gases
A) alkali metals
B) transition metals
C) halogens
D) noble gases

Unlock Deck
Unlock for access to all 30 flashcards in this deck.
Unlock Deck
k this deck
10
Which statement is NOT accurate concerning Dalton's atomic theory?
A) Elements are made of tiny, indivisible particles called atoms.
B) The atoms of each element are unique.
C) Atoms can join together in whole-number ratios to form compounds.
D) Atoms are changed in chemical reactions.
A) Elements are made of tiny, indivisible particles called atoms.
B) The atoms of each element are unique.
C) Atoms can join together in whole-number ratios to form compounds.
D) Atoms are changed in chemical reactions.

Unlock Deck
Unlock for access to all 30 flashcards in this deck.
Unlock Deck
k this deck
11
Which elements are in the same group?
A) K, Ca, and Mg
B) Li, Na, and Cs
C) F, S, and N
D) C, Si, and N
A) K, Ca, and Mg
B) Li, Na, and Cs
C) F, S, and N
D) C, Si, and N

Unlock Deck
Unlock for access to all 30 flashcards in this deck.
Unlock Deck
k this deck
12
Which element is NOT a metal?
A) Mg
B) S
C) Sn
D) Ti
A) Mg
B) S
C) Sn
D) Ti

Unlock Deck
Unlock for access to all 30 flashcards in this deck.
Unlock Deck
k this deck
13
Water has the formula H2O. Water can be made from the reaction of hydrogen and oxygen. If 2.0 g of hydrogen reacts fully with 2.0 g of oxygen, then _____ of water is made.
A) 2.0 g
B) 3.0 g
C) 4.0 g
D) 6.0 g
A) 2.0 g
B) 3.0 g
C) 4.0 g
D) 6.0 g

Unlock Deck
Unlock for access to all 30 flashcards in this deck.
Unlock Deck
k this deck
14
Which pair of atoms has similar chemical properties?
A) Li and Be
B) Li and Na
C) O and F
D) F and He
A) Li and Be
B) Li and Na
C) O and F
D) F and He

Unlock Deck
Unlock for access to all 30 flashcards in this deck.
Unlock Deck
k this deck
15
Which subatomic particle has the LOWEST mass?
A) a proton
B) a neutron
C) an electron
D) All of these subatomic particles have equal masses.
A) a proton
B) a neutron
C) an electron
D) All of these subatomic particles have equal masses.

Unlock Deck
Unlock for access to all 30 flashcards in this deck.
Unlock Deck
k this deck
16
Which subatomic particle will be attracted to a positively charged metal plate?
A) a proton
B) a neutron
C) an electron
D) All of these particles will be attracted to the metal plate.
A) a proton
B) a neutron
C) an electron
D) All of these particles will be attracted to the metal plate.

Unlock Deck
Unlock for access to all 30 flashcards in this deck.
Unlock Deck
k this deck
17
The atomic number tells us:
A) the sum of all the particles in the nucleus.
B) the number of electrons in a neutral atom.
C) the number of protons in a nucleus.
D) both the number of electrons in a neutral atom and the number of protons in a nucleus .
A) the sum of all the particles in the nucleus.
B) the number of electrons in a neutral atom.
C) the number of protons in a nucleus.
D) both the number of electrons in a neutral atom and the number of protons in a nucleus .

Unlock Deck
Unlock for access to all 30 flashcards in this deck.
Unlock Deck
k this deck
18
What two particles reside in the nucleus?
A) protons and electrons
B) electrons and neutrons
C) protons and neutrons
D) futons and protons
A) protons and electrons
B) electrons and neutrons
C) protons and neutrons
D) futons and protons

Unlock Deck
Unlock for access to all 30 flashcards in this deck.
Unlock Deck
k this deck
19
Which subatomic particle will be attracted to a negatively charged metal plate?
A) a proton
B) a neutron
C) an electron
D) All of these particles will be attracted to the metal plate.
A) a proton
B) a neutron
C) an electron
D) All of these particles will be attracted to the metal plate.

Unlock Deck
Unlock for access to all 30 flashcards in this deck.
Unlock Deck
k this deck
20
Which of these symbols represents an atom with 15 protons, 16 neutrons, and 15 electrons?
A)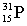
B)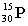
C)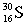
D)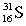
A)
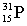
B)
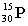
C)
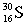
D)
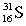

Unlock Deck
Unlock for access to all 30 flashcards in this deck.
Unlock Deck
k this deck
21
A certain element is composed of two isotopes, having the mass and abundance shown in the table. What is the average atomic mass for this element? Mass
Abundance
Isotope 1
6.0 u
7.59%
Isotope 2
7.0 u
92.41%
A) 6.0 u
B) 6.1 u
C) 7.0 u
D) 6.9 u
Abundance
Isotope 1
6.0 u
7.59%
Isotope 2
7.0 u
92.41%
A) 6.0 u
B) 6.1 u
C) 7.0 u
D) 6.9 u

Unlock Deck
Unlock for access to all 30 flashcards in this deck.
Unlock Deck
k this deck
22
Which symbol BEST represents a potassium cation?
A) K-
B) K+
C) K
D) K(cat)
A) K-
B) K+
C) K
D) K(cat)

Unlock Deck
Unlock for access to all 30 flashcards in this deck.
Unlock Deck
k this deck
23
Which scientist proposed that electrons orbit the nucleus like planets orbit the Sun?
A) Dalton
B) Rutherford
C) Bohr
D) Thomson
A) Dalton
B) Rutherford
C) Bohr
D) Thomson

Unlock Deck
Unlock for access to all 30 flashcards in this deck.
Unlock Deck
k this deck
24
Which symbol BEST represents a lithium cation?
A) Li(cat)
B) Li+
C) Li-
D) Li
A) Li(cat)
B) Li+
C) Li-
D) Li

Unlock Deck
Unlock for access to all 30 flashcards in this deck.
Unlock Deck
k this deck
25
Which symbol represents an atom with 8 protons and 10 electrons?
A) Ne-
B) O2-
C) Na+
D) Na-
A) Ne-
B) O2-
C) Na+
D) Na-

Unlock Deck
Unlock for access to all 30 flashcards in this deck.
Unlock Deck
k this deck
26
A certain element is composed of two isotopes, having the mass and abundance shown in the table. What is the average atomic mass for this element? Mass
Abundance
Isotope 1
35.0 u
75.8%
Isotope 2
37.0 u
24.2%
A) 36.0 u
B) 35.5 u
C) 37.0 u
D) 921.9 u
Abundance
Isotope 1
35.0 u
75.8%
Isotope 2
37.0 u
24.2%
A) 36.0 u
B) 35.5 u
C) 37.0 u
D) 921.9 u

Unlock Deck
Unlock for access to all 30 flashcards in this deck.
Unlock Deck
k this deck
27
An atom that loses an electron is:
A) known as a cation.
B) known as an anion.
C) still called an atom.
D) known as an isotope.
A) known as a cation.
B) known as an anion.
C) still called an atom.
D) known as an isotope.

Unlock Deck
Unlock for access to all 30 flashcards in this deck.
Unlock Deck
k this deck
28
An atom that gains an electron is:
A) known as a cation.
B) known as an anion.
C) still called an atom.
D) known as an isotope.
A) known as a cation.
B) known as an anion.
C) still called an atom.
D) known as an isotope.

Unlock Deck
Unlock for access to all 30 flashcards in this deck.
Unlock Deck
k this deck
29
Which symbol represents an atom with 9 protons and 10 electrons?
A) Ne-
B) Na+
C) Ne+
D) F-
A) Ne-
B) Na+
C) Ne+
D) F-

Unlock Deck
Unlock for access to all 30 flashcards in this deck.
Unlock Deck
k this deck
30
An atom has atomic number 92 and mass number 238. Which symbol is an ISOTOPE of this atom?
A)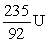
B)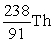
C)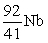
D)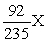
A)
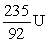
B)
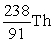
C)
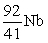
D)
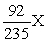

Unlock Deck
Unlock for access to all 30 flashcards in this deck.
Unlock Deck
k this deck


