Deck 7: Atoms and Spectra
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/60
Play
Full screen (f)
Deck 7: Atoms and Spectra
1
A plot of the continuous spectra of five different stars is shown in the figure below.Based on these spectra,which of the stars is the hottest? 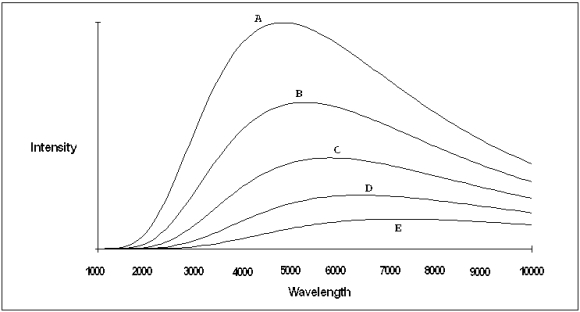
A) Star A
B) Star B
C) Star C
D) Star D
E) Star E

A) Star A
B) Star B
C) Star C
D) Star D
E) Star E
Star A
2
An atom can be ionized by ____ it.
A) removing electrons from
B) adding an electron to
C) removing a proton from
D) Either answer "a" or "b"
A) removing electrons from
B) adding an electron to
C) removing a proton from
D) Either answer "a" or "b"
Either answer "a" or "b"
3
The most massive part of the atom is(are)the ____ which has(have)a ____ charge.
A) electrons,negative
B) nucleus,negative
C) electrons,positive
D) nucleus,positive
A) electrons,negative
B) nucleus,negative
C) electrons,positive
D) nucleus,positive
nucleus,positive
4
The constant in the Stefan-Boltzmann law is equal to 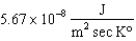 ,so that 5.67*10 - 4 J of energy is radiated each second by one square meter of a star whose temperature is 10,000 K.How much energy is radiated each second by one square meter of a star whose temperature is 5,000 ?
,so that 5.67*10 - 4 J of energy is radiated each second by one square meter of a star whose temperature is 10,000 K.How much energy is radiated each second by one square meter of a star whose temperature is 5,000 ?
A) 5.67*10 - 2 J
B) 5.67*10 - 3 J
C) 5.67*10 - 4 J
D) 1.42*10 - 4 J
E) 1.42*10 - 1 J
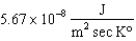 ,so that 5.67*10 - 4 J of energy is radiated each second by one square meter of a star whose temperature is 10,000 K.How much energy is radiated each second by one square meter of a star whose temperature is 5,000 ?
,so that 5.67*10 - 4 J of energy is radiated each second by one square meter of a star whose temperature is 10,000 K.How much energy is radiated each second by one square meter of a star whose temperature is 5,000 ?A) 5.67*10 - 2 J
B) 5.67*10 - 3 J
C) 5.67*10 - 4 J
D) 1.42*10 - 4 J
E) 1.42*10 - 1 J

Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
5
The nucleus is made of ____
A) electrons only.
B) protons only.
C) neutrons only.
D) a and b,usually
E) b and c,usually
A) electrons only.
B) protons only.
C) neutrons only.
D) a and b,usually
E) b and c,usually

Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
6
Absolute zero is
A) zero degrees Celsius.
B) the temperature at which atoms have no remaining energy from which we can extract heat.
C) the temperature at which water freezes.
D) both a and c
E) none of the above
A) zero degrees Celsius.
B) the temperature at which atoms have no remaining energy from which we can extract heat.
C) the temperature at which water freezes.
D) both a and c
E) none of the above

Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
7
NARRBEGIN: Diagram 07-01
Diagram 7-1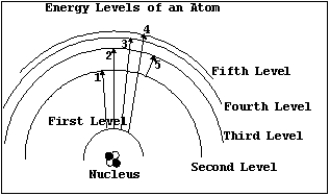 NARREND
NARREND
In Diagram 7-1,which of the transitions would absorb a photon with the shortest wavelength?
A) Transition 1
B) Transition 2
C) Transition 3
D) Transition 4
E) Transition 5
Diagram 7-1
 NARREND
NARRENDIn Diagram 7-1,which of the transitions would absorb a photon with the shortest wavelength?
A) Transition 1
B) Transition 2
C) Transition 3
D) Transition 4
E) Transition 5

Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
8
A(n)____ contains two or more atoms that are bound together by sharing electrons with each other.
A) nucleus
B) ion
C) proton
D) electron cloud
E) molecule
A) nucleus
B) ion
C) proton
D) electron cloud
E) molecule

Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
9
One star has a temperature of 10,000 K and another star has a temperature of 5000 K Compared to the cooler star,how much more energy per second will the hotter star radiate from each square meter of its surface?
A) 16 times
B) 2 times
C) 1*1016 times
D) 625 times
E) 6.3*1014 times
A) 16 times
B) 2 times
C) 1*1016 times
D) 625 times
E) 6.3*1014 times

Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
10
An atom that is excited
A) is also ionized.
B) is an isotope.
C) has had its electron moved to the lowest energy level.
D) can emit a photon when the electron moves to a lower energy level.
E) can emit a photon when the electron moves to a higher energy level.
A) is also ionized.
B) is an isotope.
C) has had its electron moved to the lowest energy level.
D) can emit a photon when the electron moves to a lower energy level.
E) can emit a photon when the electron moves to a higher energy level.

Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
11
The number of ____ in the nucleus determines what element the nucleus is.
A) protons
B) electrons
C) neutrons
D) None of these determines the element
E) All of these determines the element
A) protons
B) electrons
C) neutrons
D) None of these determines the element
E) All of these determines the element

Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
12
The lowest energy level in an atom is
A) the absolute zero temperature.
B) the ground state.
C) the ionization level.
D) responsible for Doppler shifts.
E) the energy level from which the Paschen Series of hydrogen originates.
A) the absolute zero temperature.
B) the ground state.
C) the ionization level.
D) responsible for Doppler shifts.
E) the energy level from which the Paschen Series of hydrogen originates.

Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
13
The sun has a surface temperature of approximately 5,800 K.At what wavelength does the maximum energy radiated by the sun occur?
A) 5,800 nm
B) 300 nm
C) 174 nm
D) 520 nm
E) 3,000 nm
A) 5,800 nm
B) 300 nm
C) 174 nm
D) 520 nm
E) 3,000 nm

Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
14
At what wavelength would a star radiate the greatest amount of energy if the star has a surface temperature of 10,000 K?
A) 10 nm
B) 100 nm
C) 300 nm
D) 1.0*104 nm
E) 3.0*1010 nm
A) 10 nm
B) 100 nm
C) 300 nm
D) 1.0*104 nm
E) 3.0*1010 nm

Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
15
The radiation emitted from a star has a maximum intensity at a wavelength of 500 nm.What is the temperature of this star?
A) 6,000 K
B) 5,000 K
C) 1.5*109 K
D) 500 K
E) 10,000 K
A) 6,000 K
B) 5,000 K
C) 1.5*109 K
D) 500 K
E) 10,000 K

Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
16
The neutral hydrogen atom consists of
A) one proton and one neutron.
B) one proton.
C) one proton,one neutron,and one electron.
D) one proton and one electron.
E) an isotope and an ion.
A) one proton and one neutron.
B) one proton.
C) one proton,one neutron,and one electron.
D) one proton and one electron.
E) an isotope and an ion.

Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
17
NARRBEGIN: Diagram 07-01
Diagram 7-1 NARREND
NARREND
In Diagram 7-1,which of the transitions would absorb a photon with the smallest energy?
A) Transition 1
B) Transition 2
C) Transition 3
D) Transition 4
E) Transition 5
Diagram 7-1
 NARREND
NARRENDIn Diagram 7-1,which of the transitions would absorb a photon with the smallest energy?
A) Transition 1
B) Transition 2
C) Transition 3
D) Transition 4
E) Transition 5

Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
18
The process of making an electron less tightly bound to an atom's nucleus is known as
A) excitation.
B) Doppler broadening.
C) collisional broadening.
D) a red shift.
E) quantum mechanics.
A) excitation.
B) Doppler broadening.
C) collisional broadening.
D) a red shift.
E) quantum mechanics.

Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
19
At what wavelength would a star radiate the greatest amount of energy if the star has a surface temperature of 60,000 K?
A) 50 nm
B) 500 nm
C) 300 nm
D) 1.8*1011 nm
E) 180 nm
A) 50 nm
B) 500 nm
C) 300 nm
D) 1.8*1011 nm
E) 180 nm

Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
20
The radiation emitted from a star has a maximum intensity at a wavelength of 300 nm.What is the temperature of this star?
A) 300 K
B) 100 K
C) 900,000,000 K
D) 90,000 K
E) 10,000 K
A) 300 K
B) 100 K
C) 900,000,000 K
D) 90,000 K
E) 10,000 K

Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
21
The bluer the light,the ____ each photon contains.
A) More energy
B) Less energy
C) Less speed
D) None speed
E) None of the above.
A) More energy
B) Less energy
C) Less speed
D) None speed
E) None of the above.

Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
22
The Doppler effect states that the motion of any object can
A) shift the wavelength of spectral lines.
B) change the speed of light emitted from the object.
C) enhance the chemical composition of the object.
D) make the object appear hotter.
E) make the object appear cooler.
A) shift the wavelength of spectral lines.
B) change the speed of light emitted from the object.
C) enhance the chemical composition of the object.
D) make the object appear hotter.
E) make the object appear cooler.

Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
23
Suppose the laboratory wavelength of a spectral line is 600 nm.In a star it is measured to be 599.4 nm.Is the star moving toward or away from us,relative to the line of sight? How fast is it moving? (Hint: c=300,000 km/s)..
A) toward,300 km/s
B) away,300 km/s
C) at rest,0 km/s
D) sideways,300 km/s
E) toward,150 km/s
A) toward,300 km/s
B) away,300 km/s
C) at rest,0 km/s
D) sideways,300 km/s
E) toward,150 km/s

Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
24
The two most abundant elements in the sun are
A) nitrogen and oxygen.
B) hydrogen and helium.
C) sulfur and iron.
D) carbon and hydrogen.
E) carbon and nitrogen.
A) nitrogen and oxygen.
B) hydrogen and helium.
C) sulfur and iron.
D) carbon and hydrogen.
E) carbon and nitrogen.

Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
25
If light from a star passes from an excited low density gas seen against the dark background of space to your telescope and spectroscope,____ spectrum results.
A) a dark (absorption)line
B) a bright (emission)line
C) continuous
A) a dark (absorption)line
B) a bright (emission)line
C) continuous

Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
26
Which of the following cannot be determined from the spectrum of a star?
A) chemical composition
B) surface temperature
C) radial (along line of sight)velocity
D) tangential (perpendicular to line of sight)velocity
E) Both c and d
A) chemical composition
B) surface temperature
C) radial (along line of sight)velocity
D) tangential (perpendicular to line of sight)velocity
E) Both c and d

Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
27
If a light-emitting object is moving away from you,you observe its wavelengths to be ________ its wavelengths if it were at rest..
A) longer than
B) shorter than
C) the same as
A) longer than
B) shorter than
C) the same as

Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
28
Two white dwarf stars are the same size,but one has a surface temperature twice as hot as the other.The hotter star will radiate ...
A) half as much energy per second as the cooler star.
B) the same amount of energy per second as the cooler star.
C) four times as much energy per second as the cooler star.
D) sixteen times as much energy per second as the cooler star.
A) half as much energy per second as the cooler star.
B) the same amount of energy per second as the cooler star.
C) four times as much energy per second as the cooler star.
D) sixteen times as much energy per second as the cooler star.

Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
29
Which of the following can be determined by using the Doppler Effect? 
A) I & IV
B) II & III
C) II & IV
D) I & III
E) I,III,& IV

A) I & IV
B) II & III
C) II & IV
D) I & III
E) I,III,& IV

Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
30
The binding energy of the first level in an atom is 2.2*10 - 18 J,and the binding energy of the second energy level is 1.6*0 - 18 J.What is the energy of the photon that is emitted if an electron moves from the second level to the first?
A) 3.3*10-18 J
B) 3.5*10-36 J
C) 1.4 J
D) 3.5*10-18 J
E) 6.0*10-19 J
A) 3.3*10-18 J
B) 3.5*10-36 J
C) 1.4 J
D) 3.5*10-18 J
E) 6.0*10-19 J

Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
31
You are standing near a railroad track and a train is moving toward you at 60 mph and blowing his horn.What will you notice as the train moves past you?
A) As the train approaches,the horn will sound lower in pitch than when it is moving away.
B) As the train approaches,the horn will sound higher in pitch than when it is moving away.
C) There will be no change in the pitch of the horn as it moves by.
D) The horn will get louder as the train moves away from me.
E) The horn will get quieter as the train moves toward me
A) As the train approaches,the horn will sound lower in pitch than when it is moving away.
B) As the train approaches,the horn will sound higher in pitch than when it is moving away.
C) There will be no change in the pitch of the horn as it moves by.
D) The horn will get louder as the train moves away from me.
E) The horn will get quieter as the train moves toward me

Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
32
If continuous spectrum light from a star passes through a cool low density gas on its way to your telescope and spectroscope,____ spectrum on the continuous spectrum results.
A) a dark (absorption)line
B) a bright (emission)line
C) continuous
A) a dark (absorption)line
B) a bright (emission)line
C) continuous

Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
33
A very bright UFO is seen near the moon's orbit.An astronomer takes a spectrum of it and discovers it has a bright line spectrum on a dark background.The earth's population is near panic when the astronomer announces the UFO is ...
A) a metal spacecraft.
B) a giant rock about to hit the earth.
C) a glowing cloud of gas.
D) a star like the sun.
A) a metal spacecraft.
B) a giant rock about to hit the earth.
C) a glowing cloud of gas.
D) a star like the sun.

Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
34
The H - line has a wavelength of 434.0 nm when observed in the laboratory.If the H - line appears in a stars spectrum at 434.5 nm,what is the radial velocity of the star?
A) 346 km/sec away from the observer
B) 346 km/sec toward the observer
C) 1.3*108 m/sec away from the observer
D) 1.3*108 m/sec toward the observer
E) The radial velocity of the star can not be determined from this information.
A) 346 km/sec away from the observer
B) 346 km/sec toward the observer
C) 1.3*108 m/sec away from the observer
D) 1.3*108 m/sec toward the observer
E) The radial velocity of the star can not be determined from this information.

Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
35
The ____ of a gas is a measure of the average speed of the particles in the gas.
A) heat
B) composition
C) temperature
D) blue shift
E) binding energy
A) heat
B) composition
C) temperature
D) blue shift
E) binding energy

Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
36
Each element has its own set of characteristic absorption lines because
A) the temperature of each element can varies.
B) elements can exist in different forms of matter.
C) electron energy levels differ for each element.
D) each element has a different mass.
E) absorption lines depend upon the speed of the object.
A) the temperature of each element can varies.
B) elements can exist in different forms of matter.
C) electron energy levels differ for each element.
D) each element has a different mass.
E) absorption lines depend upon the speed of the object.

Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
37
What is the order of star colors with increasing temperature?
A) Red,Yellow,Blue
B) Blue,Red,Yellow
C) Red,Blue,Yellow
D) Yellow,Red,Blue
E) Blue,Yellow,Red
A) Red,Yellow,Blue
B) Blue,Red,Yellow
C) Red,Blue,Yellow
D) Yellow,Red,Blue
E) Blue,Yellow,Red

Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
38
Which of the following is not a fundamental component of the atom?
A) proton
B) neutron
C) ion
D) electron
A) proton
B) neutron
C) ion
D) electron

Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
39
The energy of a photon is proportional to the light's
A) wavelength.
B) speed.
C) frequency.
D) intensity.
E) Two of the above.
A) wavelength.
B) speed.
C) frequency.
D) intensity.
E) Two of the above.

Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
40
The diagram illustrates a light source,a gas cloud,and three different lines of sight.Along which line of sight would an observer see an emission spectrum? 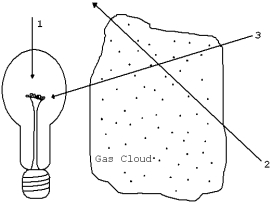
A) 1
B) 2
C) 3
D) 2 and 3
E) none of them

A) 1
B) 2
C) 3
D) 2 and 3
E) none of them

Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
41
The figure shows a color image of Betelgeuse,a red giant star.Which of the surface temperatures below best matches Betelgeuse's surface temperature? Hint: Our sun at 6000K is yellow. 
A) 64,000 K
B) 32,000 K
C) 16,000 K
D) 8000 K
E) 2000 K

A) 64,000 K
B) 32,000 K
C) 16,000 K
D) 8000 K
E) 2000 K

Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
42
____________________ is a measure of the amount of energy due to the motion of the particles in a gas,liquid,or solid.

Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
43
Blue stars are emit most of their energy at shorter wavelengths than red stars..

Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
44
Isotopes of the same element have the same number of protons.

Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
45
The ____________________ of a star can be determined from its color.

Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
46
An atom is ionized if one of its electrons jumps to a higher energy level in the atom.

Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
47
The Doppler Effect is sensitive only to motion along the line of sight.

Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
48
Which one of the hydrogen atoms below is EMITTING light? The electron jumps from the ...
A) second energy level to first (most bound)
B) third energy level to fourth
C) fourth energy level to fifth
D) second energy level to third
E) first energy level to second
A) second energy level to first (most bound)
B) third energy level to fourth
C) fourth energy level to fifth
D) second energy level to third
E) first energy level to second

Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
49
Which one of the hydrogen atoms below is EMITTING light at a wavelength in the Balmer series?
A) second energy level to first (most bound)
B) third energy level to second
C) fourth energy level to third
D) second energy level to third
E) first energy level to second
A) second energy level to first (most bound)
B) third energy level to second
C) fourth energy level to third
D) second energy level to third
E) first energy level to second

Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
50
Blue stars are hotter than red stars.

Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
51
The nucleus of the hydrogen atom consists of a single neutron.

Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
52
The Lyman series lines of hydrogen all lie in the infrared.

Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
53
The process of removing an electron from a stable nucleus is known as ____________________.

Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
54
An atom that has lost an electron is called an ion.

Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
55
When the electrons in an atom are in their lowest possible energy levels,the atom is said to be in its ____________________ state.

Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
56
An absorption spectrum is also called a bright line spectrum.

Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
57
Hydrogen alpha is the longest wavelength Balmer line.

Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
58
The wavelength of the hydrogen line from level 1 to level 2 in a hot star is ____________ the wavelength of the same line in a cool star.The stars have the same radial velocities away from the earth.
A) larger than.
B) the same as
C) smaller than
D) not enough information to answer
A) larger than.
B) the same as
C) smaller than
D) not enough information to answer

Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
59
The hotter an object,the more blue it appears.

Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
60
In the diagram below,draw the transition that would emit a photon with the smallest wavelength. 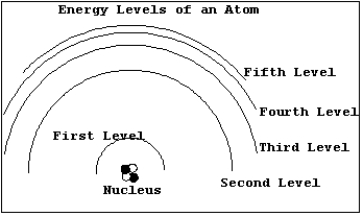


Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck


