Deck 2: The Chemical Level of Organization
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/79
Play
Full screen (f)
Deck 2: The Chemical Level of Organization
1
How many carbons are in this structural formula? 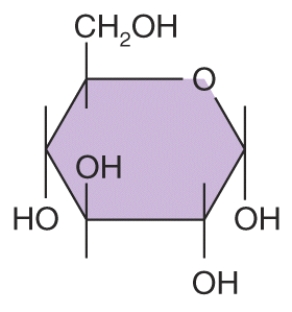
A) 1
B) 2
C) 4
D) 6
E) 9

A) 1
B) 2
C) 4
D) 6
E) 9
D
2
Most enzymes are non-specific, meaning they may bind to substrates indiscriminately and catalyze a wide range of reactions in the body.
False
3
Which of the following is one of the 4 major elements found in the body?
A) carbon
B) aluminum
C) iodine
D) calcium
E) selenium
A) carbon
B) aluminum
C) iodine
D) calcium
E) selenium
A
4
Which of the following is a good example of an acidic bodily fluid in a healthy individual?
A) semen
B) cerebrospinal fluid
C) bile
D) blood
E) gastric juice
A) semen
B) cerebrospinal fluid
C) bile
D) blood
E) gastric juice

Unlock Deck
Unlock for access to all 79 flashcards in this deck.
Unlock Deck
k this deck
5
Which of the following statements best characterizes lipid molecules?
A) In general, they have few polar covalent bonds, and are therefore hydrophobic and insoluble in water.
B) They are most commonly found in the body functioning as enzymes.
C) Glucose, fructose, and galactose are classified as triglycerides.
D) Components of these molecules are joined together by peptide bonds.
E) They are toxic in the body and should be eradicated in all forms from the diet.
A) In general, they have few polar covalent bonds, and are therefore hydrophobic and insoluble in water.
B) They are most commonly found in the body functioning as enzymes.
C) Glucose, fructose, and galactose are classified as triglycerides.
D) Components of these molecules are joined together by peptide bonds.
E) They are toxic in the body and should be eradicated in all forms from the diet.

Unlock Deck
Unlock for access to all 79 flashcards in this deck.
Unlock Deck
k this deck
6
Which of the following is the most plentiful positive ion in the extracellular fluid?
A) nitrogen
B) hydrogen
C) magnesium
D) potassium
E) sodium
A) nitrogen
B) hydrogen
C) magnesium
D) potassium
E) sodium

Unlock Deck
Unlock for access to all 79 flashcards in this deck.
Unlock Deck
k this deck
7
Ions are formed when atoms gain or lose one or more valence electrons and become positively or negatively charged.

Unlock Deck
Unlock for access to all 79 flashcards in this deck.
Unlock Deck
k this deck
8
Which of the following is NOT an example of a hydrogen bond?
A) the bonds formed between H2O molecules
B) the peptide bonds between amino acids
C) the bonds formed between base pairs of DNA and RNA nucleotides
D) the bonds formed along the polypeptide backbone of a protein to form its secondary structure, the alpha helixes and beta pleated sheets.
A) the bonds formed between H2O molecules
B) the peptide bonds between amino acids
C) the bonds formed between base pairs of DNA and RNA nucleotides
D) the bonds formed along the polypeptide backbone of a protein to form its secondary structure, the alpha helixes and beta pleated sheets.

Unlock Deck
Unlock for access to all 79 flashcards in this deck.
Unlock Deck
k this deck
9
Salts are always ___.
A) defined as dissociating in water into a hydrogen ion and an anion
B) ionic compounds
C) polar covalent compounds
D) non-polar covalent compounds
E) hydrogen bonded
A) defined as dissociating in water into a hydrogen ion and an anion
B) ionic compounds
C) polar covalent compounds
D) non-polar covalent compounds
E) hydrogen bonded

Unlock Deck
Unlock for access to all 79 flashcards in this deck.
Unlock Deck
k this deck
10
The energy needed to break an existing chemical bond or to form a new chemical bond is called the ___.
A) potential energy
B) catalysis
C) activation energy
D) chemical energy
E) mechanical energy
A) potential energy
B) catalysis
C) activation energy
D) chemical energy
E) mechanical energy

Unlock Deck
Unlock for access to all 79 flashcards in this deck.
Unlock Deck
k this deck
11
A bond in which electrons are shared unequally is called a(n) ___.
A) hydrogen bond
B) ionic bond
C) amphipathic bond
D) polar covalent bond
E) nonpolar covalent bond
A) hydrogen bond
B) ionic bond
C) amphipathic bond
D) polar covalent bond
E) nonpolar covalent bond

Unlock Deck
Unlock for access to all 79 flashcards in this deck.
Unlock Deck
k this deck
12
Which of these terms describes a large molecule created by covalent bonding of many similar small building block molecules?
A) polymer
B) colloid
C) monomer
D) electrolyte
E) polymath
A) polymer
B) colloid
C) monomer
D) electrolyte
E) polymath

Unlock Deck
Unlock for access to all 79 flashcards in this deck.
Unlock Deck
k this deck
13
Which of the following would be regarded as an organic molecule?
A) H2O
B) CH4
C) PO43-
D) NaCl
E) HCl
A) H2O
B) CH4
C) PO43-
D) NaCl
E) HCl

Unlock Deck
Unlock for access to all 79 flashcards in this deck.
Unlock Deck
k this deck
14
The sugar part of the backbone of DNA strands is called deoxyribose.

Unlock Deck
Unlock for access to all 79 flashcards in this deck.
Unlock Deck
k this deck
15
The rate of a chemical reaction is influenced by all of the following except:
A) the presence of the appropriate enzyme
B) the concentration of the reactants
C) the presence or absence of carbon
D) the temperature
E) the presence of the appropriate catalyst
A) the presence of the appropriate enzyme
B) the concentration of the reactants
C) the presence or absence of carbon
D) the temperature
E) the presence of the appropriate catalyst

Unlock Deck
Unlock for access to all 79 flashcards in this deck.
Unlock Deck
k this deck
16
The phospholipids that make up the plasma membranes of body cells have a ___ head region and a ___ tail region, a quality essential for the formation of the "lipid bilayer."
A) non-polar, hydrophilic
B) non-polar, polar
C) polar, hydrophilic
D) hydrophobic, hydrophilic
E) hydrophilic, non-polar
A) non-polar, hydrophilic
B) non-polar, polar
C) polar, hydrophilic
D) hydrophobic, hydrophilic
E) hydrophilic, non-polar

Unlock Deck
Unlock for access to all 79 flashcards in this deck.
Unlock Deck
k this deck
17
The lower the pH, the higher the H+ concentration.

Unlock Deck
Unlock for access to all 79 flashcards in this deck.
Unlock Deck
k this deck
18
Which of these statements is NOT true of fats and oils?
A) A fat is typically solid at room temperature, while an oil is liquid.
B) Fats contain fatty acids that are mostly saturated, while oils have mostly unsaturated fatty acids.
C) Fats are usually phospholipids, while oils are triglycerides.
D) Fats in our diet are typically from meat products; oils are typically from plant products and some fish.
E) Eating large amounts of saturated fat is associated with heart disease, and eating oils is believed to reduce the risk of heart disease.
A) A fat is typically solid at room temperature, while an oil is liquid.
B) Fats contain fatty acids that are mostly saturated, while oils have mostly unsaturated fatty acids.
C) Fats are usually phospholipids, while oils are triglycerides.
D) Fats in our diet are typically from meat products; oils are typically from plant products and some fish.
E) Eating large amounts of saturated fat is associated with heart disease, and eating oils is believed to reduce the risk of heart disease.

Unlock Deck
Unlock for access to all 79 flashcards in this deck.
Unlock Deck
k this deck
19
Which of the following statements is FALSE regarding enzymes?
A) They are specific; each one is able to bind only to certain substrates.
B) They lower the activation energy of a chemical reaction.
C) They are all capable of breaking down lipids.
D) They are unchanged at the end of the reaction.
E) They usually end with the suffix -ase.
A) They are specific; each one is able to bind only to certain substrates.
B) They lower the activation energy of a chemical reaction.
C) They are all capable of breaking down lipids.
D) They are unchanged at the end of the reaction.
E) They usually end with the suffix -ase.

Unlock Deck
Unlock for access to all 79 flashcards in this deck.
Unlock Deck
k this deck
20
The smallest unit of matter that retains the properties and characteristics of an element is the ___.
A) molecule
B) atom
C) electron
D) proton
E) nucleus
A) molecule
B) atom
C) electron
D) proton
E) nucleus

Unlock Deck
Unlock for access to all 79 flashcards in this deck.
Unlock Deck
k this deck
21
This term refers to the average atomic mass of all the naturally occurring isotopes of an element.
A) mass number
B) atomic number
C) atomic weight
D) ionic mass
E) covalent mass
A) mass number
B) atomic number
C) atomic weight
D) ionic mass
E) covalent mass

Unlock Deck
Unlock for access to all 79 flashcards in this deck.
Unlock Deck
k this deck
22
The following equation is an example of which of the following concepts?
ATP + H2O → ADP + Phosphate group + Energy
A) dehydration synthesis reaction
B) anabolic reaction
C) phosphorylation
D) hydrolysis
ATP + H2O → ADP + Phosphate group + Energy
A) dehydration synthesis reaction
B) anabolic reaction
C) phosphorylation
D) hydrolysis

Unlock Deck
Unlock for access to all 79 flashcards in this deck.
Unlock Deck
k this deck
23
This type of reaction will absorb more energy than it releases.
A) exergonic
B) endergonic
C) potential
D) kinetic
E) activation
A) exergonic
B) endergonic
C) potential
D) kinetic
E) activation

Unlock Deck
Unlock for access to all 79 flashcards in this deck.
Unlock Deck
k this deck
24
What region of an atom contains the protons and neutrons?
A) cloud
B) nucleus
C) element
D) ring
E) shell
A) cloud
B) nucleus
C) element
D) ring
E) shell

Unlock Deck
Unlock for access to all 79 flashcards in this deck.
Unlock Deck
k this deck
25
Non-polar covalent bonds between hydrogen and other atoms create partially charged regions on those molecules, and the attraction between other molecules with partially charged regions are called hydrogen bonds.

Unlock Deck
Unlock for access to all 79 flashcards in this deck.
Unlock Deck
k this deck
26
Which of the following particles plays the greatest role in creating chemical bonds?
A) neutron
B) electron
C) proton
D) neutron and proton
E) all of these choices
A) neutron
B) electron
C) proton
D) neutron and proton
E) all of these choices

Unlock Deck
Unlock for access to all 79 flashcards in this deck.
Unlock Deck
k this deck
27
Which of the following bodily fluids is strongly alkaline (basic)?
A) blood
B) gastric juice
C) bile
D) vaginal fluid
E) saliva
A) blood
B) gastric juice
C) bile
D) vaginal fluid
E) saliva

Unlock Deck
Unlock for access to all 79 flashcards in this deck.
Unlock Deck
k this deck
28
This is the type of bond between the atoms within a molecule of H2O.
A) nonpolar covalent bond
B) polar covalent bond
C) hydrogen bond
D) ionic bond
E) treasury bond
A) nonpolar covalent bond
B) polar covalent bond
C) hydrogen bond
D) ionic bond
E) treasury bond

Unlock Deck
Unlock for access to all 79 flashcards in this deck.
Unlock Deck
k this deck
29
Which of these conducts an electric current?
A) isotope
B) surface tension
C) compound
D) electrolyte
E) valence molecule
A) isotope
B) surface tension
C) compound
D) electrolyte
E) valence molecule

Unlock Deck
Unlock for access to all 79 flashcards in this deck.
Unlock Deck
k this deck
30
Anions are formed when atoms gain or lose one or more valence electrons and become positively or negatively charged.

Unlock Deck
Unlock for access to all 79 flashcards in this deck.
Unlock Deck
k this deck
31
This describes the number of protons in an atom.
A) mass number
B) atomic number
C) isotope
D) valence shell
E) none of these choices
A) mass number
B) atomic number
C) isotope
D) valence shell
E) none of these choices

Unlock Deck
Unlock for access to all 79 flashcards in this deck.
Unlock Deck
k this deck
32
Which of the following particles has a neutral charge?
A) neutron
B) atom
C) proton
D) both the neutron and atom
E) all of these choices
A) neutron
B) atom
C) proton
D) both the neutron and atom
E) all of these choices

Unlock Deck
Unlock for access to all 79 flashcards in this deck.
Unlock Deck
k this deck
33
This term describes the process of forming new chemical bonds or breaking bonds between atoms.
A) homeostasis
B) surface tension
C) isotopes
D) chemical reaction
E) anabolism
A) homeostasis
B) surface tension
C) isotopes
D) chemical reaction
E) anabolism

Unlock Deck
Unlock for access to all 79 flashcards in this deck.
Unlock Deck
k this deck
34
An enzyme acts to ___.
A) raise the activation energy required for a chemical reaction to occur
B) lower the activation energy required for a chemical reaction to occur
C) convert the activation energy into potential energy
D) convert the activation energy into kinetic energy
E) quench a chemical reaction
A) raise the activation energy required for a chemical reaction to occur
B) lower the activation energy required for a chemical reaction to occur
C) convert the activation energy into potential energy
D) convert the activation energy into kinetic energy
E) quench a chemical reaction

Unlock Deck
Unlock for access to all 79 flashcards in this deck.
Unlock Deck
k this deck
35
Atoms with the same number of protons and a differing number of neutrons are ___.
A) compounds
B) cations
C) anions
D) isotopes
E) molecules
A) compounds
B) cations
C) anions
D) isotopes
E) molecules

Unlock Deck
Unlock for access to all 79 flashcards in this deck.
Unlock Deck
k this deck
36
This type of bond requires a sharing of electrons.
A) covalent
B) ionic
C) hydrogen
D) bail
E) electronic
A) covalent
B) ionic
C) hydrogen
D) bail
E) electronic

Unlock Deck
Unlock for access to all 79 flashcards in this deck.
Unlock Deck
k this deck
37
Which of the following is the most plentiful positive ion in the intracellular fluid?
A) Na+
B) Cl-
C) H+
D) K+
E) Mg2+
A) Na+
B) Cl-
C) H+
D) K+
E) Mg2+

Unlock Deck
Unlock for access to all 79 flashcards in this deck.
Unlock Deck
k this deck
38
Which of the following bonds is the weakest of the chemical bonds but plays a large role in determining the three dimensional shape of large molecules such as proteins?
A) nonpolar covalent bonds
B) polar covalent bonds
C) hydrogen bonds
D) ionic bonds
E) barry bonds
A) nonpolar covalent bonds
B) polar covalent bonds
C) hydrogen bonds
D) ionic bonds
E) barry bonds

Unlock Deck
Unlock for access to all 79 flashcards in this deck.
Unlock Deck
k this deck
39
This is a negatively charged particle.
A) superoxide
B) isotope
C) catalyst
D) anion
E) cation
A) superoxide
B) isotope
C) catalyst
D) anion
E) cation

Unlock Deck
Unlock for access to all 79 flashcards in this deck.
Unlock Deck
k this deck
40
This term is defined as the capacity to do work.
A) metabolism
B) electrolytes
C) chemical reaction
D) concentration
E) energy
A) metabolism
B) electrolytes
C) chemical reaction
D) concentration
E) energy

Unlock Deck
Unlock for access to all 79 flashcards in this deck.
Unlock Deck
k this deck
41
This type of lipid is the body's long-term energy storage molecule.
A) steroid
B) phospholipid
C) cholesterol
D) triglyceride
E) lipoprotein
A) steroid
B) phospholipid
C) cholesterol
D) triglyceride
E) lipoprotein

Unlock Deck
Unlock for access to all 79 flashcards in this deck.
Unlock Deck
k this deck
42
Which of these is the solvent in a typical body solution?
A) glucose
B) lipids
C) carbon dioxide
D) water
E) electrolyte
A) glucose
B) lipids
C) carbon dioxide
D) water
E) electrolyte

Unlock Deck
Unlock for access to all 79 flashcards in this deck.
Unlock Deck
k this deck
43
Which of the following is considered a proton donor?
A) acid
B) base
C) salt
D) organic compound
E) colloid
A) acid
B) base
C) salt
D) organic compound
E) colloid

Unlock Deck
Unlock for access to all 79 flashcards in this deck.
Unlock Deck
k this deck
44
The major energy storage polysaccharide in humans is ___.
A) cellulose
B) ribose
C) lipids
D) fats
E) glycogen
A) cellulose
B) ribose
C) lipids
D) fats
E) glycogen

Unlock Deck
Unlock for access to all 79 flashcards in this deck.
Unlock Deck
k this deck
45
The primary structure of a protein contains ___.
A) alpha helix
B) beta-pleated sheets
C) substrates
D) amino acids
E) all of these choices
A) alpha helix
B) beta-pleated sheets
C) substrates
D) amino acids
E) all of these choices

Unlock Deck
Unlock for access to all 79 flashcards in this deck.
Unlock Deck
k this deck
46
Which of these is a substance that is added to solutions to add or remove hydrogen ions so that stronger acids or bases are converted into weaker acids or bases?
A) base
B) lye
C) acid
D) alkaline
E) buffer
A) base
B) lye
C) acid
D) alkaline
E) buffer

Unlock Deck
Unlock for access to all 79 flashcards in this deck.
Unlock Deck
k this deck
47
The body uses this type of lipid to create hormones.
A) cellulose
B) phospholipid
C) cholesterol
D) triglyceride
E) lipoprotein
A) cellulose
B) phospholipid
C) cholesterol
D) triglyceride
E) lipoprotein

Unlock Deck
Unlock for access to all 79 flashcards in this deck.
Unlock Deck
k this deck
48
A solute that dissolves in water is ___.
A) hydrophobic
B) hydrostatic
C) hydroelectric
D) hydrophilic
E) hydrozone
A) hydrophobic
B) hydrostatic
C) hydroelectric
D) hydrophilic
E) hydrozone

Unlock Deck
Unlock for access to all 79 flashcards in this deck.
Unlock Deck
k this deck
49
Which of the following is a monosaccharide that is important in producing energy?
A) glucose
B) sucrose
C) lactose
D) ribose
E) deoxyribose
A) glucose
B) sucrose
C) lactose
D) ribose
E) deoxyribose

Unlock Deck
Unlock for access to all 79 flashcards in this deck.
Unlock Deck
k this deck
50
Which is the function of RNA?
A) produce electrical impulses
B) storage of energy
C) transfer information for protein synthesis
D) store information for protein synthesis
E) transport of fluids
A) produce electrical impulses
B) storage of energy
C) transfer information for protein synthesis
D) store information for protein synthesis
E) transport of fluids

Unlock Deck
Unlock for access to all 79 flashcards in this deck.
Unlock Deck
k this deck
51
Which of the following is NOT true of phospholipids?
A) They contain a glycerol backbone.
B) The head group is polar.
C) The molecule is an important part of cell membranes.
D) The tail groups are nonpolar.
E) They are a major energy storage lipid.
A) They contain a glycerol backbone.
B) The head group is polar.
C) The molecule is an important part of cell membranes.
D) The tail groups are nonpolar.
E) They are a major energy storage lipid.

Unlock Deck
Unlock for access to all 79 flashcards in this deck.
Unlock Deck
k this deck
52
How does RNA differ from DNA?
A) RNA is usually single-stranded while DNA is usually part of a double-stranded helix.
B) The sugar in RNA monomers is ribose; in DNA, it is deoxyribose.
C) RNA uses the nitrogenous base, uracil, where DNA uses thymine.
D) RNA is usually single-stranded while DNA is usually part of a double-stranded helix, and RNA uses the nitrogenous base, uracil, where DNA uses thymine.
E) All of these choices.
A) RNA is usually single-stranded while DNA is usually part of a double-stranded helix.
B) The sugar in RNA monomers is ribose; in DNA, it is deoxyribose.
C) RNA uses the nitrogenous base, uracil, where DNA uses thymine.
D) RNA is usually single-stranded while DNA is usually part of a double-stranded helix, and RNA uses the nitrogenous base, uracil, where DNA uses thymine.
E) All of these choices.

Unlock Deck
Unlock for access to all 79 flashcards in this deck.
Unlock Deck
k this deck
53
This type of triglyceride contains more than one double bond in the fatty acid carbon atoms.
A) saturated
B) monounsaturated
C) polyunsaturated
D) acylglycerols
E) lipoprotein
A) saturated
B) monounsaturated
C) polyunsaturated
D) acylglycerols
E) lipoprotein

Unlock Deck
Unlock for access to all 79 flashcards in this deck.
Unlock Deck
k this deck
54
This type of reaction will convert larger reactants to smaller products.
A) synthesis
B) decomposition
C) potential
D) exchange
E) activated
A) synthesis
B) decomposition
C) potential
D) exchange
E) activated

Unlock Deck
Unlock for access to all 79 flashcards in this deck.
Unlock Deck
k this deck
55
Prostaglandins and leukotrienes are considered ___.
A) amphipathic
B) lipids
C) eicosanoids
D) both lipids and eicosanoids
E) all of these choices
A) amphipathic
B) lipids
C) eicosanoids
D) both lipids and eicosanoids
E) all of these choices

Unlock Deck
Unlock for access to all 79 flashcards in this deck.
Unlock Deck
k this deck
56
This is the most abundant and most important inorganic compound in the body.
A) water
B) oxygen gas
C) carbon dioxide
D) glucose
E) DNA
A) water
B) oxygen gas
C) carbon dioxide
D) glucose
E) DNA

Unlock Deck
Unlock for access to all 79 flashcards in this deck.
Unlock Deck
k this deck
57
Which of these best describes a solution with a pH value less than 7?
A) basic
B) salty
C) acidic
D) alkaline
E) concentrated
A) basic
B) salty
C) acidic
D) alkaline
E) concentrated

Unlock Deck
Unlock for access to all 79 flashcards in this deck.
Unlock Deck
k this deck
58
This type of reaction combines reactants to produce larger products.
A) synthesis
B) decomposition
C) potential
D) exchange
E) activated
A) synthesis
B) decomposition
C) potential
D) exchange
E) activated

Unlock Deck
Unlock for access to all 79 flashcards in this deck.
Unlock Deck
k this deck
59
Which of these molecules is made up of a nitrogenous base, five-carbon sugar, and a phosphate group?
A) amino acid
B) monosaccharide
C) nucleotide
D) fatty acid
E) triglyceride
A) amino acid
B) monosaccharide
C) nucleotide
D) fatty acid
E) triglyceride

Unlock Deck
Unlock for access to all 79 flashcards in this deck.
Unlock Deck
k this deck
60
Which of the following is true of inorganic acids, bases and salts in water?
A) An acid dissociates into one or more hydrogen ions and one or more cations.
B) A base dissociates into one or more hydroxide ions and one or more anions.
C) Salts dissociate into one or more anions and one or more cations, none of which are H+ or OH-.
D) An acid dissociates into one or more hydrogen ions and one or more cations and a base dissociates into one or more hydroxide ions and one or more anions.
E) None of these answer choices are correct.
A) An acid dissociates into one or more hydrogen ions and one or more cations.
B) A base dissociates into one or more hydroxide ions and one or more anions.
C) Salts dissociate into one or more anions and one or more cations, none of which are H+ or OH-.
D) An acid dissociates into one or more hydrogen ions and one or more cations and a base dissociates into one or more hydroxide ions and one or more anions.
E) None of these answer choices are correct.

Unlock Deck
Unlock for access to all 79 flashcards in this deck.
Unlock Deck
k this deck
61
Describe the law of conservation of energy.

Unlock Deck
Unlock for access to all 79 flashcards in this deck.
Unlock Deck
k this deck
62
In the diagram which particles are negatively charged? 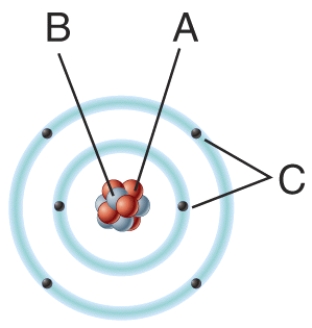
A) A
B) B
C) C
D) All of these choices
E) None of these choices

A) A
B) B
C) C
D) All of these choices
E) None of these choices

Unlock Deck
Unlock for access to all 79 flashcards in this deck.
Unlock Deck
k this deck
63
Describe what is happening at places 1, 2, and 3 in the figure. 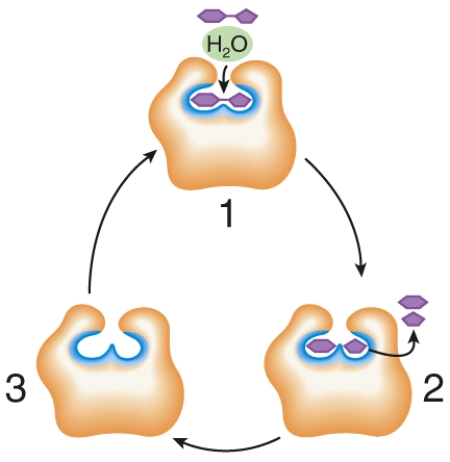


Unlock Deck
Unlock for access to all 79 flashcards in this deck.
Unlock Deck
k this deck
64
Why are most lipids insoluble in water?

Unlock Deck
Unlock for access to all 79 flashcards in this deck.
Unlock Deck
k this deck
65
Describe three factors that increase the rate of chemical reactions.

Unlock Deck
Unlock for access to all 79 flashcards in this deck.
Unlock Deck
k this deck
66
Which of these describes a function of adenosine triphosphate (ATP)?
A) produce electrical impulses
B) transfers energy for cell functions
C) surrounds the cell and separates it from environment
D) store information for protein synthesis
E) all of these choices
A) produce electrical impulses
B) transfers energy for cell functions
C) surrounds the cell and separates it from environment
D) store information for protein synthesis
E) all of these choices

Unlock Deck
Unlock for access to all 79 flashcards in this deck.
Unlock Deck
k this deck
67
An atom with 4 electrons in its valence shell is ___.
A) very stable
B) likely to ionize
C) a noble gas
D) an oxygen atom
E) a carbon atom
A) very stable
B) likely to ionize
C) a noble gas
D) an oxygen atom
E) a carbon atom

Unlock Deck
Unlock for access to all 79 flashcards in this deck.
Unlock Deck
k this deck
68
Describe what happens to a protein's structure and function when it is denatured.

Unlock Deck
Unlock for access to all 79 flashcards in this deck.
Unlock Deck
k this deck
69
Which of these describes the function of DNA?
A) produce chemical signals
B) storage of energy
C) transfer information for protein synthesis
D) store information for protein synthesis
E) transport of electrolytes
A) produce chemical signals
B) storage of energy
C) transfer information for protein synthesis
D) store information for protein synthesis
E) transport of electrolytes

Unlock Deck
Unlock for access to all 79 flashcards in this deck.
Unlock Deck
k this deck
70
Describe the functions of water in the body.

Unlock Deck
Unlock for access to all 79 flashcards in this deck.
Unlock Deck
k this deck
71
What is the difference between atomic mass, mass number and atomic number?

Unlock Deck
Unlock for access to all 79 flashcards in this deck.
Unlock Deck
k this deck
72
List the three major properties of enzymes.

Unlock Deck
Unlock for access to all 79 flashcards in this deck.
Unlock Deck
k this deck
73
List the six major functions of proteins.

Unlock Deck
Unlock for access to all 79 flashcards in this deck.
Unlock Deck
k this deck
74
Describe a hydrogen bond.

Unlock Deck
Unlock for access to all 79 flashcards in this deck.
Unlock Deck
k this deck
75
What is this molecule, where can it be found in a eukaryotic cell and what are the special properties of this molecule? 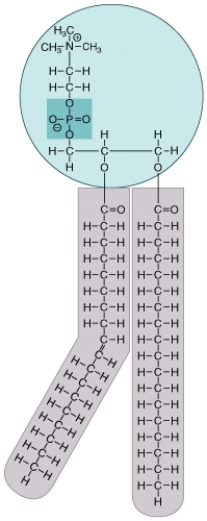


Unlock Deck
Unlock for access to all 79 flashcards in this deck.
Unlock Deck
k this deck
76
Describe what structures comprise an amino acid.

Unlock Deck
Unlock for access to all 79 flashcards in this deck.
Unlock Deck
k this deck
77
In the diagram, which pH value represents an acidic solution? 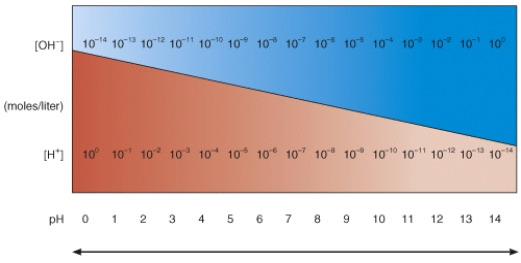
A) 12
B) 10
C) 8
D) 6
E) None of these choices

A) 12
B) 10
C) 8
D) 6
E) None of these choices

Unlock Deck
Unlock for access to all 79 flashcards in this deck.
Unlock Deck
k this deck
78
What is the monomer used to build RNA and DNA?
A) fatty acid
B) amino acid
C) glucose
D) glycerol
E) nucleotide
A) fatty acid
B) amino acid
C) glucose
D) glycerol
E) nucleotide

Unlock Deck
Unlock for access to all 79 flashcards in this deck.
Unlock Deck
k this deck
79
Give a brief description of what rule this diagram represents. 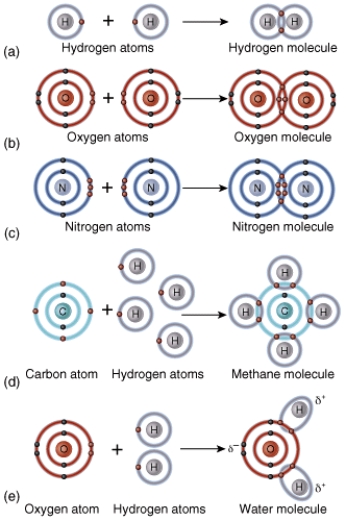


Unlock Deck
Unlock for access to all 79 flashcards in this deck.
Unlock Deck
k this deck


