Deck 15: Fusion and Fission: and a New Energy
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/77
Play
Full screen (f)
Deck 15: Fusion and Fission: and a New Energy
1
In the nuclear reaction 1H + 2H ·3He, how does the nuclear energy and the total rest- mass of the two hydrogen nuclei compare with the nuclear energy and the total rest- mass of the resulting helium nucleus?
A) Nuclear energy of helium is more, mass of helium is the same as the mass of the two hydrogen nuclei.
B) Nuclear energy of helium is more, mass of helium is more.
C) Nuclear energy of helium is less, mass of helium is the same as the mass of the two hydrogen nuclei.
D) Nuclear energy of helium, and mass of helium, are both the same as the nuclear energy and the mass of the two hydrogen nuclei.
E) Nuclear energy of helium is less, mass of helium is less.
A) Nuclear energy of helium is more, mass of helium is the same as the mass of the two hydrogen nuclei.
B) Nuclear energy of helium is more, mass of helium is more.
C) Nuclear energy of helium is less, mass of helium is the same as the mass of the two hydrogen nuclei.
D) Nuclear energy of helium, and mass of helium, are both the same as the nuclear energy and the mass of the two hydrogen nuclei.
E) Nuclear energy of helium is less, mass of helium is less.
Nuclear energy of helium is less, mass of helium is less.
2
Suppose that you fission a sulfur nucleus, 32 S, into two fragments. Referring to the graph above, does this process convert nuclear energy to other forms, or is it vice versa?
A) Nuclear energy to other forms, because this process proceeds downward, from higher to lower energy on the curve.
B) Other forms to nuclear energy, because this process proceeds upward, from lower to higher energy on the curve.
C) Other forms to nuclear energy, because this process proceeds downward, from higher to lower energy on the curve.
D) Nuclear energy to other forms, because this process proceeds upward, from lower to higher energy on the curve.
E) This is definitely the wrong answer; go back and start over.
A) Nuclear energy to other forms, because this process proceeds downward, from higher to lower energy on the curve.
B) Other forms to nuclear energy, because this process proceeds upward, from lower to higher energy on the curve.
C) Other forms to nuclear energy, because this process proceeds downward, from higher to lower energy on the curve.
D) Nuclear energy to other forms, because this process proceeds upward, from lower to higher energy on the curve.
E) This is definitely the wrong answer; go back and start over.
Other forms to nuclear energy, because this process proceeds upward, from lower to higher energy on the curve.
3
According to the nuclear energy curve, would fusion or fission or neither release nuclear energy from carbon [atomic number 6], and why?
A) Neither, because all other elements have higher nuclear energies.
B) Fusion, because then the reaction product has a lower atomic number and a higher nuclear energy.
C) Fission, because then the reaction products have higher atomic numbers and a lower nuclear energy.
D) Fission, because then the reaction products have lower atomic numbers and a higher nuclear energy.
E) Fusion, because then the reaction product has a higher atomic number and a lower nuclear energy.
A) Neither, because all other elements have higher nuclear energies.
B) Fusion, because then the reaction product has a lower atomic number and a higher nuclear energy.
C) Fission, because then the reaction products have higher atomic numbers and a lower nuclear energy.
D) Fission, because then the reaction products have lower atomic numbers and a higher nuclear energy.
E) Fusion, because then the reaction product has a higher atomic number and a lower nuclear energy.
Fusion, because then the reaction product has a higher atomic number and a lower nuclear energy.
4
Nuclear fusion is a process in which
A) a heavy nucleus is split into two or more lighter nuclei.
B) a small particle is spontaneously emitted from a nucleus.
C) two or more light nuclei are combined into a single heavier nucleus.
D) atoms combine into molecules.
E) None of the above.
A) a heavy nucleus is split into two or more lighter nuclei.
B) a small particle is spontaneously emitted from a nucleus.
C) two or more light nuclei are combined into a single heavier nucleus.
D) atoms combine into molecules.
E) None of the above.

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
5
The fusion of 6 Li and 10 N results in creation of 3 5
A) "16 O and no energy transformed to other forms."
B) "16 O and energy transformed to other forms."
C) "4 He and energy transformed to other forms."
D) "4 He and no energy transformed to other forms."
E) "salami and cheese sandwiches."
A) "16 O and no energy transformed to other forms."
B) "16 O and energy transformed to other forms."
C) "4 He and energy transformed to other forms."
D) "4 He and no energy transformed to other forms."
E) "salami and cheese sandwiches."

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
6
Fusion is often called a "thermonuclear reaction" because
A) it is a nuclear reaction that is self- sustaining a sufficiently high temperatures.
B) it produces nuclear energy.
C) it is a process that converts thermal energy into nuclear energy.
D) it is a nuclear reaction that consumes, or uses, more thermal energy than it creates.
E) it is a nuclear reaction that produces more total energy than it consumes.
A) it is a nuclear reaction that is self- sustaining a sufficiently high temperatures.
B) it produces nuclear energy.
C) it is a process that converts thermal energy into nuclear energy.
D) it is a nuclear reaction that consumes, or uses, more thermal energy than it creates.
E) it is a nuclear reaction that produces more total energy than it consumes.

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
7
Has a nuclear device ever been exploded by a terrorist?
A) No, although a terrorist has been arrested who possessed and planned to use a stolen nuclear weapon.
B) No, although a terrorist has been arrested who possessed and planned to use a dirty bomb.
C) No, in fact there are no known instances of terrorists even possessing a nuclear device.
D) Yes, a small dirty bomb was exploded during the Iraq War.
E) Yes, terrorists have entered a nuclear power plant in Pakistan and dispersed radioactive materials.
A) No, although a terrorist has been arrested who possessed and planned to use a stolen nuclear weapon.
B) No, although a terrorist has been arrested who possessed and planned to use a dirty bomb.
C) No, in fact there are no known instances of terrorists even possessing a nuclear device.
D) Yes, a small dirty bomb was exploded during the Iraq War.
E) Yes, terrorists have entered a nuclear power plant in Pakistan and dispersed radioactive materials.

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
8
Centrifuge separation, a method of separating 235U from 238U, works because of
A) the difference in the masses of the two nuclei.
B) the fact that both these nuclei are radioactive.
C) the difference in electrical charge of the two nuclei.
D) the difference in size of the two nuclei.
E) the fact that both these nuclei respond to (or feel) the strong nuclear force.
A) the difference in the masses of the two nuclei.
B) the fact that both these nuclei are radioactive.
C) the difference in electrical charge of the two nuclei.
D) the difference in size of the two nuclei.
E) the fact that both these nuclei respond to (or feel) the strong nuclear force.

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
9
Other than radioactive decay, how can energy be released from the nucleus?
A) Nuclear fusion.
B) Nuclear fission.
C) Both of the above.
D) Thermal decay.
E) All of the above.
A) Nuclear fusion.
B) Nuclear fission.
C) Both of the above.
D) Thermal decay.
E) All of the above.

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
10
Which of the following works best for fusing hydrogen?
A) Bring the hydrogen nuclei together very slowly until they hold together.
B) Heat it up to such a high temperature that collisions cause the nuclei to fuse.
C) Hit the hydrogen nucleus with a neutron.
D) Hit the hydrogen nucleus with an electron.
E) Cool the hydrogen until the nuclei are at rest and then suddenly compress them.
A) Bring the hydrogen nuclei together very slowly until they hold together.
B) Heat it up to such a high temperature that collisions cause the nuclei to fuse.
C) Hit the hydrogen nucleus with a neutron.
D) Hit the hydrogen nucleus with an electron.
E) Cool the hydrogen until the nuclei are at rest and then suddenly compress them.

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
11
Lise Meitner was
A) one of the discoverers of nuclear fission.
B) one of the discoverers of radioactivity.
C) one of the discoverers of nuclear fusion.
D) a leader in developing the world's first nuclear reactor.
E) discoverer of the ozone hole over the southern polar region.
A) one of the discoverers of nuclear fission.
B) one of the discoverers of radioactivity.
C) one of the discoverers of nuclear fusion.
D) a leader in developing the world's first nuclear reactor.
E) discoverer of the ozone hole over the southern polar region.

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
12
When 1H and 2H fuse, the result is
A) a particle with a mass less than the sum of the mass of 1H plus the mass of 2H.
B) a particle with a mass equal to the sum of the mass of 1H plus the mass of 2H.
C) crabgrass.
D) a particle with a mass greater than the sum of the mass of 1H plus the mass of 2H.
E) the annihilation of both nuclei and the production of energy in the form of radiation.
A) a particle with a mass less than the sum of the mass of 1H plus the mass of 2H.
B) a particle with a mass equal to the sum of the mass of 1H plus the mass of 2H.
C) crabgrass.
D) a particle with a mass greater than the sum of the mass of 1H plus the mass of 2H.
E) the annihilation of both nuclei and the production of energy in the form of radiation.

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
13
The astronomical phenomenon that created the elements heavier than iron is
A) neutron stars.
B) the collapse of a star to a white dwarf.
C) supernova explosions.
D) black holes.
E) the birth [or formation] of stars.
A) neutron stars.
B) the collapse of a star to a white dwarf.
C) supernova explosions.
D) black holes.
E) the birth [or formation] of stars.

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
14
When a hydrogen bomb explodes, hydrogen fuses to form helium. According to Einstein's mass- energy equivalence [that is, according to E=mc2], does this result in an change in the total mass of the material?
A) Yes, the mass increases, but by such a small amount that it would be impossible to measure it.
B) Yes, the mass decreases, and by a measurable amount.
C) Yes, the mass increases, and by a measurable amount.
D) Yes, the mass decreases, but by such a small amount that it would be impossible to measure it.
E) No, the mass remains exactly the same.
A) Yes, the mass increases, but by such a small amount that it would be impossible to measure it.
B) Yes, the mass decreases, and by a measurable amount.
C) Yes, the mass increases, and by a measurable amount.
D) Yes, the mass decreases, but by such a small amount that it would be impossible to measure it.
E) No, the mass remains exactly the same.

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
15
In order for a substance to sustain a fission chain reaction, one important condition is that
A) the nuclei must collide with enough energy to overcome the electrical repulsion between them.
B) energy must be continually supplied from the outside.
C) each fissioning nucleus must release a sufficient number of alpha- particles.
D) each fissioning nucleus must release a sufficient number of neutrons.
E) the nuclei must stay in shape by maintaining a regular jogging program.
A) the nuclei must collide with enough energy to overcome the electrical repulsion between them.
B) energy must be continually supplied from the outside.
C) each fissioning nucleus must release a sufficient number of alpha- particles.
D) each fissioning nucleus must release a sufficient number of neutrons.
E) the nuclei must stay in shape by maintaining a regular jogging program.

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
16
Which of the following works best for fissioning a uranium nucleus?
A) Heat it up to such a high temperature that collisions cause uranium nuclei to split.
B) Hit it with a neutron.
C) Hit it with a proton.
D) Hit it with another uranium nucleus.
E) Hit it with an electron.
A) Heat it up to such a high temperature that collisions cause uranium nuclei to split.
B) Hit it with a neutron.
C) Hit it with a proton.
D) Hit it with another uranium nucleus.
E) Hit it with an electron.

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
17
In a fusion bomb, what initiates, or triggers, the fusion reaction?
A) nuclear magnetic resonance
B) a laser beam
C) a sudden release of neutrons
D) a fission explosion
E) conventional chemical explosive, designed to implode instead of explode
A) nuclear magnetic resonance
B) a laser beam
C) a sudden release of neutrons
D) a fission explosion
E) conventional chemical explosive, designed to implode instead of explode

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
18
A "critical mass" is needed for a chain reaction because
A) if the mass is too small the temperature achieved during fission will be too low to sustain the reaction.
B) if the mass is too small, there will not be enough uranium to start the reaction.
C) if the mass is too large, the temperature will get too high and the entire mass will blow up without fissioning.
D) if the mass is too large, the density will be too high to sustain a chain reaction.
E) if the mass is too small, too many neutrons will leak out without causing fission.
A) if the mass is too small the temperature achieved during fission will be too low to sustain the reaction.
B) if the mass is too small, there will not be enough uranium to start the reaction.
C) if the mass is too large, the temperature will get too high and the entire mass will blow up without fissioning.
D) if the mass is too large, the density will be too high to sustain a chain reaction.
E) if the mass is too small, too many neutrons will leak out without causing fission.

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
19
In one popular type of fusion reaction, 2H fuses with 3H to yield 4He and
A) a neutron.
B) an alpha particle.
C) a beta particle.
D) a proton.
E) Actually no other particle is produced.
A) a neutron.
B) an alpha particle.
C) a beta particle.
D) a proton.
E) Actually no other particle is produced.

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
20
One nuclear reaction that occurs occasionally in the sun is the fusion of three alpha particles. What isotope is produced by this reaction? NOTE: Atomic number 6 is carbon, number 7 is nitrogen, and number 8 is oxygen.
A) 14N
B) 12O
C) 14C
D) 12C
E) 12N
A) 14N
B) 12O
C) 14C
D) 12C
E) 12N

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
21
The source of energy for the Hiroshima bomb was basically the same as the source of energy for
A) hydro- electric plants.
B) future fusion reactors.
C) the sun.
D) nuclear power reactors.
E) other chemical reactions.
A) hydro- electric plants.
B) future fusion reactors.
C) the sun.
D) nuclear power reactors.
E) other chemical reactions.

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
22
The United States originally started the fission bomb project because
A) it was afraid that Germany was developing it.
B) its military wanted the biggest bomb possible.
C) it seemed like it would be fun.
D) it thought it would need it against Japan.
E) it wanted to threaten Russia with it after the war.
A) it was afraid that Germany was developing it.
B) its military wanted the biggest bomb possible.
C) it seemed like it would be fun.
D) it thought it would need it against Japan.
E) it wanted to threaten Russia with it after the war.

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
23
What is the source of the energy for the creation of heavy elements [heavier than iron]?
A) Fission.
B) Hydrogen fusion.
C) Helium fusion.
D) Gravitational collapse.
E) The shock of a supernova explosion.
A) Fission.
B) Hydrogen fusion.
C) Helium fusion.
D) Gravitational collapse.
E) The shock of a supernova explosion.

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
24
Recalling that iron lies at the lowest point of the "nuclear energy curve," which of the following nuclear reactions would "release" [that is, convert to other forms] nuclear energy from iron?
A) Fission
B) Fusion
C) Both of the above.
D) None of the above.
E) This is the wrong answer.
A) Fission
B) Fusion
C) Both of the above.
D) None of the above.
E) This is the wrong answer.

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
25
The A- bomb is based on
A) nuclear fission.
B) a chemical reaction.
C) nuclear fusion.
D) popcorn.
E) radioactive decay.
A) nuclear fission.
B) a chemical reaction.
C) nuclear fusion.
D) popcorn.
E) radioactive decay.

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
26
The naturally- occurring isotope that will sustain a fission chain reaction is
A) 238U.
B) 235U.
C) 239Pu.
D) 14C.
E) tritium, i.e., 3H.
A) 238U.
B) 235U.
C) 239Pu.
D) 14C.
E) tritium, i.e., 3H.

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
27
The fission reaction involves
A) the splitting of a large molecule into smaller molecules.
B) the combining of two low- mass nuclei.
C) the splitting of a heavy nucleus.
D) the combining of two lighter molecules into a heavier molecule.
E) the splitting of a light nucleus.
A) the splitting of a large molecule into smaller molecules.
B) the combining of two low- mass nuclei.
C) the splitting of a heavy nucleus.
D) the combining of two lighter molecules into a heavier molecule.
E) the splitting of a light nucleus.

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
28
The four plausible routes by which terrorists might use nuclear devices include
A) obtaining weapons- grade uranium or plutonium and building a nuclear weapon from it.
B) seizing an intact nuclear weapon.
C) Both of the above.
D) sabotaging a nuclear power plant.
E) All of the above.
A) obtaining weapons- grade uranium or plutonium and building a nuclear weapon from it.
B) seizing an intact nuclear weapon.
C) Both of the above.
D) sabotaging a nuclear power plant.
E) All of the above.

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
29
The sun's energy comes from
A) the fission of helium into hydrogen.
B) the fission of uranium.
C) the fusion of helium into hydrogen.
D) the fusion of hydrogen into helium.
E) None of the above.
A) the fission of helium into hydrogen.
B) the fission of uranium.
C) the fusion of helium into hydrogen.
D) the fusion of hydrogen into helium.
E) None of the above.

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
30
The term "dirty bomb" refers to
A) a bomb that uses conventional explosive to disperse radioactive materials.
B) any nuclear device that is used for terrorist purposes.
C) a conventional chemical bomb that disperses pieces of metal in order to harm the maximum number of people.
D) a nuclear device that releases neutrons in order to kill people without harming buildings and other structures.
E) a nuclear device that is optimized for the release of radioactive materials.
A) a bomb that uses conventional explosive to disperse radioactive materials.
B) any nuclear device that is used for terrorist purposes.
C) a conventional chemical bomb that disperses pieces of metal in order to harm the maximum number of people.
D) a nuclear device that releases neutrons in order to kill people without harming buildings and other structures.
E) a nuclear device that is optimized for the release of radioactive materials.

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
31
Except for hydrogen, helium, and a little lithium, the lower- mass elements (lighter than iron) that are spread throughout the dust of the universe were created
A) in the original creation of the universe.
B) during the fusion process that powers the stars, and then released in supernova explosions.
C) in Kansas.
D) during the shock of a supernova explosion.
E) in hot white dwarf stars.
A) in the original creation of the universe.
B) during the fusion process that powers the stars, and then released in supernova explosions.
C) in Kansas.
D) during the shock of a supernova explosion.
E) in hot white dwarf stars.

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
32
The first laboratory discovery of nuclear fission occurred
A) in London in 1930.
B) in Germany in 1938.
C) in the United States in 1940.
D) in New Mexico in 1944
E) in Marie Curie's lab in the early 1900s.
A) in London in 1930.
B) in Germany in 1938.
C) in the United States in 1940.
D) in New Mexico in 1944
E) in Marie Curie's lab in the early 1900s.

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
33
Regarding the size, or total megatonnage, of a hydrogen bomb:
A) It is limited to about 10 megatons by the fact that tritium has a short half- life and thus must react very quickly.
B) It is unlimited, because there is no limit to the amount of fusible material that can be put together.
C) It is unlimited, because there is no limit to the amount of chain- reacting material that can be put together.
D) It is limited, because of the existence of a "critical mass" for fusion, to about one megaton.
E) It is limited, because of the existence of a "critical mass" for fusion, to about 20 kilotons.
A) It is limited to about 10 megatons by the fact that tritium has a short half- life and thus must react very quickly.
B) It is unlimited, because there is no limit to the amount of fusible material that can be put together.
C) It is unlimited, because there is no limit to the amount of chain- reacting material that can be put together.
D) It is limited, because of the existence of a "critical mass" for fusion, to about one megaton.
E) It is limited, because of the existence of a "critical mass" for fusion, to about 20 kilotons.

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
34
Most of the hydrogen bomb's energy comes from
A) fission.
B) fusion.
C) a chemical reaction.
D) magic.
E) radioactive decay.
A) fission.
B) fusion.
C) a chemical reaction.
D) magic.
E) radioactive decay.

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
35
The element iron, having atomic number 26, is the "crossover point" between fusion and fission. Why?
A) Because all elements heavier than iron are radioactive, implying that they will fission easier than they will fuse.
B) Because for elements lighter than iron, fusion converts nuclear energy to non- nuclear energy, whereas for elements heavier than iron, fission converts nuclear energy to non- nuclear energy.
C) Because for elements lighter than iron, fusion converts non- nuclear energy to nuclear energy, whereas for elements heavier than iron, fission converts non- nuclear energy to nuclear energy.
D) Because neutrons will only split the elements that are heavier than iron.
E) Because of the neutron balance that results from splitting a nucleus.
A) Because all elements heavier than iron are radioactive, implying that they will fission easier than they will fuse.
B) Because for elements lighter than iron, fusion converts nuclear energy to non- nuclear energy, whereas for elements heavier than iron, fission converts nuclear energy to non- nuclear energy.
C) Because for elements lighter than iron, fusion converts non- nuclear energy to nuclear energy, whereas for elements heavier than iron, fission converts non- nuclear energy to nuclear energy.
D) Because neutrons will only split the elements that are heavier than iron.
E) Because of the neutron balance that results from splitting a nucleus.

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
36
In a fission reaction, such as occurs in a nuclear power plant,
A) nuclear energy is converted to thermal energy and other forms of energy.
B) energy is created.
C) nuclear energy is created.
D) thermal energy and other forms of energy are converted to nuclear energy.
E) heat is converted to work.
A) nuclear energy is converted to thermal energy and other forms of energy.
B) energy is created.
C) nuclear energy is created.
D) thermal energy and other forms of energy are converted to nuclear energy.
E) heat is converted to work.

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
37
According to the nuclear energy curve shown in the graph above, nuclear energy can be transformed to other forms by
A) fusing nuclei heavier than iron.
B) fusing nuclei lighter than iron.
C) fissioning nuclei lighter than iron.
D) ironing your clothes.
E) fissioning any nuclei including iron.
A) fusing nuclei heavier than iron.
B) fusing nuclei lighter than iron.
C) fissioning nuclei lighter than iron.
D) ironing your clothes.
E) fissioning any nuclei including iron.

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
38
The nuclear energy curve shown in the graph above tells us that iron is
A) the most stable nucleus, because the least work is needed to pull it apart into separate particles.
B) the most stable nucleus, because the most work is needed to pull it apart into separate particles.
C) the least stable nucleus, because the least work is needed to pull it apart into separate particles.
D) good to eat.
E) the least stable nucleus, because the most work is needed to pull it apart into separate particles.
A) the most stable nucleus, because the least work is needed to pull it apart into separate particles.
B) the most stable nucleus, because the most work is needed to pull it apart into separate particles.
C) the least stable nucleus, because the least work is needed to pull it apart into separate particles.
D) good to eat.
E) the least stable nucleus, because the most work is needed to pull it apart into separate particles.

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
39
The two nuclei used for the fuel in fission bombs are
A) "235Th and 235U."
B) "234Th and 239Pu."
C) "235U and 239Pu."
D) "238U and 239Pu."
E) "234Th and 238U."
A) "235Th and 235U."
B) "234Th and 239Pu."
C) "235U and 239Pu."
D) "238U and 239Pu."
E) "234Th and 238U."

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
40
In fission bombs, a certain "critical mass" is needed because
A) the fissionable material will not reach a sufficiently high temperature unless this much material is present.
B) too small an amount of uranium will not hold together throughout the reaction, and thus the uranium falls apart and the reaction goes out before it is completed.
C) for smaller masses the pressure is so low that neutrons move too slowly through the uranium to sustain a chain reaction.
D) if the mass is too small, too many neutrons pass through the uranium and out the sides without hitting a nucleus.
E) it is impossible to fission a uranium nucleus unless it is near a large number of other uranium nuclei.
A) the fissionable material will not reach a sufficiently high temperature unless this much material is present.
B) too small an amount of uranium will not hold together throughout the reaction, and thus the uranium falls apart and the reaction goes out before it is completed.
C) for smaller masses the pressure is so low that neutrons move too slowly through the uranium to sustain a chain reaction.
D) if the mass is too small, too many neutrons pass through the uranium and out the sides without hitting a nucleus.
E) it is impossible to fission a uranium nucleus unless it is near a large number of other uranium nuclei.

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
41
The source of the sun's energy is
A) gravitational energy.
B) nuclear fusion.
C) nuclear fission.
D) granola bars.
E) chemical reactions.
A) gravitational energy.
B) nuclear fusion.
C) nuclear fission.
D) granola bars.
E) chemical reactions.

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
42
According to the nuclear energy curve, would fusion or fission or neither release nuclear energy from gold [atomic number 79], and why?
A) Fusion, because then the reaction product has a lower atomic number and thus a higher nuclear energy.
B) Neither, because all other elements have higher nuclear energies than carbon.
C) Fission, because then the reaction products have lower atomic numbers and thus a lower nuclear energy.
D) Fusion, because then the reaction product has a higher atomic number and thus a lower nuclear energy.
E) Fission, because then the reaction products have higher atomic numbers and thus a higher nuclear energy.
A) Fusion, because then the reaction product has a lower atomic number and thus a higher nuclear energy.
B) Neither, because all other elements have higher nuclear energies than carbon.
C) Fission, because then the reaction products have lower atomic numbers and thus a lower nuclear energy.
D) Fusion, because then the reaction product has a higher atomic number and thus a lower nuclear energy.
E) Fission, because then the reaction products have higher atomic numbers and thus a higher nuclear energy.

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
43
A neutron makes a better "nuclear bullet", for fissioning a nucleus, than does a proton. This is because
A) it is easy to accelerate neutrons to high speeds in nuclear accelerators.
B) the negative charge on the neutron attracts it to the nucleus.
C) unlike the proton, the neutron is not repelled by the charge on the nucleus.
D) protons do not feel the strong nuclear force and thus do not interact appreciably with the nucleus.
E) neutrons are so much more massive than protons.
A) it is easy to accelerate neutrons to high speeds in nuclear accelerators.
B) the negative charge on the neutron attracts it to the nucleus.
C) unlike the proton, the neutron is not repelled by the charge on the nucleus.
D) protons do not feel the strong nuclear force and thus do not interact appreciably with the nucleus.
E) neutrons are so much more massive than protons.

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
44
In which of the following nuclear processes are other forms of energy converted into nuclear energy? In other words, in which of these processes is nuclear energy created?
A) Exothermic ["heat producing"] chemical reactions.
B) Endothermic ["heat consuming"] chemical reactions.
C) In nuclear fission of the lighter elements and nuclear fusion of the heavier elements.
D) In nuclear fusion of the lighter elements and nuclear fission of the heavier elements.
E) Supernova explosions, i.e., explosions of stars.
A) Exothermic ["heat producing"] chemical reactions.
B) Endothermic ["heat consuming"] chemical reactions.
C) In nuclear fission of the lighter elements and nuclear fusion of the heavier elements.
D) In nuclear fusion of the lighter elements and nuclear fission of the heavier elements.
E) Supernova explosions, i.e., explosions of stars.

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
45
The four plausible routes by which terrorists might use nuclear devices do not include
A) seizing an intact nuclear weapon.
B) sabotaging a nuclear power plant.
C) enriching natural uranium to weapons grade uranium, and building a nuclear weapon from it.
D) acquiring radioactive material and using conventional explosive to disperse this material.
E) obtaining weapons- grade uranium or plutonium and building a nuclear weapon from it.
A) seizing an intact nuclear weapon.
B) sabotaging a nuclear power plant.
C) enriching natural uranium to weapons grade uranium, and building a nuclear weapon from it.
D) acquiring radioactive material and using conventional explosive to disperse this material.
E) obtaining weapons- grade uranium or plutonium and building a nuclear weapon from it.

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
46
All fission bombs are based on
A) either uranium or plutonium or both.
B) isotopes of hydrogen.
C) laughing gas.
D) uranium.
E) either isotopes of hydrogen or uranium or both.
A) either uranium or plutonium or both.
B) isotopes of hydrogen.
C) laughing gas.
D) uranium.
E) either isotopes of hydrogen or uranium or both.

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
47
Edward Teller was
A) the head of the program to develop the world's first fission bomb.
B) the head of the program to develop the world's first nuclear reactor.
C) the discoverer of nuclear fission.
D) the inventor of radiation therapy.
E) the head of the program to develop the world's first fusion bomb.
A) the head of the program to develop the world's first fission bomb.
B) the head of the program to develop the world's first nuclear reactor.
C) the discoverer of nuclear fission.
D) the inventor of radiation therapy.
E) the head of the program to develop the world's first fusion bomb.

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
48
In a self- sustaining fusion reaction, such as occurs in the sun,
A) nuclear energy is converted to other forms of energy.
B) energy is created.
C) thermal energy is converted to nuclear energy.
D) energy is destroyed.
E) nuclear energy is created from other forms of energy.
A) nuclear energy is converted to other forms of energy.
B) energy is created.
C) thermal energy is converted to nuclear energy.
D) energy is destroyed.
E) nuclear energy is created from other forms of energy.

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
49
The four plausible routes by which terrorists might use nuclear devices include
A) acquiring radioactive material and using conventional explosive to disperse this material.
B) seizing an intact nuclear weapon.
C) Both of the above.
D) enriching natural uranium to weapons grade uranium, and building a nuclear weapon from it.
E) All of the above.
A) acquiring radioactive material and using conventional explosive to disperse this material.
B) seizing an intact nuclear weapon.
C) Both of the above.
D) enriching natural uranium to weapons grade uranium, and building a nuclear weapon from it.
E) All of the above.

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
50
Energetically, a fusion reaction can be self- sustaining whenever
A) the reaction proceeds from lower to higher energies on the nuclear energy curve.
B) the reaction proceeds from higher to lower energies on the nuclear energy curve.
C) the reaction proceeds from lighter nuclei to heavier nuclei on the nuclear energy curve.
D) the reaction proceeds from heavier to lighter nuclei on the nuclear energy curve.
E) the reaction obeys the principle of conservation of total energy.
A) the reaction proceeds from lower to higher energies on the nuclear energy curve.
B) the reaction proceeds from higher to lower energies on the nuclear energy curve.
C) the reaction proceeds from lighter nuclei to heavier nuclei on the nuclear energy curve.
D) the reaction proceeds from heavier to lighter nuclei on the nuclear energy curve.
E) the reaction obeys the principle of conservation of total energy.

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
51
Which situation has more energy, a 2 H nucleus, or a separated proton and neutron?
A) They both have the same energy, because energy is always conserved in any physical process.
B) A separated proton and neutron, because work is required to put a 2 H nucleus together.
C) A 2 H nucleus, because work is required to put it together beginning from a separated proton and neutron.
D) A separated proton and neutron has more energy, because work is required to pull a 2 H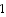 nucleus apart.
nucleus apart.
E) A 2 H nucleus, because work is required to pull a 2 H nucleus apart.
A) They both have the same energy, because energy is always conserved in any physical process.
B) A separated proton and neutron, because work is required to put a 2 H nucleus together.
C) A 2 H nucleus, because work is required to put it together beginning from a separated proton and neutron.
D) A separated proton and neutron has more energy, because work is required to pull a 2 H
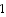 nucleus apart.
nucleus apart.E) A 2 H nucleus, because work is required to pull a 2 H nucleus apart.

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
52
In the fusion of low- mass nuclei to make heavier nuclei
A) thermal energy is produced.
B) the final nucleus has a smaller rest- mass than the total rest- mass of the original nuclei.
C) nuclear energy is converted to other forms.
D) All of the above.
A) thermal energy is produced.
B) the final nucleus has a smaller rest- mass than the total rest- mass of the original nuclei.
C) nuclear energy is converted to other forms.
D) All of the above.

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
53
Hydrogen must be raised to a high temperature before it will fuse. This is because
A) high temperatures are needed to overcome the strong nuclear force between hydrogen nuclei.
B) high temperatures are needed to overcome the electric repulsion between hydrogen nuclei.
C) fusion and all other forms of chemical combustion must be initiated by high temperatures.
D) this reaction consumes more thermal energy than it produces.
E) this reaction must consume a large amount of thermal energy in order to produce nuclear energy.
A) high temperatures are needed to overcome the strong nuclear force between hydrogen nuclei.
B) high temperatures are needed to overcome the electric repulsion between hydrogen nuclei.
C) fusion and all other forms of chemical combustion must be initiated by high temperatures.
D) this reaction consumes more thermal energy than it produces.
E) this reaction must consume a large amount of thermal energy in order to produce nuclear energy.

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
54
Fallout from nuclear weapons or from a nuclear reactor accident is due to
A) the uranium nuclei used in the fission reaction.
B) the radioactive "fusion products" produced during the fusion reaction.
C) the neutrons produced during the fission reaction.
D) the radioactive "fission products" produced during the fission reaction.
E) chickens flying overhead.
A) the uranium nuclei used in the fission reaction.
B) the radioactive "fusion products" produced during the fusion reaction.
C) the neutrons produced during the fission reaction.
D) the radioactive "fission products" produced during the fission reaction.
E) chickens flying overhead.

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
55
The two nuclei that can sustain a nuclear chain- reaction are
A) "235U and plutonium."
B) "thorium and plutonium."
C) "thorium and 235U."
D) "238U and plutonium."
E) "Actually, there is only one nucleus that can sustain a nuclear chain- reaction."
A) "235U and plutonium."
B) "thorium and plutonium."
C) "thorium and 235U."
D) "238U and plutonium."
E) "Actually, there is only one nucleus that can sustain a nuclear chain- reaction."

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
56
When two 4 He nuclei fuse, the resulting nucleus will have
A) atomic number 4 and mass number 8.
B) mass number 4 and atomic number 8.
C) atomic number 4 and mass number 4.
D) atomic number 2 and mass number 8.
E) None of the above.
A) atomic number 4 and mass number 8.
B) mass number 4 and atomic number 8.
C) atomic number 4 and mass number 4.
D) atomic number 2 and mass number 8.
E) None of the above.

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
57
In a nuclear reactor, how many neutrons [on the average] created during the fission of a 235U nucleus cause further fission in other uranium nuclei?
A) None
B) Less than one
C) More than one
D) One
E) Ten
A) None
B) Less than one
C) More than one
D) One
E) Ten

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
58
The two most plentiful elements in the universe are
A) hydrogen and helium.
B) nitrogen and helium.
C) lithium and helium.
D) carbon and oxygen.
E) hydrogen and oxygen.
A) hydrogen and helium.
B) nitrogen and helium.
C) lithium and helium.
D) carbon and oxygen.
E) hydrogen and oxygen.

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
59
What material was used as fuel in the world's first nuclear reactor?
A) 239Pu
B) 238U
C) 14C
D) 235U
E) 2H
A) 239Pu
B) 238U
C) 14C
D) 235U
E) 2H

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
60
Most of the elements heavier than iron that are spread throughout the dust of the universe were created
A) during the fusion process that powers the stars, and then released in supernova explosions.
B) during the shock of a supernova explosion.
C) in the original creation of the universe.
D) in college cafeterias.
E) in hot white dwarf stars.
A) during the fusion process that powers the stars, and then released in supernova explosions.
B) during the shock of a supernova explosion.
C) in the original creation of the universe.
D) in college cafeterias.
E) in hot white dwarf stars.

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
61
J. Robert Oppenheimer was
A) the head of the program to develop the world's first nuclear reactor.
B) the inventor of radiation therapy.
C) the head of the program to develop the world's first fusion bomb.
D) the discoverer of nuclear fission.
E) the head of the program to develop the world's first fission bomb.
A) the head of the program to develop the world's first nuclear reactor.
B) the inventor of radiation therapy.
C) the head of the program to develop the world's first fusion bomb.
D) the discoverer of nuclear fission.
E) the head of the program to develop the world's first fission bomb.

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
62
Nuclear fusion creates
A) thermal energy.
B) radiant energy.
C) both thermal and radiant energy.
D) nuclear energy.
E) all types of energy.
A) thermal energy.
B) radiant energy.
C) both thermal and radiant energy.
D) nuclear energy.
E) all types of energy.

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
63
Which of the following is needed in order to produce a uranium fission bomb?
A) deuterium and tritium
B) plutonium
C) an isotope separation, or enrichment, facility
D) All of the above.
E) None of the above.
A) deuterium and tritium
B) plutonium
C) an isotope separation, or enrichment, facility
D) All of the above.
E) None of the above.

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
64
In a fission bomb explosion, how many neutrons [on the average] created during the fission of a 235U nucleus cause further fission in other uranium nuclei?
A) None
B) Less than one
C) More than one
D) One
E) Ten
A) None
B) Less than one
C) More than one
D) One
E) Ten

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
65
Naturally occurring uranium is mostly
A) 14U.
B) 235U.
C) 233U.
D) 238U.
E) good to eat.
A) 14U.
B) 235U.
C) 233U.
D) 238U.
E) good to eat.

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
66
When 1H fuses with 2H to form 3He, the energy transformation could best be described as follows:
A) nuclear energy is converted into chemical energy.
B) thermal and radiant energy is converted into nuclear energy.
C) nuclear energy is converted into thermal and radiant energy.
D) chemical energy is converted into thermal energy.
E) electromagnetic energy is converted into nuclear energy.
A) nuclear energy is converted into chemical energy.
B) thermal and radiant energy is converted into nuclear energy.
C) nuclear energy is converted into thermal and radiant energy.
D) chemical energy is converted into thermal energy.
E) electromagnetic energy is converted into nuclear energy.

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
67
The bombs used against Japan were
A) a uranium bomb and a plutonium bomb.
B) two plutonium bombs.
C) two uranium bombs.
D) two fusion bombs.
E) an fission bomb and a fusion bomb.
A) a uranium bomb and a plutonium bomb.
B) two plutonium bombs.
C) two uranium bombs.
D) two fusion bombs.
E) an fission bomb and a fusion bomb.

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
68
When two 4 He nuclei fuse, the energy transformation is
A) radiant E + thermal E ·nuclear E.
B) thermal E + nuclear E ·radiant E.
C) nuclear E ·radiant E + thermal E.
D) chemical E ·radiant E + thermal E.
E) thermal E ·nuclear E + radiant E.
A) radiant E + thermal E ·nuclear E.
B) thermal E + nuclear E ·radiant E.
C) nuclear E ·radiant E + thermal E.
D) chemical E ·radiant E + thermal E.
E) thermal E ·nuclear E + radiant E.

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
69
The four plausible routes by which terrorists might use nuclear devices include
A) acquiring radioactive material and using conventional explosive to disperse this material.
B) enriching natural uranium to weapons grade uranium, and building a nuclear weapon from it.
C) obtaining plutonium by the chemical reprocessing of used fuel rods, and building a nuclear weapon from it.
D) All of the above.
E) None of the above.
A) acquiring radioactive material and using conventional explosive to disperse this material.
B) enriching natural uranium to weapons grade uranium, and building a nuclear weapon from it.
C) obtaining plutonium by the chemical reprocessing of used fuel rods, and building a nuclear weapon from it.
D) All of the above.
E) None of the above.

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
70
The percentage of 235U, as a fraction of the total uranium content, in a uranium fission bomb is closest to
A) 20 percent.
B) 50 percent.
C) 90 percent.
D) 5 percent.
E) 1 percent.
A) 20 percent.
B) 50 percent.
C) 90 percent.
D) 5 percent.
E) 1 percent.

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
71
The percentage of 235U, as a fraction of the total uranium content, present in natural uranium is closest to
A) 50 percent.
B) 20 percent.
C) 1 percent.
D) 5 percent.
E) 99 percent.
A) 50 percent.
B) 20 percent.
C) 1 percent.
D) 5 percent.
E) 99 percent.

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
72
When we fission the U- 235 nucleus, the total rest- mass of all the resulting fragments of matter
A) is less than the mass of the original nucleus.
B) is more than the mass of the original nucleus
C) is turned into broccoli.
D) is the same as the mass of the original nucleus.
E) None of the above.
A) is less than the mass of the original nucleus.
B) is more than the mass of the original nucleus
C) is turned into broccoli.
D) is the same as the mass of the original nucleus.
E) None of the above.

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
73
In the nuclear energy curve shown in the graph above, what quantity is graphed along the vertical axis?
A) the amount of thermal energy released [per nuclear particle] by nuclear reactions of each nucleus.
B) the amount of nuclear energy [per nuclear particle] in each nucleus.
C) Both of the above.
D) the amount of work required [per nuclear particle] to fuse or fission each nucleus.
E) All of the above.
A) the amount of thermal energy released [per nuclear particle] by nuclear reactions of each nucleus.
B) the amount of nuclear energy [per nuclear particle] in each nucleus.
C) Both of the above.
D) the amount of work required [per nuclear particle] to fuse or fission each nucleus.
E) All of the above.

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
74
Enrico Fermi was
A) the head of the program to develop the world's first fusion bomb.
B) the discoverer of nuclear fission.
C) the inventor of radiation therapy.
D) the head of the program to develop the world's first nuclear reactor.
E) the head of the program to develop the world's first fission bomb.
A) the head of the program to develop the world's first fusion bomb.
B) the discoverer of nuclear fission.
C) the inventor of radiation therapy.
D) the head of the program to develop the world's first nuclear reactor.
E) the head of the program to develop the world's first fission bomb.

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
75
A "supernova" is
A) a large new star.
B) a new dance step.
C) an exploding galaxy.
D) an exploding star.
E) a slight flare- up of an aged star.
A) a large new star.
B) a new dance step.
C) an exploding galaxy.
D) an exploding star.
E) a slight flare- up of an aged star.

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
76
Who headed the project to construct the world's first human- made nuclear reactor?
A) Leo Szilard
B) J. Robert Oppenheimer
C) Enrico Fermi
D) Edward Teller
E) Albert Einstein
A) Leo Szilard
B) J. Robert Oppenheimer
C) Enrico Fermi
D) Edward Teller
E) Albert Einstein

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
77
Which particle plays the key role in sustaining a fission reaction?
A) neutron
B) quark
C) gamma photon
D) proton
E) electron
A) neutron
B) quark
C) gamma photon
D) proton
E) electron

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck


