Deck 14: The Nucleus and Radioactivity: an New Force
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/77
Play
Full screen (f)
Deck 14: The Nucleus and Radioactivity: an New Force
1
How do the masses of the nuclei of 3 H and 3 He compare? 1 2
A) "3 He is approximately three times as massive as 3 H. 2 1"
B) "3 He is approximately 5/4 times as massive as 3 H. 2 1"
C) "Their masses are approximately the same."
D) "3 He is approximately twice as massive as 3 H. 2 1"
E) "None of the above."
A) "3 He is approximately three times as massive as 3 H. 2 1"
B) "3 He is approximately 5/4 times as massive as 3 H. 2 1"
C) "Their masses are approximately the same."
D) "3 He is approximately twice as massive as 3 H. 2 1"
E) "None of the above."
"Their masses are approximately the same."
2
Quantum uncertainties are the source of
A) the unpredictability of radioactive decay.
B) the unpredictability of games of chance such as flipping coins.
C) Both of the above.
D) None of the above.
A) the unpredictability of radioactive decay.
B) the unpredictability of games of chance such as flipping coins.
C) Both of the above.
D) None of the above.
the unpredictability of radioactive decay.
3
90 Sr is a radioactive isotope that decays by beta- decay. Its daughter nucleus is
A) "86 Kr."
B) "89 Y."
C) "90 Y."
D) "89 Rb."
E) "88 Rb."
A) "86 Kr."
B) "89 Y."
C) "90 Y."
D) "89 Rb."
E) "88 Rb."
"90 Y."
4
Nuclear energies are much larger than chemical energies because
A) the electromagnetic force has such a long range compared to the nuclear force.
B) the nuclear force is so much stronger than the electromagnetic force.
C) there are so many sub- atomic particles in the nucleus.
D) the nuclear force is so much stronger than the gravitational force.
E) nuclear forces act over such a long range.
A) the electromagnetic force has such a long range compared to the nuclear force.
B) the nuclear force is so much stronger than the electromagnetic force.
C) there are so many sub- atomic particles in the nucleus.
D) the nuclear force is so much stronger than the gravitational force.
E) nuclear forces act over such a long range.

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
5
Can an element decay "backward" in the periodic table, to a lower atomic number?
A) No, in fact elements cannot alter their atomic number by radioactive decay.
B) No, but an element can decay to a higher atomic number.
C) Yes, by alpha decay.
D) Yes, by beta decay.
E) Yes, radioactive decay always causes elements to decay to a lower atomic number.
A) No, in fact elements cannot alter their atomic number by radioactive decay.
B) No, but an element can decay to a higher atomic number.
C) Yes, by alpha decay.
D) Yes, by beta decay.
E) Yes, radioactive decay always causes elements to decay to a lower atomic number.

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
6
Of the following list, the one that gives the smallest average ionizing radiation dose per person per year is
A) cosmic rays.
B) nuclear power plants in normal operation.
C) radon gas.
D) medical X- rays.
E) the rocks and soil of Earth.
A) cosmic rays.
B) nuclear power plants in normal operation.
C) radon gas.
D) medical X- rays.
E) the rocks and soil of Earth.

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
7
The isotope 226 Ra alpha decays to the daughter nucleus
A) "224 Rn."
B) "226 Ac."
C) "222 Rn."
D) "222 Po."
E) "222 Ra."
A) "224 Rn."
B) "226 Ac."
C) "222 Rn."
D) "222 Po."
E) "222 Ra."

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
8
It takes 6400 years for one gram of radium to decay away to only 1/16-one- sixteenth-of a gram. The half- life of radium is
A) not possible to determine from this information.
B) 800 years.
C) 400 years.
D) 3200 years.
E) 1600 years.
A) not possible to determine from this information.
B) 800 years.
C) 400 years.
D) 3200 years.
E) 1600 years.

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
9
Radioactive isotopes are useful for
A) tracing the path of certain chemicals.
B) cancer therapy.
C) dating the ages of objects.
D) All of the above.
E) None of the above.
A) tracing the path of certain chemicals.
B) cancer therapy.
C) dating the ages of objects.
D) All of the above.
E) None of the above.

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
10
Radon has a 4- day half- life. Starting with 10 radon atoms, how many will remain after 4 days?
A) About 5, but this prediction is uncertain due to the inaccuracies of our measuring devices.
B) Precisely five.
C) Either four, or five, or six.
D) Precisely zero.
E) About 5, but this prediction is uncertain due to quantum uncertainties.
A) About 5, but this prediction is uncertain due to the inaccuracies of our measuring devices.
B) Precisely five.
C) Either four, or five, or six.
D) Precisely zero.
E) About 5, but this prediction is uncertain due to quantum uncertainties.

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
11
The radioactive isotope 3H is a beta- emitter. When it decays, the result is
A) 4He.
B) 3He.
C) 1H.
D) 3H.
E) 2H.
A) 4He.
B) 3He.
C) 1H.
D) 3H.
E) 2H.

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
12
One of the radioactive isotopes that fell on the land and got into the food chain after the Chernobyl accident was 131I. Its half- life is 8 days. How long was it, following its deposit on the land, before its radioactivity had fallen to 1/32-- about 3%-- of its initial value?
A) 48 days
B) 40 days
C) several years
D) 16 days
E) It is impossible to say, even approximately.
A) 48 days
B) 40 days
C) several years
D) 16 days
E) It is impossible to say, even approximately.

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
13
The scientific consensus regarding the age of Earth is that
A) there is insufficient data to determine Earth's age.
B) Earth is about 5 thousand years old.
C) Earth is about 5 million years old.
D) Earth is about 5 billion years old.
E) Earth is about 100 billion years old.
A) there is insufficient data to determine Earth's age.
B) Earth is about 5 thousand years old.
C) Earth is about 5 million years old.
D) Earth is about 5 billion years old.
E) Earth is about 100 billion years old.

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
14
Radioactive decay occurs when
A) bacteria and other microscopic organisms digest nuclear particles.
B) a nucleus is struck and broken into pieces by a fast moving particle.
C) an atom loses one or more orbital electrons.
D) a nucleus emits a particle spontaneously
E) two or more nuclei fuse together.
A) bacteria and other microscopic organisms digest nuclear particles.
B) a nucleus is struck and broken into pieces by a fast moving particle.
C) an atom loses one or more orbital electrons.
D) a nucleus emits a particle spontaneously
E) two or more nuclei fuse together.

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
15
How does the mass of a 4 He nucleus compare with the mass of a 1 H nucleus? 2 1
A) "4 He is half as massive."
B) "4 He has about the same mass and 1 H. 2 1"
C) "4 He is four times as massive."
D) "4 He is twice as massive."
E) "It is impossible to answer this without further information."
A) "4 He is half as massive."
B) "4 He has about the same mass and 1 H. 2 1"
C) "4 He is four times as massive."
D) "4 He is twice as massive."
E) "It is impossible to answer this without further information."

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
16
Marie Curie is famous for
A) her invention of the electron microscope.
B) her studies of nuclear fusion.
C) her invention of the X- ray laser.
D) her discovery of fission.
E) her studies of radioactivity.
A) her invention of the electron microscope.
B) her studies of nuclear fusion.
C) her invention of the X- ray laser.
D) her discovery of fission.
E) her studies of radioactivity.

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
17
A common opinion concerning the creationism versus evolution controversy is the idea that Earth was created fairly recently but that it was created with fossils and other evidence of great age already present, so that Earth has the appearance of being very old even though it is really quite young. This opinion is best classified as
A) a scientific opinion that is certainly incorrect.
B) a scientific hypothesis for which there is currently insufficient evidence.
C) nonscientific because there is no conceivable scientific way to disprove it.
D) a scientific opinion that is probably incorrect.
E) nonscientific because it contradicts common sense.
A) a scientific opinion that is certainly incorrect.
B) a scientific hypothesis for which there is currently insufficient evidence.
C) nonscientific because there is no conceivable scientific way to disprove it.
D) a scientific opinion that is probably incorrect.
E) nonscientific because it contradicts common sense.

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
18
Which of the following is the strongest fundamental force acting between microscopic particles?
A) weak force
B) gravity
C) electromagnetic force
D) strong force
E) Elmer's glue
A) weak force
B) gravity
C) electromagnetic force
D) strong force
E) Elmer's glue

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
19
According to our best present predictions about the medical consequences of the Chernobyl nuclear power plant accident, the longer- term cancer deaths caused by that accident will
A) be medically indistinguishable from cancer deaths produced by other causes.
B) be very difficult, or impossible, to detect statistically among the large number of other cancer deaths.
C) be much more numerous than the roughly 40 deaths caused directly by short- term radiation effects.
D) All of the above.
E) None of the above.
A) be medically indistinguishable from cancer deaths produced by other causes.
B) be very difficult, or impossible, to detect statistically among the large number of other cancer deaths.
C) be much more numerous than the roughly 40 deaths caused directly by short- term radiation effects.
D) All of the above.
E) None of the above.

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
20
Can an element decay "forward" in the periodic table, to a higher atomic number?
A) No, in fact elements cannot alter their atomic number by radioactive decay.
B) No, but an element can decay to a lower atomic number.
C) Yes, by beta decay.
D) Yes, by alpha decay.
E) Yes, this can occur by means of both beta decay and alpha decay.
A) No, in fact elements cannot alter their atomic number by radioactive decay.
B) No, but an element can decay to a lower atomic number.
C) Yes, by beta decay.
D) Yes, by alpha decay.
E) Yes, this can occur by means of both beta decay and alpha decay.

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
21
234 Th beta- decays. The resulting nucleus has mass number
A) 234.
B) 230.
C) 91.
D) 88.
E) 90.
A) 234.
B) 230.
C) 91.
D) 88.
E) 90.

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
22
Except for such nuclear processes as nuclear reactors, nuclear bombs, and radioactivity, all of the common forces we see around us are due to just two underlying or "fundamental" forces. One of these two forces is the gravitational force. The other is
A) the color force.
B) the chemical force.
C) contact forces.
D) the frictional force.
E) the electromagnetic force.
A) the color force.
B) the chemical force.
C) contact forces.
D) the frictional force.
E) the electromagnetic force.

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
23
14C has a half- life of 6000 years. An ancient corpse is uncovered and found to have 1/32-- one thirty- second-- of the normal amount of 14C found in the bodies of people now living. How long has the body been dead?
A) 30,000 years
B) 24,000 years
C) 12,000 years
D) 36,000 years
E) 6000 years
A) 30,000 years
B) 24,000 years
C) 12,000 years
D) 36,000 years
E) 6000 years

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
24
Reasoning from the fact that it is difficult to pull a nucleus apart, we can conclude that the nuclear force must
A) be repulsive and very strong.
B) operate only between neutrons, and not between protons.
C) be attractive and very strong.
D) have a very long range.
E) have a very short range.
A) be repulsive and very strong.
B) operate only between neutrons, and not between protons.
C) be attractive and very strong.
D) have a very long range.
E) have a very short range.

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
25
The isotope 3 H has
A) 1 neutron and 2 protons.
B) 1 proton and 2 neutrons.
C) 1 neutron and 3 protons.
D) 1 proton and 3 neutrons.
E) None of the above.
A) 1 neutron and 2 protons.
B) 1 proton and 2 neutrons.
C) 1 neutron and 3 protons.
D) 1 proton and 3 neutrons.
E) None of the above.

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
26
How do the mass and charge of a 14C nucleus compare with the mass and charge of a 12C nucleus?
A) Both the mass and charge of 14C are one- sixth larger.
B) The mass of 14C is one- sixth larger, while the charge is the same.
C) The mass is the same, while the charge of 14C is one- sixth larger.
D) The mass of 14C is 50% larger, while the charge is the same.
E) Both the mass and the charge of 14C are the same as those of 12C.
A) Both the mass and charge of 14C are one- sixth larger.
B) The mass of 14C is one- sixth larger, while the charge is the same.
C) The mass is the same, while the charge of 14C is one- sixth larger.
D) The mass of 14C is 50% larger, while the charge is the same.
E) Both the mass and the charge of 14C are the same as those of 12C.

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
27
Which of the 4 fundamental forces binds the nucleus together, and which binds the atom [the electron orbits] together?
A) Gravity binds the nucleus, electric force binds the atom.
B) Strong nuclear force binds the nucleus, weak nuclear force binds the atom.
C) Electric force binds the nucleus, strong nuclear force binds the atom.
D) Weak nuclear force binds the nucleus, strong nuclear force binds the atom.
E) Strong nuclear force binds the nucleus, electric force binds the atom.
A) Gravity binds the nucleus, electric force binds the atom.
B) Strong nuclear force binds the nucleus, weak nuclear force binds the atom.
C) Electric force binds the nucleus, strong nuclear force binds the atom.
D) Weak nuclear force binds the nucleus, strong nuclear force binds the atom.
E) Strong nuclear force binds the nucleus, electric force binds the atom.

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
28
The isotope 2 H has
A) 1 proton and 3 neutrons.
B) 1 proton and 2 neutrons.
C) 1 proton and 1 neutron.
D) 1 neutron and 2 protons.
E) None of the above.
A) 1 proton and 3 neutrons.
B) 1 proton and 2 neutrons.
C) 1 proton and 1 neutron.
D) 1 neutron and 2 protons.
E) None of the above.

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
29
How old is Earth, according to the vast majority of scientists?
A) a few million years
B) about a billion years
C) about 100 billion years
D) a few thousand years
E) a few billion years
A) a few million years
B) about a billion years
C) about 100 billion years
D) a few thousand years
E) a few billion years

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
30
Reasoning from the fact that the nucleus is very small, we can conclude that the nuclear force must
A) be attractive and very strong.
B) have a very long range.
C) operate only between neutrons, and not between protons.
D) have a very short range.
E) be repulsive and very strong.
A) be attractive and very strong.
B) have a very long range.
C) operate only between neutrons, and not between protons.
D) have a very short range.
E) be repulsive and very strong.

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
31
The biological damage caused by radioactive materials is due to
A) nuclear fusion occurring within biological cells.
B) thermal energy created by radioactive decay within biological cells.
C) nuclear fission occurring within biological cells.
D) the fast- moving alpha, beta, and gamma particles passing through biological cells.
E) the fast- moving daughter nuclei [or residual nuclei] passing through biological cells.
A) nuclear fusion occurring within biological cells.
B) thermal energy created by radioactive decay within biological cells.
C) nuclear fission occurring within biological cells.
D) the fast- moving alpha, beta, and gamma particles passing through biological cells.
E) the fast- moving daughter nuclei [or residual nuclei] passing through biological cells.

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
32
Among the following items, the largest single source of radiation in the environment is
A) fallout.
B) cosmic rays.
C) medical diagnosis.
D) radon.
E) nuclear power plants.
A) fallout.
B) cosmic rays.
C) medical diagnosis.
D) radon.
E) nuclear power plants.

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
33
234 Th beta- decays. The resulting nucleus has atomic number
A) 230.
B) 90.
C) 234.
D) 91.
E) 88.
A) 230.
B) 90.
C) 234.
D) 91.
E) 88.

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
34
The forces that hold the nucleus together
A) act over only very short distances.
B) are much stronger than electromagnetic forces.
C) Both of the above.
D) are attractive, that is, they pull in the inward direction.
E) All of the above.
A) act over only very short distances.
B) are much stronger than electromagnetic forces.
C) Both of the above.
D) are attractive, that is, they pull in the inward direction.
E) All of the above.

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
35
The distinction between an "element" and an "isotope" is
A) an element has a specific number of neutrons; an isotope has a specific number of both protons and neutrons.
B) an element has a specific number of protons; an isotope has a specific number of neutrons.
C) an element has a specific number of protons; an isotope has a specific number of both protons and neutrons.
D) an element has a specific number of both protons and neutrons; an isotope has a specific number of protons.
E) these two words have the same meaning, but "element" is used by chemists while "isotope" is used by physicists.
A) an element has a specific number of neutrons; an isotope has a specific number of both protons and neutrons.
B) an element has a specific number of protons; an isotope has a specific number of neutrons.
C) an element has a specific number of protons; an isotope has a specific number of both protons and neutrons.
D) an element has a specific number of both protons and neutrons; an isotope has a specific number of protons.
E) these two words have the same meaning, but "element" is used by chemists while "isotope" is used by physicists.

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
36
In what way or ways do 3H and 3He differ from each other?
A) They have different numbers of neutrons.
B) They have different numbers of protons.
C) Both of the above.
D) They have different mass numbers.
E) All of the above.
A) They have different numbers of neutrons.
B) They have different numbers of protons.
C) Both of the above.
D) They have different mass numbers.
E) All of the above.

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
37
What heats the water in a naturally heated hot spring?
A) radioactivity in the Earth
B) gamma radiation from the sun
C) nuclear fission within the Earth
D) nuclear fusion within the Earth
E) the high pressures existing within the Earth
A) radioactivity in the Earth
B) gamma radiation from the sun
C) nuclear fission within the Earth
D) nuclear fusion within the Earth
E) the high pressures existing within the Earth

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
38
The fundamental force responsible for the chemical properties of atoms is
A) the strong nuclear force.
B) gravity.
C) friction.
D) the weak nuclear force.
E) the electromagnetic force.
A) the strong nuclear force.
B) gravity.
C) friction.
D) the weak nuclear force.
E) the electromagnetic force.

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
39
A bone is dug up and analyzed and found to contain 1/16-- one sixteenth-- of the amount of 14C radioactivity of normal bones in living bodies. The half- life of 14C is 6000 years. Roughly how long has the individual been dead?
A) 24, 000 years
B) 30,000 years
C) 6000 years
D) 36,000 years
E) Impossible to tell from the given information.
A) 24, 000 years
B) 30,000 years
C) 6000 years
D) 36,000 years
E) Impossible to tell from the given information.

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
40
What is the difference between 239Pu and 240Pu?
A) "240Pu has one additional neutron."
B) "239Pu has one less beta- particle."
C) "239Pu has different chemical properties from 240Pu."
D) "240Pu has one additional proton."
E) "There is no actual physical difference."
A) "240Pu has one additional neutron."
B) "239Pu has one less beta- particle."
C) "239Pu has different chemical properties from 240Pu."
D) "240Pu has one additional proton."
E) "There is no actual physical difference."

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
41
Except for such nuclear processes as nuclear reactors, nuclear bombs, and radioactivity, all of the common forces we see around us daily are due to just two underlying or "fundamental" forces. One of these two forces is the electromagnetic force. The other is
A) the strong nuclear force.
B) the frictional force.
C) the chemical force.
D) contact forces.
E) the gravitational force.
A) the strong nuclear force.
B) the frictional force.
C) the chemical force.
D) contact forces.
E) the gravitational force.

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
42
How does the mass of a 4 He nucleus compare with the mass of a 3 H nucleus?
2 1
A) 4 He is 4/3 times as massive, i.e., 33% more massive.
B) They have about the same mass.
C) 4 He is four times as massive.
D) 4 He is twice as massive.
E) 4 He is 3/2 times as massive, i.e., 50% more massive.
2 1
A) 4 He is 4/3 times as massive, i.e., 33% more massive.
B) They have about the same mass.
C) 4 He is four times as massive.
D) 4 He is twice as massive.
E) 4 He is 3/2 times as massive, i.e., 50% more massive.

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
43
Mutations are
A) important in the operation of evolution.
B) sometimes caused by radioactivity.
C) Both of the above.
D) None of the above.
E) mostly caused by eating pizza.
A) important in the operation of evolution.
B) sometimes caused by radioactivity.
C) Both of the above.
D) None of the above.
E) mostly caused by eating pizza.

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
44
The isotope 238 U alpha decays to the daughter nucleus
A) "234 U."
B) "238 Np."
C) "234 Th."
D) "234 u."
E) "236 Th.
92 93"
A) "234 U."
B) "238 Np."
C) "234 Th."
D) "234 u."
E) "236 Th.
92 93"

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
45
At Chernobyl, one of the harmful radioactive isotopes was 131I, a beta emitter with a halflife of 8 days. The mass number of the nucleus that remains after this isotope decays is
A) 131.
B) 129.
C) 132.
D) 130.
E) 127.
A) 131.
B) 129.
C) 132.
D) 130.
E) 127.

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
46
Two different isotopes of the same element have
A) different chemical properties.
B) different numbers of protons but the same number of neutrons.
C) different numbers of electrons.
D) the same number of protons, but different numbers of neutrons.
E) different numbers of both protons and neutrons.
A) different chemical properties.
B) different numbers of protons but the same number of neutrons.
C) different numbers of electrons.
D) the same number of protons, but different numbers of neutrons.
E) different numbers of both protons and neutrons.

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
47
How many protons and how many neutrons are in the 56 Fe nucleus?
A) 26 protons and 30 neutrons
B) 30 protons and 26 neutrons
C) 26 protons and 56 neutrons
D) 56 protons and 26 neutrons
E) None of the above.
A) 26 protons and 30 neutrons
B) 30 protons and 26 neutrons
C) 26 protons and 56 neutrons
D) 56 protons and 26 neutrons
E) None of the above.

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
48
If you start with one gram of pure 14C [half- life 6000 years], the amount remaining after 36,000 years will be
A) 1/128 gram.
B) 1/6 gram.
C) 1/32 gram.
D) 1/64 gram.
E) None.
A) 1/128 gram.
B) 1/6 gram.
C) 1/32 gram.
D) 1/64 gram.
E) None.

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
49
The discoverer of radium was
A) Enrico Fermi.
B) Max Planck.
C) Lise Meitner.
D) Marie Curie.
E) J. Robert Oppenheimer.
A) Enrico Fermi.
B) Max Planck.
C) Lise Meitner.
D) Marie Curie.
E) J. Robert Oppenheimer.

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
50
Compared to natural background radiation, the amount of radiation received by U.S. citizens from normally operating nuclear power plants is
A) very much less.
B) considerably more.
C) roughly the same amount.
D) absolutely zero.
E) about 10% as much.
A) very much less.
B) considerably more.
C) roughly the same amount.
D) absolutely zero.
E) about 10% as much.

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
51
When the radioactive isotope 14 C undergoes beta- decay, the atomic number of the resulting nucleus is
A) 8.
B) 6.
C) 5.
D) 7.
E) 4.
A) 8.
B) 6.
C) 5.
D) 7.
E) 4.

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
52
Consider the three following forces: gravitational, electromagnetic, and strong nuclear. Of these three, which one or ones have significant effects [i.e., experimentally detectable effects] within the nucleus?
A) electromagnetic and strong nuclear only
B) all three
C) electromagnetic only
D) gravitational and strong nuclear only
E) strong nuclear only
A) electromagnetic and strong nuclear only
B) all three
C) electromagnetic only
D) gravitational and strong nuclear only
E) strong nuclear only

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
53
The half- life of a radioactive isotope is
A) the time required for a nucleus of that isotope to partially decay.
B) half the time required for a nucleus of that isotope to decay.
C) half the time that it takes for a radioactive isotope to eat a large pizza.
D) the time required for the decay of half of a large sample of nuclei of that isotope.
E) half the time required for the decay of a large sample of nuclei of that isotope.
A) the time required for a nucleus of that isotope to partially decay.
B) half the time required for a nucleus of that isotope to decay.
C) half the time that it takes for a radioactive isotope to eat a large pizza.
D) the time required for the decay of half of a large sample of nuclei of that isotope.
E) half the time required for the decay of a large sample of nuclei of that isotope.

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
54
One way in which the ionizing radiation occurring naturally in the environment has been beneficial to the human race is
A) in the operation of the electron microscope.
B) in making nuclear fission possible.
C) in producing the mutations that are essential for the process of evolution.
D) the application of solar radiation to the treatment of cancer.
E) nonsense-- there are no beneficial uses of naturally- occurring high- energy radiation.
A) in the operation of the electron microscope.
B) in making nuclear fission possible.
C) in producing the mutations that are essential for the process of evolution.
D) the application of solar radiation to the treatment of cancer.
E) nonsense-- there are no beneficial uses of naturally- occurring high- energy radiation.

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
55
If a radioactive isotope has a one year halflife, what fraction will remain after 5 years?
A) None
B) Half
C) 1/32
D) 1/5 [one fifth]
E) 1/64
A) None
B) Half
C) 1/32
D) 1/5 [one fifth]
E) 1/64

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
56
The half- life of radium is
A) half of the total time during which a quantity of radium will radioactively decay.
B) the time during which half of a large number of radium atoms will decay.
C) half the lifetime of the individual radium atom.
D) the life- time of the individual radium atom.
E) the time after which an individual radium atom splits in half.
A) half of the total time during which a quantity of radium will radioactively decay.
B) the time during which half of a large number of radium atoms will decay.
C) half the lifetime of the individual radium atom.
D) the life- time of the individual radium atom.
E) the time after which an individual radium atom splits in half.

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
57
Regarding the question of the precise time at which a radioactive nucleus will decay:
A) The time is completely unpredictable (i.e., we cannot know anything about it), because we cannot know anything about an object as small as an individual nucleus.
B) The time is precisely predictable, provided we have sufficient information about the condition of the nucleus.
C) The time is only statistically predictable, because our measuring instruments are not yet sufficiently precise.
D) The time is precisely predictable for any particular isotope, regardless our knowledge of its precise state.
E) The time is only statistically predictable, because of unavoidable uncertainties associated with quantum theory.
A) The time is completely unpredictable (i.e., we cannot know anything about it), because we cannot know anything about an object as small as an individual nucleus.
B) The time is precisely predictable, provided we have sufficient information about the condition of the nucleus.
C) The time is only statistically predictable, because our measuring instruments are not yet sufficiently precise.
D) The time is precisely predictable for any particular isotope, regardless our knowledge of its precise state.
E) The time is only statistically predictable, because of unavoidable uncertainties associated with quantum theory.

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
58
A saber- toothed- tiger's bones contain 1/32 of the amount of 14C that is contained in the bone material of living animals. The half- life of 14C is 6000 years. From this information, we can conclude
A) that the tiger died about 30,000 years ago.
B) that the tiger died about 18,000 years ago.
C) nothing at all, because of the uncertainties associated with radioactive decay.
D) nothing at all, because 14C dating is known to be inaccurate.
E) that the tiger died about [i.e., plus or minus a few thousand years] 42,000 years ago.
A) that the tiger died about 30,000 years ago.
B) that the tiger died about 18,000 years ago.
C) nothing at all, because of the uncertainties associated with radioactive decay.
D) nothing at all, because 14C dating is known to be inaccurate.
E) that the tiger died about [i.e., plus or minus a few thousand years] 42,000 years ago.

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
59
Where does most of Earth's 14C come from?
A) It is continually being formed as a result of the photosynthesis process
B) It is continually being formed by the impact of cosmic rays on the atmosphere.
C) It comes to Earth from outer space along with other cosmic rays.
D) It was deposited on Earth when the solar system first evolved.
E) It was deposited on Earth many years ago by meteors.
A) It is continually being formed as a result of the photosynthesis process
B) It is continually being formed by the impact of cosmic rays on the atmosphere.
C) It comes to Earth from outer space along with other cosmic rays.
D) It was deposited on Earth when the solar system first evolved.
E) It was deposited on Earth many years ago by meteors.

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
60
How do 3 H and 3 He differ? 1 2
A) in their number of protons
B) in their number of neutrons
C) in both of the above ways
D) in their chemical behavior
E) in all of the above ways
A) in their number of protons
B) in their number of neutrons
C) in both of the above ways
D) in their chemical behavior
E) in all of the above ways

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
61
The radioactive isotope 14 C beta- decays to the daughter nucleus
A) "13 C."
B) "13 N."
C) "15 C."
D) "14 N."
E) "None of the above."
A) "13 C."
B) "13 N."
C) "15 C."
D) "14 N."
E) "None of the above."

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
62
In a nuclear accident such as occurred at Chernobyl, the long- term biological damage is caused by
A) nuclear fission occurring within the biological cells.
B) alpha, beta, and gamma particles passing through biological cells.
C) the thermal energy produced by radioactive decay within biological cells.
D) the high energy vibrations of the water molecule that are caused by radioactive isotopes.
E) nuclear fusion occurring within the biological cells.
A) nuclear fission occurring within the biological cells.
B) alpha, beta, and gamma particles passing through biological cells.
C) the thermal energy produced by radioactive decay within biological cells.
D) the high energy vibrations of the water molecule that are caused by radioactive isotopes.
E) nuclear fusion occurring within the biological cells.

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
63
Radon is
A) the radioactive substance that will probably be used to irradiate and sterilize food.
B) the main component in the radioactive fallout from nuclear weapons and nuclear power plant accidents.
C) a type of electromagnetic radiation that is produced by the sun and partially penetrates Earth's atmosphere.
D) a radioactive gas produced by the rocks and soil that can filter into houses.
E) the chemically active element in asbestos.
A) the radioactive substance that will probably be used to irradiate and sterilize food.
B) the main component in the radioactive fallout from nuclear weapons and nuclear power plant accidents.
C) a type of electromagnetic radiation that is produced by the sun and partially penetrates Earth's atmosphere.
D) a radioactive gas produced by the rocks and soil that can filter into houses.
E) the chemically active element in asbestos.

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
64
12C and 13C differ in their
A) number of neutrons.
B) chemical behavior.
C) number of protons.
D) number of electrons in orbit around the nucleus.
E) All of the above.
A) number of neutrons.
B) chemical behavior.
C) number of protons.
D) number of electrons in orbit around the nucleus.
E) All of the above.

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
65
According to most scientists, when did the first hominids-- direct human forbears that were fully separated from the apes-- appear on Earth?
A) about a billion years ago
B) a few billion years ago
C) a few million years ago
D) a few thousand years ago
E) about 100 billion years ago
A) about a billion years ago
B) a few billion years ago
C) a few million years ago
D) a few thousand years ago
E) about 100 billion years ago

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
66
At Chernobyl, one of the harmful radioactive isotopes was 131I, a beta emitter with a halflife of 8 days. Suppose some of this isotope is deposited on a field. What can we say about this field 40 days later?
A) Its radioactivity level has decreased to 1/32 of its original level.
B) Its radioactivity level has decreased to 1/64-- one sixty- forth-- of its original level.
C) Its radioactivity has decreased to harmless levels.
D) Its radioactivity level has decreased to 1/16 of its original level.
E) It is just as radioactive as it was to begin with.
A) Its radioactivity level has decreased to 1/32 of its original level.
B) Its radioactivity level has decreased to 1/64-- one sixty- forth-- of its original level.
C) Its radioactivity has decreased to harmless levels.
D) Its radioactivity level has decreased to 1/16 of its original level.
E) It is just as radioactive as it was to begin with.

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
67
Which of the following is classified as "ionizing radiation"?
A) alpha and beta radiation
B) gamma rays
C) Both of the above.
D) X-rays
E) All of the above.
A) alpha and beta radiation
B) gamma rays
C) Both of the above.
D) X-rays
E) All of the above.

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
68
Of the four fundamental forces that act in the universe, which one supports a book that is at rest on a table?
A) the gravitational force
B) the photon force
C) the perpendicular force
D) the strong nuclear force
E) the electromagnetic force
A) the gravitational force
B) the photon force
C) the perpendicular force
D) the strong nuclear force
E) the electromagnetic force

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
69
The carbon in a certain ancient ax- handle is only 1/8-- one- eighth-- as radioactive as the carbon in living wood. The half- life of 14C is 6000 years. From these facts we can conclude that
A) the ax- handle is about 48,000 years old.
B) the ax- handle is about 12,000 years old.
C) the ax- handle was made from an unusual type of wood that was less radioactive than normal wood.
D) the ax- handle is about 18,000 years old.
E) the natural carbon- radioactivity at the time the ax- handle was made was only one- eighth of what it is today.
A) the ax- handle is about 48,000 years old.
B) the ax- handle is about 12,000 years old.
C) the ax- handle was made from an unusual type of wood that was less radioactive than normal wood.
D) the ax- handle is about 18,000 years old.
E) the natural carbon- radioactivity at the time the ax- handle was made was only one- eighth of what it is today.

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
70
Radioactive dating methods and other dating methods indicate that hominids have been on Earth for about
A) 5 billion years.
B) 5000 years.
C) 5 million years.
D) 15 billion years.
E) 100,000 years.
A) 5 billion years.
B) 5000 years.
C) 5 million years.
D) 15 billion years.
E) 100,000 years.

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
71
Which of the following is not one of the four fundamental forces in nature?
A) frictional force
B) weak nuclear force
C) gravitational force
D) strong nuclear force
E) electromagnetic force
A) frictional force
B) weak nuclear force
C) gravitational force
D) strong nuclear force
E) electromagnetic force

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
72
In every nuclear reaction,
A) nuclear structure is altered.
B) molecules combine with other molecules.
C) electrons combine with each other.
D) a nucleus is split.
E) nuclei are brought together and permanently combined with other nuclei.
A) nuclear structure is altered.
B) molecules combine with other molecules.
C) electrons combine with each other.
D) a nucleus is split.
E) nuclei are brought together and permanently combined with other nuclei.

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
73
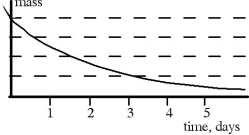 The graph above shows the decay curve for a certain radioactive substance. The half- life of this substance is approximately
The graph above shows the decay curve for a certain radioactive substance. The half- life of this substance is approximatelyA) 0.5 days.
B) 2 days.
C) 1.5 days.
D) 1 day.
E) 3 days.

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
74
Suppose that Al the physicist observes a single radioactive nucleus. The length of time until that particular nucleus decays is
A) unpredictable because of fundamental uncertainties arising from quantum physics
B) predictable, provided Al knows the precise nuclear state of this nucleus.
C) predictable from the known half- life of the nucleus.
D) unpredictable because of the practical difficulties of obtaining all the information needed to make the prediction.
E) unpredictable because of fundamental uncertainties arising from Newtonian physics.
A) unpredictable because of fundamental uncertainties arising from quantum physics
B) predictable, provided Al knows the precise nuclear state of this nucleus.
C) predictable from the known half- life of the nucleus.
D) unpredictable because of the practical difficulties of obtaining all the information needed to make the prediction.
E) unpredictable because of fundamental uncertainties arising from Newtonian physics.

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
75
Suppose that a certain saber- toothed- tiger contained one gram of 14C [half- life 6000 years] when it died. The approximate 14C content of the remains of this tiger 24,000 years later would be
A) zero grams.
B) 1/32 gram.
C) 1/16 gram.
D) 1/8 gram.
E) impossible to predict.
A) zero grams.
B) 1/32 gram.
C) 1/16 gram.
D) 1/8 gram.
E) impossible to predict.

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
76
We cannot predict the precise decay time of a particular radioactive 14C nucleus because
A) of the quantum entanglement of its protons and neutrons.
B) a single nucleus is too small to be seen or directly detected.
C) of quantum uncertainties.
D) the calculation is too difficult for today's computers.
E) of the difficulty of determining the precise initial states of all its protons and neutron.
A) of the quantum entanglement of its protons and neutrons.
B) a single nucleus is too small to be seen or directly detected.
C) of quantum uncertainties.
D) the calculation is too difficult for today's computers.
E) of the difficulty of determining the precise initial states of all its protons and neutron.

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
77
Two different isotopes of the same element have
A) the same chemical properties, but different properties in nuclear processes.
B) different chemical properties, but the same properties in nuclear processes.
C) the same chemical properties, and also the same properties in nuclear processes.
D) different chemical properties, and also different properties in nuclear processes.
E) None of the above.
A) the same chemical properties, but different properties in nuclear processes.
B) different chemical properties, but the same properties in nuclear processes.
C) the same chemical properties, and also the same properties in nuclear processes.
D) different chemical properties, and also different properties in nuclear processes.
E) None of the above.

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck


