Deck 6: Air a Study of the Gases in Our Atmosphere
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/42
Play
Full screen (f)
Deck 6: Air a Study of the Gases in Our Atmosphere
1
Which is true about the air at a high altitude versus the air at a lower altitude?
A)Air contains a higher number of nitrogen molecules at higher altitudes.
B)There are fewer overall molecules of air at high altitude.
C)The percent of each gas present in air changes from high to low altitude.
D)The number of ozone molecules at high altitude is higher than at low altitude because the ozone layer is closer.
A)Air contains a higher number of nitrogen molecules at higher altitudes.
B)There are fewer overall molecules of air at high altitude.
C)The percent of each gas present in air changes from high to low altitude.
D)The number of ozone molecules at high altitude is higher than at low altitude because the ozone layer is closer.
There are fewer overall molecules of air at high altitude.
2
When methane forms in the ground, it forms from
A)the decomposition of organic matter.
B)the reaction of water and carbon dioxide in the water table and below.
C)out-gassing of compounds released from the earth's core.
D)the combination of formaldehyde that is released from the mantle with other compounds from the Earth's surface.
A)the decomposition of organic matter.
B)the reaction of water and carbon dioxide in the water table and below.
C)out-gassing of compounds released from the earth's core.
D)the combination of formaldehyde that is released from the mantle with other compounds from the Earth's surface.
the decomposition of organic matter.
3
Which location will have the highest atmospheric pressure?
A)Chicago airport (619 feet)
B)Grand Canyon National Park (6606 feet)
C)San Francisco airport (11 feet)
D)Atlanta airport (1025 feet)
E)Boise airport (2858 feet)
A)Chicago airport (619 feet)
B)Grand Canyon National Park (6606 feet)
C)San Francisco airport (11 feet)
D)Atlanta airport (1025 feet)
E)Boise airport (2858 feet)
San Francisco airport (11 feet)
4
List the three phases of matter, from slowest to fastest particle speed.
A)solid, liquid, gas
B)liquid, solid, gas
C)gas, liquid, solid
D)solid, gas, liquid
A)solid, liquid, gas
B)liquid, solid, gas
C)gas, liquid, solid
D)solid, gas, liquid

Unlock Deck
Unlock for access to all 42 flashcards in this deck.
Unlock Deck
k this deck
5
Which gas makes up the largest portion of clean, unpolluted air?
A)oxygen
B)nitrogen
C)carbon dioxide
D)carbon monoxide
E)argon
A)oxygen
B)nitrogen
C)carbon dioxide
D)carbon monoxide
E)argon

Unlock Deck
Unlock for access to all 42 flashcards in this deck.
Unlock Deck
k this deck
6
Gases mix thoroughly with one another.

Unlock Deck
Unlock for access to all 42 flashcards in this deck.
Unlock Deck
k this deck
7
At sea level, the atmospheric pressure is usually equal to about
A)100 millimeters of mercury
B)2 atmospheres
C)350 millimeters of mercury
D)760 millimeters of mercury
E)None of the above
A)100 millimeters of mercury
B)2 atmospheres
C)350 millimeters of mercury
D)760 millimeters of mercury
E)None of the above

Unlock Deck
Unlock for access to all 42 flashcards in this deck.
Unlock Deck
k this deck
8
Explain why atmospheric pressure changes with altitude.
A)as altitude increases, atmospheric pressure decreases
B)as altitude increases, atmospheric pressure increases
C)as altitude decreases, atmospheric pressure decreases
D)none of the above
A)as altitude increases, atmospheric pressure decreases
B)as altitude increases, atmospheric pressure increases
C)as altitude decreases, atmospheric pressure decreases
D)none of the above

Unlock Deck
Unlock for access to all 42 flashcards in this deck.
Unlock Deck
k this deck
9
If the pressure in Denver is 0.83 atm, how many mm of mercury (Hg)is this?
A)760 mm Hg
B)0.83 mm Hg
C)1 mm Hg
D)631 mm Hg
E)22.4 mm Hg
A)760 mm Hg
B)0.83 mm Hg
C)1 mm Hg
D)631 mm Hg
E)22.4 mm Hg

Unlock Deck
Unlock for access to all 42 flashcards in this deck.
Unlock Deck
k this deck
10
Which instrument is used to measure atmospheric pressure?
A)a gas chromatograph
B)a thermometer
C)a barometer
D)a pH meter
E)a voltmeter
A)a gas chromatograph
B)a thermometer
C)a barometer
D)a pH meter
E)a voltmeter

Unlock Deck
Unlock for access to all 42 flashcards in this deck.
Unlock Deck
k this deck
11
Due to the force of gravity, which is true about the particles of a sample of gas at sea level versus those at higher altitude?
A)The atoms and molecules of a gas will move more quickly at sea level than at higher altitude.
B)The mean free path between atoms and molecules of a gas will be longer at sea level.
C)The volume of a sample of gas will be larger at sea level than at higher altitude.
D)The number of particles in a particular volume will be greater at higher altitude than at sea level.
E)The particles of the gas at sea level will be closer together than those at higher altitude.
A)The atoms and molecules of a gas will move more quickly at sea level than at higher altitude.
B)The mean free path between atoms and molecules of a gas will be longer at sea level.
C)The volume of a sample of gas will be larger at sea level than at higher altitude.
D)The number of particles in a particular volume will be greater at higher altitude than at sea level.
E)The particles of the gas at sea level will be closer together than those at higher altitude.

Unlock Deck
Unlock for access to all 42 flashcards in this deck.
Unlock Deck
k this deck
12
Gases undergo a higher number of collisions when the atmospheric pressure is higher.

Unlock Deck
Unlock for access to all 42 flashcards in this deck.
Unlock Deck
k this deck
13
Which location will have the lowest atmospheric pressure?
A)Chicago airport (619 feet)
B)Grand Canyon National Park (6606 feet)
C)San Francisco airport (11 feet)
D)Atlanta airport (1025 feet)
E)Boise airport (2858 feet)
A)Chicago airport (619 feet)
B)Grand Canyon National Park (6606 feet)
C)San Francisco airport (11 feet)
D)Atlanta airport (1025 feet)
E)Boise airport (2858 feet)

Unlock Deck
Unlock for access to all 42 flashcards in this deck.
Unlock Deck
k this deck
14
During the process of diffusion, gas particles move in a straight line from one point to another (for example from one side of a room to the other).

Unlock Deck
Unlock for access to all 42 flashcards in this deck.
Unlock Deck
k this deck
15
The primary component of natural gas is
A)octane
B)propane
C)methane
D)butane
A)octane
B)propane
C)methane
D)butane

Unlock Deck
Unlock for access to all 42 flashcards in this deck.
Unlock Deck
k this deck
16
Identify whether the substance described is a solid, liquid or gas at room temperature: A substance that must be stored in a sealed, leak-proof container.
A)solid
B)liquid
C)gas
A)solid
B)liquid
C)gas

Unlock Deck
Unlock for access to all 42 flashcards in this deck.
Unlock Deck
k this deck
17
A person wearing strong perfume enters a room where you are sitting.At what time of year would you smell this perfume more quickly?
A)You would smell this more quickly in the winter when the heat is on (keeping the room at a toasty 74 degrees).
B)You would smell this more quickly in the summer when the air conditioner is running (keeping the room at a nice, cool 68 degrees).
C)None of the above
A)You would smell this more quickly in the winter when the heat is on (keeping the room at a toasty 74 degrees).
B)You would smell this more quickly in the summer when the air conditioner is running (keeping the room at a nice, cool 68 degrees).
C)None of the above

Unlock Deck
Unlock for access to all 42 flashcards in this deck.
Unlock Deck
k this deck
18
Mercury is a better choice for use in barometers than water because
A)It is less dense.
B)It vaporizes much more slowly.
C)It is cheaper.
D)It is more colorful and easier to see.
E)It is poisonous.
A)It is less dense.
B)It vaporizes much more slowly.
C)It is cheaper.
D)It is more colorful and easier to see.
E)It is poisonous.

Unlock Deck
Unlock for access to all 42 flashcards in this deck.
Unlock Deck
k this deck
19
The percent composition of air is different at higher altitude than it is at sea level.

Unlock Deck
Unlock for access to all 42 flashcards in this deck.
Unlock Deck
k this deck
20
All particles of a gas in a particular sample will move at the same speed.

Unlock Deck
Unlock for access to all 42 flashcards in this deck.
Unlock Deck
k this deck
21
If the average molecular speed of argon gas at 25°C is 350 m/s, estimate the average molecular speed at 35°C.
A)100 m/s
B)200 m/s
C)450 m/s
A)100 m/s
B)200 m/s
C)450 m/s

Unlock Deck
Unlock for access to all 42 flashcards in this deck.
Unlock Deck
k this deck
22
Indicate which gas particles are moving at the faster speed.
A)8 billion He gas atoms at 298 K
B)8 billion He gas atoms at 95°F
A)8 billion He gas atoms at 298 K
B)8 billion He gas atoms at 95°F

Unlock Deck
Unlock for access to all 42 flashcards in this deck.
Unlock Deck
k this deck
23
Which substance would be most likely to be counted using the mole?
A)donuts
B)marbles
C)oxygen molecules
D)cars
E)telephones
A)donuts
B)marbles
C)oxygen molecules
D)cars
E)telephones

Unlock Deck
Unlock for access to all 42 flashcards in this deck.
Unlock Deck
k this deck
24
Which describes an experiment in which only pressure and temperature change?
A)taking a shampoo bottle from sea level to altitude
B)putting air in your tires
C)heating a sealed glass sphere
D)putting a balloon in liquid nitrogen
E)none of these
A)taking a shampoo bottle from sea level to altitude
B)putting air in your tires
C)heating a sealed glass sphere
D)putting a balloon in liquid nitrogen
E)none of these

Unlock Deck
Unlock for access to all 42 flashcards in this deck.
Unlock Deck
k this deck
25
Methane is more dangerous in certain ways than carbon dioxide because
A)it is lighter and moves more easily through the atmosphere.
B)it dissolves in water more quickly.
C)it can be produced in a home laboratory without sophisticated equipment.
D)it is a more potent greenhouse gas.
A)it is lighter and moves more easily through the atmosphere.
B)it dissolves in water more quickly.
C)it can be produced in a home laboratory without sophisticated equipment.
D)it is a more potent greenhouse gas.

Unlock Deck
Unlock for access to all 42 flashcards in this deck.
Unlock Deck
k this deck
26
If a balloon at STP has a volume of 89.6 L, how many moles of gas does it contain?
A)22.4 moles
B)1.0 mole
C)89.6 moles
D)4.0 moles
E)6.02 × 1023 moles
A)22.4 moles
B)1.0 mole
C)89.6 moles
D)4.0 moles
E)6.02 × 1023 moles

Unlock Deck
Unlock for access to all 42 flashcards in this deck.
Unlock Deck
k this deck
27
The lungs of a scuba diver expand as he rises from 100 m below the surface to 10 m below the surface.The gas law that describes this scenario is _____.

Unlock Deck
Unlock for access to all 42 flashcards in this deck.
Unlock Deck
k this deck
28
What volume will 10.0 moles of a gas fill at STP?
A)2.4 L
B)22.4 L
C)224 L
D)100 L
E)10.0 L
A)2.4 L
B)22.4 L
C)224 L
D)100 L
E)10.0 L

Unlock Deck
Unlock for access to all 42 flashcards in this deck.
Unlock Deck
k this deck
29
A beach containing 3.0 moles of sand grains would have how many individual grains of sand?
A)6.02 × 1023
B)3.0
C)3.0 × 6.02 × 1023
D)1.8 × 1023
A)6.02 × 1023
B)3.0
C)3.0 × 6.02 × 1023
D)1.8 × 1023

Unlock Deck
Unlock for access to all 42 flashcards in this deck.
Unlock Deck
k this deck
30
If a sealed box at STP holds 8.0 moles of clean air, how many molecules of nitrogen does it contain?
A)8.4 × 1023 oxygen molecules
B)6.02 × 1023 oxygen molecules
C)1.3 × 1023 oxygen molecules
D)3.8 × 1024 oxygen molecules
E)4.8 × 1024 oxygen molecules
A)8.4 × 1023 oxygen molecules
B)6.02 × 1023 oxygen molecules
C)1.3 × 1023 oxygen molecules
D)3.8 × 1024 oxygen molecules
E)4.8 × 1024 oxygen molecules

Unlock Deck
Unlock for access to all 42 flashcards in this deck.
Unlock Deck
k this deck
31
A balloon shrinks when taken from inside a hot car and put into a freezer.The gas law that describes this scenario is_____.

Unlock Deck
Unlock for access to all 42 flashcards in this deck.
Unlock Deck
k this deck
32
Which of the following gases would move at the fastest speed, assuming the temperature of each gas is the same?
A)oxygen
B)nitrogen
C)carbon dioxide
A)oxygen
B)nitrogen
C)carbon dioxide

Unlock Deck
Unlock for access to all 42 flashcards in this deck.
Unlock Deck
k this deck
33
Which describes an experiment in which only moles of gas and pressure change?
A)taking a sealed shampoo bottle from sea level to altitude
B)putting air in your tires
C)heating a sealed glass sphere
D)pouring liquid nitrogen over a balloon
E)none of these
A)taking a sealed shampoo bottle from sea level to altitude
B)putting air in your tires
C)heating a sealed glass sphere
D)pouring liquid nitrogen over a balloon
E)none of these

Unlock Deck
Unlock for access to all 42 flashcards in this deck.
Unlock Deck
k this deck
34
If a sealed box at STP holds a total of 6.7 moles of clean air, how many oxygen molecules does it contain?
A)8.4 × 1023 oxygen molecules
B)6.02 × 1023 oxygen molecules
C)1.3 × 1023 oxygen molecules
D)1.4 × 1024 oxygen molecules
E)4.0 × 1024 oxygen molecules
A)8.4 × 1023 oxygen molecules
B)6.02 × 1023 oxygen molecules
C)1.3 × 1023 oxygen molecules
D)1.4 × 1024 oxygen molecules
E)4.0 × 1024 oxygen molecules

Unlock Deck
Unlock for access to all 42 flashcards in this deck.
Unlock Deck
k this deck
35
At what temperature is a gas considered to be at STP?
A)25°C
B)298 K
C)0°C
D)0 K
E)100°C
A)25°C
B)298 K
C)0°C
D)0 K
E)100°C

Unlock Deck
Unlock for access to all 42 flashcards in this deck.
Unlock Deck
k this deck
36
A rigid, sealed box at 25°C is heated to 50°C.Which gas variable changes?
A)pressure
B)volume
C)moles of gas
A)pressure
B)volume
C)moles of gas

Unlock Deck
Unlock for access to all 42 flashcards in this deck.
Unlock Deck
k this deck
37
A balloon at STP holds 2.4 × 1024 ozone molecules.About how many moles of ozone does it contain?
A)1.0 mole
B)2.0 moles
C)3.0 moles
D)4.0 moles
E)22.4 moles
A)1.0 mole
B)2.0 moles
C)3.0 moles
D)4.0 moles
E)22.4 moles

Unlock Deck
Unlock for access to all 42 flashcards in this deck.
Unlock Deck
k this deck
38
Of the four gas variables, which are inversely proportional to one another?
A)P and V
B)P and T
C)V and T
D)V and n
A)P and V
B)P and T
C)V and T
D)V and n

Unlock Deck
Unlock for access to all 42 flashcards in this deck.
Unlock Deck
k this deck
39
Another name for hydraulic fracturing, the process by which natural gas is removed from underground is
A)hydrolyzing.
B)fracking.
C)shale cracking.
D)benzenosis.
A)hydrolyzing.
B)fracking.
C)shale cracking.
D)benzenosis.

Unlock Deck
Unlock for access to all 42 flashcards in this deck.
Unlock Deck
k this deck
40
Which describes an experiment in which only volume and temperature change?
A)taking a shampoo bottle from sea level to altitude
B)putting air in your tires
C)heating a sealed glass sphere
D)pouring liquid nitrogen over a balloon
E)none of these
A)taking a shampoo bottle from sea level to altitude
B)putting air in your tires
C)heating a sealed glass sphere
D)pouring liquid nitrogen over a balloon
E)none of these

Unlock Deck
Unlock for access to all 42 flashcards in this deck.
Unlock Deck
k this deck
41
For the image shown below, choose which line represents the gas at the lowest temperature.The x-axis represents the molecular speed in m/s and the y-axis represents the number of atoms. 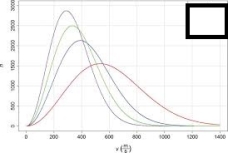
A)The tallest, narrowest line
B)The shortest, widest line
C)The second tallest, second narrowest line
D)The third tallest, third narrowest line

A)The tallest, narrowest line
B)The shortest, widest line
C)The second tallest, second narrowest line
D)The third tallest, third narrowest line

Unlock Deck
Unlock for access to all 42 flashcards in this deck.
Unlock Deck
k this deck
42
For the image shown below, choose which line represents the gas with the lowest molar mass.The x-axis represents the molecular speed in m/s and the y-axis represents the number of atoms. 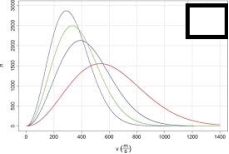
A)The tallest, narrowest line
B)The shortest, widest line
C)The second tallest, second narrowest line
D)The third tallest, third narrowest line

A)The tallest, narrowest line
B)The shortest, widest line
C)The second tallest, second narrowest line
D)The third tallest, third narrowest line

Unlock Deck
Unlock for access to all 42 flashcards in this deck.
Unlock Deck
k this deck


