Deck 18: Thermodynamics: Spontaneous and Nonspontaneous Reactions and Processes
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/157
Play
Full screen (f)
Deck 18: Thermodynamics: Spontaneous and Nonspontaneous Reactions and Processes
1
During a spontaneous chemical reaction, it is found that Ssys < 0. This means ________
A) ( surr < 0 and its magnitude is < Ssys.)
B) ( Ssurr < 0 and its magnitude is > Ssys.)
C) ( Ssurr > 0 and its magnitude is < Ssys.)
D) ( Ssurr > 0 and its magnitude is > Ssys.)
E) an error has been made, because Ssys > 0 by necessity for a spontaneous process.
A) ( surr < 0 and its magnitude is < Ssys.)
B) ( Ssurr < 0 and its magnitude is > Ssys.)
C) ( Ssurr > 0 and its magnitude is < Ssys.)
D) ( Ssurr > 0 and its magnitude is > Ssys.)
E) an error has been made, because Ssys > 0 by necessity for a spontaneous process.
( Ssurr > 0 and its magnitude is > Ssys.)
2
Heat transfer from the system to the surroundings has a large effect on Ssurr ________
A) when the temperature of the surroundings is low.
B) when the temperature of the surroundings is high.
C) when the temperature of the system is low.
D) when the temperature of the system is high.
E) at any temperature, because the amount of heat transferred is independent of temperature.
A) when the temperature of the surroundings is low.
B) when the temperature of the surroundings is high.
C) when the temperature of the system is low.
D) when the temperature of the system is high.
E) at any temperature, because the amount of heat transferred is independent of temperature.
when the temperature of the surroundings is low.
3
What is the entropy change to the surroundings when 1 mol of ice melts in someone's hand if the hand temperature is 32°C? Assume a final temperature for the water of 0°C. The heat of fusion of ice is 6.01 kJ/mol.
A) -188 J/K
B) -22.0 J/K
C) -19.7 J/K
D) +19.7 J/K
E) +188 J/K
A) -188 J/K
B) -22.0 J/K
C) -19.7 J/K
D) +19.7 J/K
E) +188 J/K
-19.7 J/K
4
Which of the following processes are spontaneous?
I.Iron in the open air rusts.
II.Liquid water in a freezer turns to ice.
III.A spark ignites a mixture of propane and air.
A) I only
B) I and II only
C) I and III only
D) II and III only
E) I, II, and III are all spontaneous.
I.Iron in the open air rusts.
II.Liquid water in a freezer turns to ice.
III.A spark ignites a mixture of propane and air.
A) I only
B) I and II only
C) I and III only
D) II and III only
E) I, II, and III are all spontaneous.

Unlock Deck
Unlock for access to all 157 flashcards in this deck.
Unlock Deck
k this deck
5
Care must be taken when dissolving solid pellets of sodium hydroxide (NaOH) in water, because the temperature of the water can rise dramatically. Taking NaOH as the system, what can you deduce about the signs of the entropy change of the system ( Ssys) and surroundings ( Ssurr) from this?
A) ( Ssys < 0 and Ssurr < 0)
B) ( Ssys < 0 and Ssurr > 0)
C) ( Ssys > 0 and Ssurr < 0)
D) ( Ssys > 0 and Ssurr > 0)
E) Nothing can be deduced from this limited information.
A) ( Ssys < 0 and Ssurr < 0)
B) ( Ssys < 0 and Ssurr > 0)
C) ( Ssys > 0 and Ssurr < 0)
D) ( Ssys > 0 and Ssurr > 0)
E) Nothing can be deduced from this limited information.

Unlock Deck
Unlock for access to all 157 flashcards in this deck.
Unlock Deck
k this deck
6
Which of the following must be true for a spontaneous exothermic process?
A) only that Ssys < 0
B) only that Ssys > 0
C) both Ssys < 0 and the magnitude of Ssys < the magnitude of Ssurr
D) both Ssys < 0 and the magnitude of Ssys > the magnitude of Ssurr
E) either Ssys > 0 or Ssys < 0 and the magnitude of Ssys < the magnitude of Ssurr
A) only that Ssys < 0
B) only that Ssys > 0
C) both Ssys < 0 and the magnitude of Ssys < the magnitude of Ssurr
D) both Ssys < 0 and the magnitude of Ssys > the magnitude of Ssurr
E) either Ssys > 0 or Ssys < 0 and the magnitude of Ssys < the magnitude of Ssurr

Unlock Deck
Unlock for access to all 157 flashcards in this deck.
Unlock Deck
k this deck
7
If 1 mol of ice melts at its melting point of 273 K, the entropy change for the ice is 22.0 J/K. If the ice melts in someone's hand at 34°C, what is the change in the entropy of the universe? Assume a final temperature for the water of 0°C. The enthalpy of fusion for ice is 6.01 kJ/mol.
A) +19.6 J/K
B) -19.6 J/K
C) +2.4 J/K
D) -2.4 J/K
E) +41.5 J/K
A) +19.6 J/K
B) -19.6 J/K
C) +2.4 J/K
D) -2.4 J/K
E) +41.5 J/K

Unlock Deck
Unlock for access to all 157 flashcards in this deck.
Unlock Deck
k this deck
8
In a spontaneous process, which of the following always increases?
A) the entropy of the system
B) the entropy of the surroundings
C) the entropy of the universe
D) the entropy of the system and the universe
E) the entropy of the system, the surroundings, and the universe
A) the entropy of the system
B) the entropy of the surroundings
C) the entropy of the universe
D) the entropy of the system and the universe
E) the entropy of the system, the surroundings, and the universe

Unlock Deck
Unlock for access to all 157 flashcards in this deck.
Unlock Deck
k this deck
9
The entropy change in a system ( Ssys) during a spontaneous process must be ________
A) greater than zero.
B) less than zero.
C) equal to zero.
D) greater than or equal to zero.
E) greater than, less than, or equal to zero.
A) greater than zero.
B) less than zero.
C) equal to zero.
D) greater than or equal to zero.
E) greater than, less than, or equal to zero.

Unlock Deck
Unlock for access to all 157 flashcards in this deck.
Unlock Deck
k this deck
10
The gas above the liquid in a sealed bottle of soda is primarily carbon dioxide. Carbon dioxide is also dissolved in the soda. When the distribution of carbon dioxide between the gas and liquid is at equilibrium, molecules of carbon dioxide in the gas phase can still dissolve in the liquid phase if they strike the surface and are captured. Similarly molecules of carbon dioxide can escape from the liquid phase. What is the entropy change of the universe, Suniv, for the dissolution of carbon dioxide under these conditions?
A) ( Suniv < 0, because the dissolved carbon dioxide has fewer accessible states.)
B) ( Suniv > 0, because the dissolved carbon dioxide has fewer accessible states.)
C) ( Suniv = 0, because this is an equilibrium situation.)
D) ( Suniv < 0, because the gas dissolves spontaneously.)
E) ( Suniv > 0, because the gas dissolves spontaneously.)
A) ( Suniv < 0, because the dissolved carbon dioxide has fewer accessible states.)
B) ( Suniv > 0, because the dissolved carbon dioxide has fewer accessible states.)
C) ( Suniv = 0, because this is an equilibrium situation.)
D) ( Suniv < 0, because the gas dissolves spontaneously.)
E) ( Suniv > 0, because the gas dissolves spontaneously.)

Unlock Deck
Unlock for access to all 157 flashcards in this deck.
Unlock Deck
k this deck
11
The enthalpy of fusion for benzene (C6H6) is 127.40 kJ/kg, and its melting point is 5.5°C. What is the entropy change when 1 mole of benzene melts at 5.5°C?
A) 9.95 kJ/K
B) 35.7 J/K
C) 1,809 J/K
D) 1.81 J/K
E) 127.40 kJ/K
A) 9.95 kJ/K
B) 35.7 J/K
C) 1,809 J/K
D) 1.81 J/K
E) 127.40 kJ/K

Unlock Deck
Unlock for access to all 157 flashcards in this deck.
Unlock Deck
k this deck
12
Which of the following processes are reversible in the thermodynamic sense?
I. Iron in the open air rusts.
II. NaCl is dissolved in water and then recovered by the evaporation of the water.
III. The ice in a mixture of ice and water at 0°C and 1 atm melts.
A) I only
B) II only
C) III only
D) II and III only
E) I, II and III are all reversible.
I. Iron in the open air rusts.
II. NaCl is dissolved in water and then recovered by the evaporation of the water.
III. The ice in a mixture of ice and water at 0°C and 1 atm melts.
A) I only
B) II only
C) III only
D) II and III only
E) I, II and III are all reversible.

Unlock Deck
Unlock for access to all 157 flashcards in this deck.
Unlock Deck
k this deck
13
The entropy change of the surroundings, Ssurr, is related to heat transfer, q, with respect to the system and temperature T by ________
A) -q/Tsys = Ssurr.
B) +q/Tsys = Ssurr.
C) -q/Tsurr = Ssurr.
D) q/Tsurr = Ssurr.
E) None of these, unless the system undergoes a reversible process.
A) -q/Tsys = Ssurr.
B) +q/Tsys = Ssurr.
C) -q/Tsurr = Ssurr.
D) q/Tsurr = Ssurr.
E) None of these, unless the system undergoes a reversible process.

Unlock Deck
Unlock for access to all 157 flashcards in this deck.
Unlock Deck
k this deck
14
Which of the processes A-D will lead to a positive change in the entropy of the system? If all of these processes lead to a positive change in the entropy of the system, select E.
A) Sodium chloride crystals form as saltwater evaporates.
B) Helium gas escapes from the hole in a balloon.
C) Stalactites form in a cave.
D) Water freezes in a freezer.
E) All of these lead to a positive change in entropy of the system, because they are all spontaneous.
A) Sodium chloride crystals form as saltwater evaporates.
B) Helium gas escapes from the hole in a balloon.
C) Stalactites form in a cave.
D) Water freezes in a freezer.
E) All of these lead to a positive change in entropy of the system, because they are all spontaneous.

Unlock Deck
Unlock for access to all 157 flashcards in this deck.
Unlock Deck
k this deck
15
Which of the following processes will lead to a decrease in the entropy of the system?
A) Salt crystals dissolve in water.
B) Air escapes from a hole in a balloon.
C) Iron and oxygen react to form rust.
D) Ice melts in your hand.
E) None of these leads to a negative change in the entropy of the system, because they are all spontaneous.
A) Salt crystals dissolve in water.
B) Air escapes from a hole in a balloon.
C) Iron and oxygen react to form rust.
D) Ice melts in your hand.
E) None of these leads to a negative change in the entropy of the system, because they are all spontaneous.

Unlock Deck
Unlock for access to all 157 flashcards in this deck.
Unlock Deck
k this deck
16
Ssys can be directly related to the heat, q. Which of statements A-D is not true regarding this relationship? If all are true, select E.
A) ( Ssys can always be determined from the heat transferred during the actual process.)
B) For a reversible spontaneous endothermic process, both q and Ssys will be positive.
C) The more heat that is transferred, the larger the magnitude of the entropy change.
D) The higher the temperature at which heat is transferred, the lower the entropy change.
E) All of the above are true.
A) ( Ssys can always be determined from the heat transferred during the actual process.)
B) For a reversible spontaneous endothermic process, both q and Ssys will be positive.
C) The more heat that is transferred, the larger the magnitude of the entropy change.
D) The higher the temperature at which heat is transferred, the lower the entropy change.
E) All of the above are true.

Unlock Deck
Unlock for access to all 157 flashcards in this deck.
Unlock Deck
k this deck
17
In a spontaneous process, the entropy of the universe ________
A) always increases.
B) always decreases.
C) does not change.
D) may decrease if the entropy of the system decreases sufficiently.
E) may decrease if the entropy of the system increases sufficiently.
A) always increases.
B) always decreases.
C) does not change.
D) may decrease if the entropy of the system decreases sufficiently.
E) may decrease if the entropy of the system increases sufficiently.

Unlock Deck
Unlock for access to all 157 flashcards in this deck.
Unlock Deck
k this deck
18
An ice cube at 0°C melts in a swimming pool at 20°C. What is the change in the entropy of the universe as a result? The ice cube was 1.00 inch on a side. Assume ice has a density of 0.917 g/cm3 and that the enthalpy of fusion of water is 6.01 kJ/mol.
A) +1.3 J/K
B) -1.3 J/K
C) 0.0 J/K
D) +18.4 J/K
E) +7.9 J/K
A) +1.3 J/K
B) -1.3 J/K
C) 0.0 J/K
D) +18.4 J/K
E) +7.9 J/K

Unlock Deck
Unlock for access to all 157 flashcards in this deck.
Unlock Deck
k this deck
19
Which, if any, of statements A through D is not true of entropy? If they are all true, select E.
A) It is a measure of the distribution of energy in a system at a specific temperature.
B) It is a measure of the number of accessible microstates in a pure substance.
C) It is a property of the universe that increases during a spontaneous process.
D) It is a property of a system that may increase or decrease during a spontaneous process.
E) All of the above are true statements.
A) It is a measure of the distribution of energy in a system at a specific temperature.
B) It is a measure of the number of accessible microstates in a pure substance.
C) It is a property of the universe that increases during a spontaneous process.
D) It is a property of a system that may increase or decrease during a spontaneous process.
E) All of the above are true statements.

Unlock Deck
Unlock for access to all 157 flashcards in this deck.
Unlock Deck
k this deck
20
According to the second law of thermodynamics, the change in the entropy of the universe ( Suniv) during a spontaneous reaction is ________
A) zero.
B) negative.
C) positive.
D) less than the change in entropy of the system ( Ssys).
E) greater than the change in entropy of the system ( Ssys).
A) zero.
B) negative.
C) positive.
D) less than the change in entropy of the system ( Ssys).
E) greater than the change in entropy of the system ( Ssys).

Unlock Deck
Unlock for access to all 157 flashcards in this deck.
Unlock Deck
k this deck
21
Indicate which one of the following reactions most certainly results in a negative Ssys.
A) H2O(g) H2O(s)
B) CaCO3(s) CaO(s) + CO2(g)
C) CuSO4 · 5H2O(s) CuSO4(s) + 5H2O(g)
D) 14O2(g) + 3NH4NO3(s) + C10H22(l) 3N2(g) + 17H2O(g)+10CO2(g)
E) CO2(aq) CO2(g)
A) H2O(g) H2O(s)
B) CaCO3(s) CaO(s) + CO2(g)
C) CuSO4 · 5H2O(s) CuSO4(s) + 5H2O(g)
D) 14O2(g) + 3NH4NO3(s) + C10H22(l) 3N2(g) + 17H2O(g)+10CO2(g)
E) CO2(aq) CO2(g)

Unlock Deck
Unlock for access to all 157 flashcards in this deck.
Unlock Deck
k this deck
22
Determine S for H2(g) + I2(g) 2HI(g) given the following information:
A) -41.10 J/K
B) -165.29 J/K
C) +398.75 J/K
D) +165.29 J/K
E) +41.10 J/K
A) -41.10 J/K
B) -165.29 J/K
C) +398.75 J/K
D) +165.29 J/K
E) +41.10 J/K

Unlock Deck
Unlock for access to all 157 flashcards in this deck.
Unlock Deck
k this deck
23
Determine S °rxn for Zn(s) + 2HCl(aq) ZnCl2(aq) + H2(g) given the following information:
A) -39.6 J/K
B) 0 J/K
C) +39.6 J/K
D) -38.2 J/K
E) +38.2 J/K
A) -39.6 J/K
B) 0 J/K
C) +39.6 J/K
D) -38.2 J/K
E) +38.2 J/K

Unlock Deck
Unlock for access to all 157 flashcards in this deck.
Unlock Deck
k this deck
24
Determine the entropy change for the reaction SO2(g) + O2(g) SO3(g) given the following information:
A) -196.4 J/K
B) +196.4 J/K
C) -93.9 J/K
D) +93.9 J/K
E) +401.4 J/K
A) -196.4 J/K
B) +196.4 J/K
C) -93.9 J/K
D) +93.9 J/K
E) +401.4 J/K

Unlock Deck
Unlock for access to all 157 flashcards in this deck.
Unlock Deck
k this deck
25
If 3.500 g of Ni are reacted with excess oxygen to form nickel oxide (NiO) under standard state conditions, what is the entropy change for the reaction?
2Ni(s) + O2 2NiO(s)
A) -49.3 J/K
B) -24.7 J/K
C) -14.7 J/K
D) +49.3 J/K
E) -10.4 J/K
2Ni(s) + O2 2NiO(s)
A) -49.3 J/K
B) -24.7 J/K
C) -14.7 J/K
D) +49.3 J/K
E) -10.4 J/K

Unlock Deck
Unlock for access to all 157 flashcards in this deck.
Unlock Deck
k this deck
26
The standard molar entropy of lead(II) bromide (PbBr2) is 161 J/(mol .K). What is the entropy of 2.45 g of PbBr2?
A) +1.07 J/K
B) -1.07 J/K
C) +161 J/K
D) -161 J/K
E) 0 J/K
A) +1.07 J/K
B) -1.07 J/K
C) +161 J/K
D) -161 J/K
E) 0 J/K

Unlock Deck
Unlock for access to all 157 flashcards in this deck.
Unlock Deck
k this deck
27
The following figures represent distributions of two types of gas molecules between two containers connected by an open tube. In which figure is the entropy of the system maximized?
A)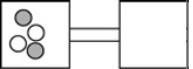
B)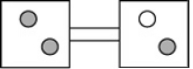
C)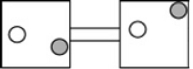
D)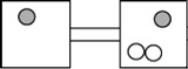
A)

B)

C)

D)


Unlock Deck
Unlock for access to all 157 flashcards in this deck.
Unlock Deck
k this deck
28
Perfect crystals of carbon monoxide (CO) are difficult to prepare because the very small dipole moment allows a few molecules to align in a pattern such as CO OC CO instead of CO CO CO. If such disordered crystals were cooled to 0 K, what would be the value of their absolute entropy?
A) > 0
B) = 0
C) < 0
D) > 0, = 0, or < 0, depending on how carefully it was cooled
E) > 0 or = 0, depending on how carefully it was cooled
A) > 0
B) = 0
C) < 0
D) > 0, = 0, or < 0, depending on how carefully it was cooled
E) > 0 or = 0, depending on how carefully it was cooled

Unlock Deck
Unlock for access to all 157 flashcards in this deck.
Unlock Deck
k this deck
29
Which of the following is in the correct order of standard state entropy?
I.Diamond < graphite
II.Liquid water < solid water
III.NH3 < H2
A) I only
B) II only
C) III only
D) I and II only
E) I and III only
I.Diamond < graphite
II.Liquid water < solid water
III.NH3 < H2
A) I only
B) II only
C) III only
D) I and II only
E) I and III only

Unlock Deck
Unlock for access to all 157 flashcards in this deck.
Unlock Deck
k this deck
30
The following figures represent distributions of gas molecules between two containers connected by an open tube. In which figure is the entropy of the system maximized?
A)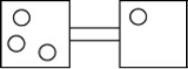
B)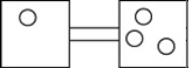
C)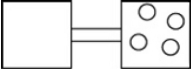
D)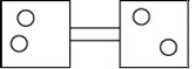
A)

B)

C)

D)


Unlock Deck
Unlock for access to all 157 flashcards in this deck.
Unlock Deck
k this deck
31
At 0 K, the entropy of a perfect crystal is ________
A) > 0.
B) = 0.
C) < 0.
D) > 0, = 0, or < 0, depending on the chemical structure of the crystal.
E) > 0 or = 0, depending on the chemical structure of the crystal.
A) > 0.
B) = 0.
C) < 0.
D) > 0, = 0, or < 0, depending on the chemical structure of the crystal.
E) > 0 or = 0, depending on the chemical structure of the crystal.

Unlock Deck
Unlock for access to all 157 flashcards in this deck.
Unlock Deck
k this deck
32
Which of the following will have the greatest standard molar entropy (S°)?
A) NH3(g)
B) He(g)
C) C(s, graphite)
D) H2O(l)
E) CaCO3(s)
A) NH3(g)
B) He(g)
C) C(s, graphite)
D) H2O(l)
E) CaCO3(s)

Unlock Deck
Unlock for access to all 157 flashcards in this deck.
Unlock Deck
k this deck
33
Indicate which of the following has the smallest standard molar entropy (S°).
A) CH4(g)
B) CH3CH2OH(l)
C) H2O(s)
D) Na(s)
E) He(g)
A) CH4(g)
B) CH3CH2OH(l)
C) H2O(s)
D) Na(s)
E) He(g)

Unlock Deck
Unlock for access to all 157 flashcards in this deck.
Unlock Deck
k this deck
34
When a molecule of ethylenediamine replaces two molecules of NH3 in Co(NH3)63+, the entropy of the system ________
A) increases.
B) decreases.
C) remains the same.
D) cannot be determined.
E) is irrelevant.
A) increases.
B) decreases.
C) remains the same.
D) cannot be determined.
E) is irrelevant.

Unlock Deck
Unlock for access to all 157 flashcards in this deck.
Unlock Deck
k this deck
35
Indicate which of the following has the highest entropy at 298 K.
A) 0.5 g of HCN
B) 1 mol of HCN
C) 2 kg of HCN
D) 2 mol of HCN
E) All of the above have the same entropy at 298 K.
A) 0.5 g of HCN
B) 1 mol of HCN
C) 2 kg of HCN
D) 2 mol of HCN
E) All of the above have the same entropy at 298 K.

Unlock Deck
Unlock for access to all 157 flashcards in this deck.
Unlock Deck
k this deck
36
The entropy of a NaCl crystal is ________
A) an intensive property and a state function.
B) an intensive property and a path function.
C) an extensive property and a state function.
D) an extensive property and a path function.
E) not appropriately described in terms of an intensive property, an extensive property, a state function, or a path function.
A) an intensive property and a state function.
B) an intensive property and a path function.
C) an extensive property and a state function.
D) an extensive property and a path function.
E) not appropriately described in terms of an intensive property, an extensive property, a state function, or a path function.

Unlock Deck
Unlock for access to all 157 flashcards in this deck.
Unlock Deck
k this deck
37
Determine S °rxn for N2O4(g) 2NO2(g) given the following information:
A) +176.7 J/K
B) -63.8 J/K
C) +63.8 J/K
D) -50.7 J/K
E) -176.7 J/K
A) +176.7 J/K
B) -63.8 J/K
C) +63.8 J/K
D) -50.7 J/K
E) -176.7 J/K

Unlock Deck
Unlock for access to all 157 flashcards in this deck.
Unlock Deck
k this deck
38
Indicate which one of the following reactions results in a positive Ssys.
A) AgNO3(aq) + NaCl(aq) AgCl(s) + NaNO3(aq)
B) HCl(g) + H2O( HCl(aq)
C) H2(g) + I2(g) 2 HI(g)
D) C2H2O2(g) 2 CO(g) + H2(g)
E) H2O(g) H2O( )
A) AgNO3(aq) + NaCl(aq) AgCl(s) + NaNO3(aq)
B) HCl(g) + H2O( HCl(aq)
C) H2(g) + I2(g) 2 HI(g)
D) C2H2O2(g) 2 CO(g) + H2(g)
E) H2O(g) H2O( )

Unlock Deck
Unlock for access to all 157 flashcards in this deck.
Unlock Deck
k this deck
39
Consider a closed container containing a 1 M solution of HCl, above which is air that contains water vapor at its equilibrium vapor pressure. Assume the pressure of the air and water vapor is
1 bar and the temperature of the system is 298 K. Which of the following are in their thermodynamic standard state?
I.The liquid water
II.The HCl solution
III.The water vapor
A) I only
B) II only
C) III only
D) I and II only
E) I, II, and III are all in their standard state.
1 bar and the temperature of the system is 298 K. Which of the following are in their thermodynamic standard state?
I.The liquid water
II.The HCl solution
III.The water vapor
A) I only
B) II only
C) III only
D) I and II only
E) I, II, and III are all in their standard state.

Unlock Deck
Unlock for access to all 157 flashcards in this deck.
Unlock Deck
k this deck
40
Which of the following graphs best depicts the entropy of a pure substance as the temperature is raised from its solid form through its liquid and gaseous forms?
A)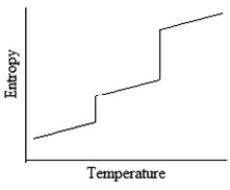
B)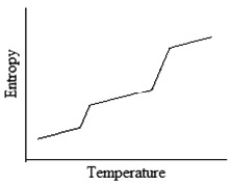
C)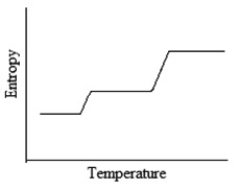
D)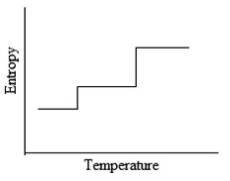
A)

B)

C)

D)


Unlock Deck
Unlock for access to all 157 flashcards in this deck.
Unlock Deck
k this deck
41
NO gas is converted to NO2 gas according to the following reaction:
NO(g) + O2(g) NO2(g)
What is the standard entropy change when 0.5 mol of NO gas reacts with 0.5 mol of O2 gas?
A) -36.6 J/K
B) -175.7 J/K
C) -83.4 J/K
D) +83.4 J/K
E) +36.6 J/K
NO(g) + O2(g) NO2(g)
What is the standard entropy change when 0.5 mol of NO gas reacts with 0.5 mol of O2 gas?
A) -36.6 J/K
B) -175.7 J/K
C) -83.4 J/K
D) +83.4 J/K
E) +36.6 J/K

Unlock Deck
Unlock for access to all 157 flashcards in this deck.
Unlock Deck
k this deck
42
Which of the following are true for a reversible process at equilibrium?
I. Suniv = 0
II. Ssys = 0
III. Gsys = 0
A) I only
B) II only
C) III only
D) I and III only
E) I, II, and III are all true.
I. Suniv = 0
II. Ssys = 0
III. Gsys = 0
A) I only
B) II only
C) III only
D) I and III only
E) I, II, and III are all true.

Unlock Deck
Unlock for access to all 157 flashcards in this deck.
Unlock Deck
k this deck
43
The reaction
Cr(NH3)63+(aq) + 3en(aq) Cr(en)33+(aq) + 6 NH3(aq)
Where en represents ethylenediamine, has a small value for the enthalpy change, Hrxn, yet the free-energy change is large because ________
A) the reaction rate is fast.
B) the entropy change is large and positive.
C) the enthalpy change is large enough to matter.
D) the entropy change is large and negative.
E) ethylenediamine has amino groups that are stronger bases than ammonia.
Cr(NH3)63+(aq) + 3en(aq) Cr(en)33+(aq) + 6 NH3(aq)
Where en represents ethylenediamine, has a small value for the enthalpy change, Hrxn, yet the free-energy change is large because ________
A) the reaction rate is fast.
B) the entropy change is large and positive.
C) the enthalpy change is large enough to matter.
D) the entropy change is large and negative.
E) ethylenediamine has amino groups that are stronger bases than ammonia.

Unlock Deck
Unlock for access to all 157 flashcards in this deck.
Unlock Deck
k this deck
44
Determine the value of G° for the reaction
2NO2(g) N2O4(g)
Given
A) -4.8 kJ
B) +4.8 kJ
C) +52.3 kJ
D) -52.3 kJ
E) -43 kJ
2NO2(g) N2O4(g)
Given
A) -4.8 kJ
B) +4.8 kJ
C) +52.3 kJ
D) -52.3 kJ
E) -43 kJ

Unlock Deck
Unlock for access to all 157 flashcards in this deck.
Unlock Deck
k this deck
45
Processes are always spontaneous when ________ (H and S refer to the system).
A) ( H > 0 and S < 0)
B) ( H < 0 and S < 0)
C) ( H > 0 and S > 0)
D) ( H < 0 and S > 0)
E) None of these is true, because temperature must always be taken into account.
A) ( H > 0 and S < 0)
B) ( H < 0 and S < 0)
C) ( H > 0 and S > 0)
D) ( H < 0 and S > 0)
E) None of these is true, because temperature must always be taken into account.

Unlock Deck
Unlock for access to all 157 flashcards in this deck.
Unlock Deck
k this deck
46
The equilibrium vapor pressure for benzene is 94.4 mm Hg. When liquid benzene is in equilibrium with its vapor, we must have ________
A) ( G = 0 and G° = 0.)
B) ( G = 0 and G° > 0.)
C) ( G = 0 and G° < 0.)
D) ( G > 0 and G° = 0.)
E) ( G < 0 and G° = 0.)
A) ( G = 0 and G° = 0.)
B) ( G = 0 and G° > 0.)
C) ( G = 0 and G° < 0.)
D) ( G > 0 and G° = 0.)
E) ( G < 0 and G° = 0.)

Unlock Deck
Unlock for access to all 157 flashcards in this deck.
Unlock Deck
k this deck
47
Determine the change in the standard entropy of the universe for the reaction
H2(g) + O2(g) H2O(g)
Given
A) -767 J/K
B) +197.4 J/K
C) -197.4 J/K
D) +767 J/K
E) +665 J/K
H2(g) + O2(g) H2O(g)
Given
A) -767 J/K
B) +197.4 J/K
C) -197.4 J/K
D) +767 J/K
E) +665 J/K

Unlock Deck
Unlock for access to all 157 flashcards in this deck.
Unlock Deck
k this deck
48
What is the standard entropy change when 10.0 g of methane reacts with 10.0 g of oxygen?
CH4(g) + 2O2(g) CO2(g) + 2H2O(l)
A) -121 J/K
B) -37.9 J/K
C) -242.6 J/K
D) -154.4 J/K
E) -16.8 J/K
CH4(g) + 2O2(g) CO2(g) + 2H2O(l)
A) -121 J/K
B) -37.9 J/K
C) -242.6 J/K
D) -154.4 J/K
E) -16.8 J/K

Unlock Deck
Unlock for access to all 157 flashcards in this deck.
Unlock Deck
k this deck
49
A reaction is at equilibrium at a given temperature and constant pressure when ________
A) ( Srxn = 0.)
B) ( S = 0)
C) ( Grxn = 0.)
D) ( G = 0))
E) ( Hrxn = 0.)
A) ( Srxn = 0.)
B) ( S = 0)
C) ( Grxn = 0.)
D) ( G = 0))
E) ( Hrxn = 0.)

Unlock Deck
Unlock for access to all 157 flashcards in this deck.
Unlock Deck
k this deck
50
In an experiment, 1.00 atm of H2(g) in a 10.0 L container at 25°C was reacted under standard state conditions with a stoichiometric quantity of O2(g) to form water vapor:
H2(g) + O2(g) H2O(g). What is the entropy change for the reaction?
A) -146.5 J/K
B) -44.3 J/K
C) -18.1 J/K
D) +18.1 J/K
E) +44.25 J/K
H2(g) + O2(g) H2O(g). What is the entropy change for the reaction?
A) -146.5 J/K
B) -44.3 J/K
C) -18.1 J/K
D) +18.1 J/K
E) +44.25 J/K

Unlock Deck
Unlock for access to all 157 flashcards in this deck.
Unlock Deck
k this deck
51
What is the entropy change if 4.500 g of CaCO3(s) is placed in a container and allowed to decompose to CaO(s) and CO2(g) according to the following reaction?
CaCO3(s) CaO(s) + CO2(g)
A) +7.2 J/K
B) -160.5 J/K
C) +35.7 J/K
D) +160.5 J/K
E) +3.57 J/K
CaCO3(s) CaO(s) + CO2(g)
A) +7.2 J/K
B) -160.5 J/K
C) +35.7 J/K
D) +160.5 J/K
E) +3.57 J/K

Unlock Deck
Unlock for access to all 157 flashcards in this deck.
Unlock Deck
k this deck
52
What is the difference between G and G ?
A) ( G refers to the formation of a compound from its elements; G can be defined for any reaction.)
B) ( G refers to the formation of a pure compound; G can be defined for an impure compound.)
C) ( G refers to a reaction that goes to completion; G is defined for a reaction that goes to any extent.)
D) ( G refers to the conversion of reactants in their standard state to products in their standard state; G is defined for a reaction under any conditions.)
E) ( G refers to reactions of one mole quantities of reactants; G is defined for any quantity of reactants.)
A) ( G refers to the formation of a compound from its elements; G can be defined for any reaction.)
B) ( G refers to the formation of a pure compound; G can be defined for an impure compound.)
C) ( G refers to a reaction that goes to completion; G is defined for a reaction that goes to any extent.)
D) ( G refers to the conversion of reactants in their standard state to products in their standard state; G is defined for a reaction under any conditions.)
E) ( G refers to reactions of one mole quantities of reactants; G is defined for any quantity of reactants.)

Unlock Deck
Unlock for access to all 157 flashcards in this deck.
Unlock Deck
k this deck
53
Which of the following statements about equilibrium are true?
I. Gsys = 0
II. Ssys = 0
III. Suniverse = 0
A) I only
B) II only
C) III only
D) both I and II
E) both I and III
I. Gsys = 0
II. Ssys = 0
III. Suniverse = 0
A) I only
B) II only
C) III only
D) both I and II
E) both I and III

Unlock Deck
Unlock for access to all 157 flashcards in this deck.
Unlock Deck
k this deck
54
Which of the relationships between the free-energy change of a system and associated entropy changes is true?
A) ( Gsys = +T Ssystem)
B) ( Gsys = -T Ssystem)
C) ( Gsys = +T Suniverse)
D) ( Gsys = -T Ssurroundings)
E) ( Gsys = -T Suniverse)
A) ( Gsys = +T Ssystem)
B) ( Gsys = -T Ssystem)
C) ( Gsys = +T Suniverse)
D) ( Gsys = -T Ssurroundings)
E) ( Gsys = -T Suniverse)

Unlock Deck
Unlock for access to all 157 flashcards in this deck.
Unlock Deck
k this deck
55
The symbol G
(CH4, g) refers to which of the following reactions?
A) CH4(l) CH4(g)
B) CH4(g) CH4(l)
C) CH4(g) C(g) + 4H(g)
D) C(g) + 4H(g) CH4(g)
E) C(s, graphite) + 2H2(g) CH4(g)
(CH4, g) refers to which of the following reactions?
A) CH4(l) CH4(g)
B) CH4(g) CH4(l)
C) CH4(g) C(g) + 4H(g)
D) C(g) + 4H(g) CH4(g)
E) C(s, graphite) + 2H2(g) CH4(g)

Unlock Deck
Unlock for access to all 157 flashcards in this deck.
Unlock Deck
k this deck
56
Alcohols for use as biofuels can be produced from glucose that is obtained from starch and cellulose in plants. Use the information in the table below to determine the free-energy change and whether or not this reaction is spontaneous at 78°C, which is the boiling point of an ethanol-water azeotrope.
C6H12O6(s) 2CH3CH2OH(l) + 2CO2(g)
A) -6 kJ, spontaneous
B) +76 kJ, not spontaneous
C) -76 kJ, spontaneous
D) -258 kJ, not spontaneous
E) -258 kJ, spontaneous
C6H12O6(s) 2CH3CH2OH(l) + 2CO2(g)
A) -6 kJ, spontaneous
B) +76 kJ, not spontaneous
C) -76 kJ, spontaneous
D) -258 kJ, not spontaneous
E) -258 kJ, spontaneous

Unlock Deck
Unlock for access to all 157 flashcards in this deck.
Unlock Deck
k this deck
57
Hydrochloric acid (HCl) reacts with sodium hydroxide (NaOH) to form sodium chloride (NaCl)
And water. If H° = -56.13 kJ/mol and S° = 79.11 J/mol . K, what is G° for this reaction at 20°C?
A) -79.31 kJ/mol
B) -77.73 kJ/mol
C) -2.324 * 104 kJ/mol
D) 79.31 kJ/mol
E) -1,638 kJ/mol
And water. If H° = -56.13 kJ/mol and S° = 79.11 J/mol . K, what is G° for this reaction at 20°C?
A) -79.31 kJ/mol
B) -77.73 kJ/mol
C) -2.324 * 104 kJ/mol
D) 79.31 kJ/mol
E) -1,638 kJ/mol

Unlock Deck
Unlock for access to all 157 flashcards in this deck.
Unlock Deck
k this deck
58
In an experiment, 1.00 mol of sodium metal is placed in a container and reacts with an excess of chlorine gas to form sodium chloride under standard state conditions. Determine Srxn given the following information:
A) -181.2 J/K
B) -90.6 J/K
C) -724.8 J/K
D) -45.3 J/K
E) -202.1 J/K
A) -181.2 J/K
B) -90.6 J/K
C) -724.8 J/K
D) -45.3 J/K
E) -202.1 J/K

Unlock Deck
Unlock for access to all 157 flashcards in this deck.
Unlock Deck
k this deck
59
Hydrogen reacts with nitrogen to form ammonia (NH3) according to the reaction
3H2(g) + N2(g) 2NH3(g)
The value of H° is -92.38 kJ/mol, and that of S° is -198.2 J/(mol . K). Determine G° at 25°C.
A) +5.897 * 104 kJ/mol
B) +297.8 kJ/mol
C) -33.32 kJ/mol
D) -16.66 kJ/mol
E) +49.5 kJ/mol
3H2(g) + N2(g) 2NH3(g)
The value of H° is -92.38 kJ/mol, and that of S° is -198.2 J/(mol . K). Determine G° at 25°C.
A) +5.897 * 104 kJ/mol
B) +297.8 kJ/mol
C) -33.32 kJ/mol
D) -16.66 kJ/mol
E) +49.5 kJ/mol

Unlock Deck
Unlock for access to all 157 flashcards in this deck.
Unlock Deck
k this deck
60
At constant T and P, any reaction will be spontaneous if ________
A) ( Gsys > 0.)
B) ( Gsys < 0.)
C) ( sys > 0.)
D) ( Ssys < 0.)
E) ( Hsys < 0.)
A) ( Gsys > 0.)
B) ( Gsys < 0.)
C) ( sys > 0.)
D) ( Ssys < 0.)
E) ( Hsys < 0.)

Unlock Deck
Unlock for access to all 157 flashcards in this deck.
Unlock Deck
k this deck
61
One of the following statements A-D may be incorrect. If so, identify it.
A) If both H and S are positive, then a reaction will be spontaneous and produce products only at a sufficiently high temperature.
B) If both H and S are negative, then a reaction will be spontaneous and produce products only at a sufficiently low temperature.
C) If H > 0 and S < 0, then a reaction is not spontaneous and will not produce products at any temperature.
D) If H < 0 and S > 0, then a reaction is spontaneous and will produce products at any temperature.
E) All of the statements A-D are correct.
A) If both H and S are positive, then a reaction will be spontaneous and produce products only at a sufficiently high temperature.
B) If both H and S are negative, then a reaction will be spontaneous and produce products only at a sufficiently low temperature.
C) If H > 0 and S < 0, then a reaction is not spontaneous and will not produce products at any temperature.
D) If H < 0 and S > 0, then a reaction is spontaneous and will produce products at any temperature.
E) All of the statements A-D are correct.

Unlock Deck
Unlock for access to all 157 flashcards in this deck.
Unlock Deck
k this deck
62
The dissolution of ammonium nitrate in water is a spontaneous endothermic process. It is spontaneous because the system undergoes ________
A) a decrease in enthalpy.
B) an increase in entropy.
C) an increase in enthalpy.
D) a decrease in entropy.
E) an increase in free energy.
A) a decrease in enthalpy.
B) an increase in entropy.
C) an increase in enthalpy.
D) a decrease in entropy.
E) an increase in free energy.

Unlock Deck
Unlock for access to all 157 flashcards in this deck.
Unlock Deck
k this deck
63
Calcium sulfate is a desiccant used for storage of samples and equipment in a dry atmosphere because it absorbs water from air. The relevant thermodynamic reaction equation is given below. At what temperature will this reaction reverse to release the water and regenerate the dry desiccant?
CaSO4(s) + 2H2O(g) CaSO4.H2O(s)
H° = -104.9 kJ/mol, S° = -291.2 J/(mol K)
A) 58°C
B) 78°C
C) 98°C
D) 68°C
E) 87°C
CaSO4(s) + 2H2O(g) CaSO4.H2O(s)
H° = -104.9 kJ/mol, S° = -291.2 J/(mol K)
A) 58°C
B) 78°C
C) 98°C
D) 68°C
E) 87°C

Unlock Deck
Unlock for access to all 157 flashcards in this deck.
Unlock Deck
k this deck
64
Which statement characterizes the following table?
Temperature Dependence of Reaction Spontaneity
A) All entries are correct
B) All entries are incorrect.
C) One entry is incorrect.
D) Two entries are incorrect.
E) Three entries are incorrect.
Temperature Dependence of Reaction Spontaneity
A) All entries are correct
B) All entries are incorrect.
C) One entry is incorrect.
D) Two entries are incorrect.
E) Three entries are incorrect.

Unlock Deck
Unlock for access to all 157 flashcards in this deck.
Unlock Deck
k this deck
65
Which of the following must be true for a reaction to proceed to form products?
A) Q > K, G < 0, and G° < 0
B) Q < K and G < 0, G° can be anything
C) Q > K and G° < 0, G can be anything
D) Q < K, G < 0, G° < 0
E) Q < K and G° < 0, G can be anything
A) Q > K, G < 0, and G° < 0
B) Q < K and G < 0, G° can be anything
C) Q > K and G° < 0, G can be anything
D) Q < K, G < 0, G° < 0
E) Q < K and G° < 0, G can be anything

Unlock Deck
Unlock for access to all 157 flashcards in this deck.
Unlock Deck
k this deck
66
The entropy of vaporization of water is 109.0 J/mol · K. What is the enthalpy of vaporization of water at its normal boiling point?
A) +10.90 kJ/mol
B) -40.66 kJ/mol
C) +3.42 kJ/mol
D) +40.66 kJ/mol
E) -10.90 kJ/mol
A) +10.90 kJ/mol
B) -40.66 kJ/mol
C) +3.42 kJ/mol
D) +40.66 kJ/mol
E) -10.90 kJ/mol

Unlock Deck
Unlock for access to all 157 flashcards in this deck.
Unlock Deck
k this deck
67
A reaction with a low enthalpy of reaction value is not spontaneous at low temperature but becomes spontaneous at high temperature. What are the signs for H° and S°, respectively?
A) +, -
B) -, -
C) -, +
D) +, +
E) Insufficient data is provided to answer this question.
A) +, -
B) -, -
C) -, +
D) +, +
E) Insufficient data is provided to answer this question.

Unlock Deck
Unlock for access to all 157 flashcards in this deck.
Unlock Deck
k this deck
68
At body temperature, many proteins have a well-defined structure that is essential to their
Function. However, as the temperature is raised, the structure changes and the protein is no longer functional. This process is referred to as protein denaturation. What can be deduced from this information about the signs of the enthalpy and entropy changes for denaturation?
A) ( H > 0 and S > 0)
B) ( H > 0 and S < 0)
C) ( H < 0 and S > 0)
D) ( H < 0 and S < 0)
E) There is insufficient information to deduce anything about the signs of H and S.
Function. However, as the temperature is raised, the structure changes and the protein is no longer functional. This process is referred to as protein denaturation. What can be deduced from this information about the signs of the enthalpy and entropy changes for denaturation?
A) ( H > 0 and S > 0)
B) ( H > 0 and S < 0)
C) ( H < 0 and S > 0)
D) ( H < 0 and S < 0)
E) There is insufficient information to deduce anything about the signs of H and S.

Unlock Deck
Unlock for access to all 157 flashcards in this deck.
Unlock Deck
k this deck
69
Some pure metals can be obtained from their ores simply by heating to a high temperature to drive off the oxygen, but iron ore usually is refined by reacting it with carbon monoxide. Use the information in the following table to determine whether or not iron ore could be refined by heating to a high temperature and, if so, how high the temperature must be. The oxidation reaction producing iron ore is given below.
4Fe(s) + 3O2(g) 2Fe2O3(s)
Thermodynamic Properties
A) Iron ore cannot be refined by heating because the oxidation reaction is spontaneous in the forward direction at all temperatures.
B) Iron ore could be refined by heating, but temperatures in the range 500-1000 K are needed.
C) Iron ore could be refined by heating, but temperatures in the range 2000-3000 K are needed.
D) Iron ore could be refined by heating, but temperatures in the range 1000-2000 K are needed.
E) Iron ore could be refined by heating, but the temperature must be greater than 3000 K.
4Fe(s) + 3O2(g) 2Fe2O3(s)
Thermodynamic Properties
A) Iron ore cannot be refined by heating because the oxidation reaction is spontaneous in the forward direction at all temperatures.
B) Iron ore could be refined by heating, but temperatures in the range 500-1000 K are needed.
C) Iron ore could be refined by heating, but temperatures in the range 2000-3000 K are needed.
D) Iron ore could be refined by heating, but temperatures in the range 1000-2000 K are needed.
E) Iron ore could be refined by heating, but the temperature must be greater than 3000 K.

Unlock Deck
Unlock for access to all 157 flashcards in this deck.
Unlock Deck
k this deck
70
Alcohols for use as biofuels can be produced from glucose that is obtained from starch and cellulose in plants. Use the information in the table below to determine the temperature range, if any, at which this reaction is spontaneous.
C6H12O6(s) 2CH3CH2OH(l) + 2CO2(g)
A) Reaction is spontaneous at high temperatures.
B) Reaction is spontaneous at low temperatures.
C) Reaction is spontaneous at all temperatures.
D) Reaction is not spontaneous at any temperature.
E) Whether the reaction is spontaneous or not cannot be predicted from the information provided.
C6H12O6(s) 2CH3CH2OH(l) + 2CO2(g)
A) Reaction is spontaneous at high temperatures.
B) Reaction is spontaneous at low temperatures.
C) Reaction is spontaneous at all temperatures.
D) Reaction is not spontaneous at any temperature.
E) Whether the reaction is spontaneous or not cannot be predicted from the information provided.

Unlock Deck
Unlock for access to all 157 flashcards in this deck.
Unlock Deck
k this deck
71
When a solution of DNA in water is heated, the double helix separates into two single strands. This process is called melting. What can be deduced from this information about the signs of the enthalpy and entropy changes for DNA melting?
A) ( H > 0 and S > 0)
B) ( H > 0 and S < 0)
C) ( H < 0 and S > 0)
D) ( H < 0 and S < 0)
E) This information is not sufficient to determine the signs of H and S.
A) ( H > 0 and S > 0)
B) ( H > 0 and S < 0)
C) ( H < 0 and S > 0)
D) ( H < 0 and S < 0)
E) This information is not sufficient to determine the signs of H and S.

Unlock Deck
Unlock for access to all 157 flashcards in this deck.
Unlock Deck
k this deck
72
Determine Grxn for C4H10(l) + O2(g) 4CO2(g) + 5H2O(g) given the following information:
A) -2705 kJ
B) -608.0 kJ
C) -1,791 kJ
D) -3,457 kJ
E) +608.0 kJ
A) -2705 kJ
B) -608.0 kJ
C) -1,791 kJ
D) -3,457 kJ
E) +608.0 kJ

Unlock Deck
Unlock for access to all 157 flashcards in this deck.
Unlock Deck
k this deck
73
Which statement characterizes the following table?
Temperature Dependence of Reaction Spontaneity
A) All entries are correct.
B) All entries are incorrect.
C) One entry is incorrect.
D) Two entries are incorrect.
E) Three entries are incorrect.
Temperature Dependence of Reaction Spontaneity
A) All entries are correct.
B) All entries are incorrect.
C) One entry is incorrect.
D) Two entries are incorrect.
E) Three entries are incorrect.

Unlock Deck
Unlock for access to all 157 flashcards in this deck.
Unlock Deck
k this deck
74
What is the maximum amount of work that can be done by the reaction
CH4(g) + 2O2(g) CO2(g) + 2H2O(g)
Given
A) -50.8 kJ/mol
B) -751 kJ/mol
C) +113 kJ/mol
D) -115 kJ/mol
E) -807 kJ/mol
CH4(g) + 2O2(g) CO2(g) + 2H2O(g)
Given
A) -50.8 kJ/mol
B) -751 kJ/mol
C) +113 kJ/mol
D) -115 kJ/mol
E) -807 kJ/mol

Unlock Deck
Unlock for access to all 157 flashcards in this deck.
Unlock Deck
k this deck
75
At what temperature does the Fe(s) Fe(g) phase transition occur?
H = 415.5 kJ/mol; S = 153.4 J/mol · K.
A) 2162°F
B) 2435°F
C) 4352°F
D) 4416°F
E) 2709°F
H = 415.5 kJ/mol; S = 153.4 J/mol · K.
A) 2162°F
B) 2435°F
C) 4352°F
D) 4416°F
E) 2709°F

Unlock Deck
Unlock for access to all 157 flashcards in this deck.
Unlock Deck
k this deck
76
Which statement characterizes the following table?
Temperature Dependence of Reaction Spontaneity
A) All entries are correct.
B) All entries are incorrect.
C) One entry is incorrect.
D) Two entries are incorrect.
E) Three entries are incorrect.
Temperature Dependence of Reaction Spontaneity
A) All entries are correct.
B) All entries are incorrect.
C) One entry is incorrect.
D) Two entries are incorrect.
E) Three entries are incorrect.

Unlock Deck
Unlock for access to all 157 flashcards in this deck.
Unlock Deck
k this deck
77
Alcohols for use as biofuels can be produced from glucose that is obtained from starch and cellulose in plants. For example, 1 mol of glucose can produce 2 mol of ethanol. Use the information in the table below to determine the maximum amount of work and energy that can be produced by the combustion of glucose compared with that by the combustion of 2 mol of ethanol.
C6H12O6(s) 2CH3CH2OH(l) + 2CO2(g)
A) 1 mol glucose = 2,830 kJ, 2 mol ethanol = 2,600 kJ
B) 1 mol glucose = 2,830 kJ, 2 mol ethanol = 1,300 kJ
C) 1 mol glucose = 2,190 kJ, 2 mol ethanol = 2,780 kJ
D) 1 mol glucose = 1,420 kJ, 2 mol ethanol = 1,390 kJ
E) 1 mol glucose = 2,190 kJ, 2 mol ethanol = 1,300 kJ
C6H12O6(s) 2CH3CH2OH(l) + 2CO2(g)
A) 1 mol glucose = 2,830 kJ, 2 mol ethanol = 2,600 kJ
B) 1 mol glucose = 2,830 kJ, 2 mol ethanol = 1,300 kJ
C) 1 mol glucose = 2,190 kJ, 2 mol ethanol = 2,780 kJ
D) 1 mol glucose = 1,420 kJ, 2 mol ethanol = 1,390 kJ
E) 1 mol glucose = 2,190 kJ, 2 mol ethanol = 1,300 kJ

Unlock Deck
Unlock for access to all 157 flashcards in this deck.
Unlock Deck
k this deck
78
Dinitrogen tetroxide (N2O4) decomposes to nitrogen dioxide (NO2). If H° = 58.02 kJ/mol and S° = 176.1 J/mol · K, at what temperature are reactants and products in their standard states at equilibrium?
A) +56.5°C
B) +329.5°C
C) -272.7°C
D) +25.0°C
E) +98.3°C
A) +56.5°C
B) +329.5°C
C) -272.7°C
D) +25.0°C
E) +98.3°C

Unlock Deck
Unlock for access to all 157 flashcards in this deck.
Unlock Deck
k this deck
79
The enthalpy and entropy of vaporization of ethanol are 38.6 kJ/mol and 109.8 J/mol · K, respectively. What is the boiling point of ethanol under equilibrium conditions, in °C?
A) 352°C
B) 78.5°C
C) 2.84°C
D) 624°C
E) Not enough information is given to answer the question.
A) 352°C
B) 78.5°C
C) 2.84°C
D) 624°C
E) Not enough information is given to answer the question.

Unlock Deck
Unlock for access to all 157 flashcards in this deck.
Unlock Deck
k this deck
80
Because the triple point of water is 273 K, what must be true of the change in enthalpy and the change in entropy for ice converting to water at this temperature?
A) ( H/ S = 273 K)
B) ( S/ H = 273 K)
C)( H - S = 273 K)
D) ( H + S = 273 K)
E) ( H)( S) = 273 K)
A) ( H/ S = 273 K)
B) ( S/ H = 273 K)
C)( H - S = 273 K)
D) ( H + S = 273 K)
E) ( H)( S) = 273 K)

Unlock Deck
Unlock for access to all 157 flashcards in this deck.
Unlock Deck
k this deck


