Deck 1: Covalent Bonding and Shapes of Molecules
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/95
Play
Full screen (f)
Deck 1: Covalent Bonding and Shapes of Molecules
1
Which of the following species has an atom that has an unfilled valence shell of electrons?
A) molecular bromine, Br2
B) fluoride anion, F-
C) ammonia, NH3
D) aluminum trichloride, AlCl3
A) molecular bromine, Br2
B) fluoride anion, F-
C) ammonia, NH3
D) aluminum trichloride, AlCl3
aluminum trichloride, AlCl3
2
How many electrons can the shell with a principal quantum number of 2 hold?
A) 1
B) 2
C) 4
D) 8
A) 1
B) 2
C) 4
D) 8
8
3
Which of the following compounds is an alcohol?
A) CH3CH2COOH
B) CH3CH2OCH3
C) CH3CH2CH2OH
D) CH3CH2CHO
A) CH3CH2COOH
B) CH3CH2OCH3
C) CH3CH2CH2OH
D) CH3CH2CHO
CH3CH2CH2OH
4
How many electrons can the shell with a principal quantum number of 1 hold?
A) 1
B) 2
C) 4
D) 8
A) 1
B) 2
C) 4
D) 8

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
5
Which of the following species possesses a formal charge?
A) CCl4
B) SiCl4
C) AlCl4
D) PCl3
A) CCl4
B) SiCl4
C) AlCl4
D) PCl3

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
6
Which of the following is trigonal planar?
A) boron trifluoride, BF3
B) methyl anion, CH3-
C) methane, CH4
D) ammonia, NH3
A) boron trifluoride, BF3
B) methyl anion, CH3-
C) methane, CH4
D) ammonia, NH3

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
7
What is the ground-state electronic configuration of a fluorine atom (fluorine: atomic number 9)?
A) 1s12s12p7
B) 1s22s22p5
C) 1s22s22p6
D) 1s02s22p7
A) 1s12s12p7
B) 1s22s22p5
C) 1s22s22p6
D) 1s02s22p7

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
8
Which of the following is a tertiary alcohol?
A) CH3CH2OCH3
B) (CH3)3COH
C) (CH3)2CHOH
D) CH3CH2CH2OH
A) CH3CH2OCH3
B) (CH3)3COH
C) (CH3)2CHOH
D) CH3CH2CH2OH

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
9
Which of the following compounds is an aldehyde?
A) CH3CH2CH2COOH
B) CH3CH2CHO
C) CH3CH2CH2OH
D) CH3CH2COCH3
A) CH3CH2CH2COOH
B) CH3CH2CHO
C) CH3CH2CH2OH
D) CH3CH2COCH3

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
10
What is the ground-state electronic configuration of a sodium cation (sodium: atomic number 11)?
A) 1s22s22p63s1
B) 1s22s22p53s1
C) 1s22s22p6
D) 1s22s22p63s2
A) 1s22s22p63s1
B) 1s22s22p53s1
C) 1s22s22p6
D) 1s22s22p63s2

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
11
Which of the following compounds is a carboxylic acid?
A) CH3CH2COOH
B) CH3CH2OCH3
C) CH3CH2CH2OH
D) CH3CH2CHO
A) CH3CH2COOH
B) CH3CH2OCH3
C) CH3CH2CH2OH
D) CH3CH2CHO

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
12
What is the ground-state electronic configuration of a nitrogen atom (nitrogen: atomic number 7)?
A) 1s22s12p4
B) 1s22s22p3
C) 1s12s12p5
D) 1s22s22p2
A) 1s22s12p4
B) 1s22s22p3
C) 1s12s12p5
D) 1s22s22p2

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
13
Which of the following compounds is a carboxylic ester?
A) CH3CH2COOH
B) CH3CH2OCH3
C) CH3CH2COOCH3
D) CH3CH2COCH3
A) CH3CH2COOH
B) CH3CH2OCH3
C) CH3CH2COOCH3
D) CH3CH2COCH3

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
14
What is the ground-state electronic configuration of a fluoride anion (fluorine: atomic number 9)?
A) 1s22s22p2
B) 1s22s22p5
C) 1s22s22p6
D) 1s22s22p7
A) 1s22s22p2
B) 1s22s22p5
C) 1s22s22p6
D) 1s22s22p7

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
15
Which of the following species has an atom that has an unfilled valence shell of electrons?
A) molecular hydrogen, H2
B) hydroxide anion, HO-
C) boron trifluoride, BF3
D) water, H2O
A) molecular hydrogen, H2
B) hydroxide anion, HO-
C) boron trifluoride, BF3
D) water, H2O

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
16
Which of the following is a tertiary amine?
A) CH3CH2N(CH3)2
B) (CH3)3CNH2
C) CH3CH2NHCH3
D) CH3CH2NHCH(CH3)2
A) CH3CH2N(CH3)2
B) (CH3)3CNH2
C) CH3CH2NHCH3
D) CH3CH2NHCH(CH3)2

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
17
Which of the following is a primary amine?
A) CH3CH2NHCH3
B) CH3CH2NHCH(CH3)2
C) CH3CH2N(CH3)2
D) (CH3)3CNH2
A) CH3CH2NHCH3
B) CH3CH2NHCH(CH3)2
C) CH3CH2N(CH3)2
D) (CH3)3CNH2

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
18
Which of the following species possesses a formal charge?
A) BH3
B) BH4
C) CCl4
D) H2S
A) BH3
B) BH4
C) CCl4
D) H2S

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
19
Which of the following compounds is a ketone?
A) CH3CH2COOH
B) CH3CH2CHO
C) CH3CH2CH2OH
D) CH3COCH3
A) CH3CH2COOH
B) CH3CH2CHO
C) CH3CH2CH2OH
D) CH3COCH3

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
20
Which of the following compounds is a ketone?
A) CH3CH2COOH
B) CH3CH2CHO
C) CH3CH2CH2OH
D) CH3COCH3
A) CH3CH2COOH
B) CH3CH2CHO
C) CH3CH2CH2OH
D) CH3COCH3

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
21
Which of the following is a polar covalent bond?
A) Na-F
B) C-H
C) C-O
D) Cl-Cl
A) Na-F
B) C-H
C) C-O
D) Cl-Cl

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
22
Which of the following molecules has a molecular dipole?
A) H2O
B) CO2
C) HCºCH
D) Cl2
A) H2O
B) CO2
C) HCºCH
D) Cl2

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
23
Which of the following molecules is not linear?
A) H2O
B) CO2
C) HCºCH
D) Cl2
A) H2O
B) CO2
C) HCºCH
D) Cl2

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
24
Which of the following elements has the highest electronegativity?
A) N
B) C
C) O
D) S
A) N
B) C
C) O
D) S

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
25
Which of the following best represents the shape of the 2s atomic orbital of carbon? 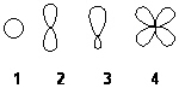
A) 1
B) 2
C) 3
D) 4

A) 1
B) 2
C) 3
D) 4

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
26
Which of the following molecules has a molecular dipole?
A) CO2
B) BF3
C) NH3
D) CH4
A) CO2
B) BF3
C) NH3
D) CH4

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
27
Which of the following elements has the highest electronegativity?
A) C
B) P
C) Si
D) Cl
A) C
B) P
C) Si
D) Cl

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
28
Which of the following molecules has a molecular dipole? 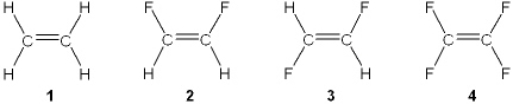
A) 1
B) 2
C) 3
D) 4

A) 1
B) 2
C) 3
D) 4

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
29
Which of the following molecules has a molecular dipole? 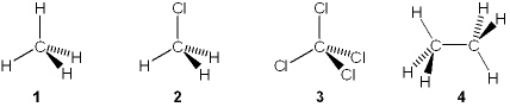
A) 1
B) 2
C) 3
D) 4

A) 1
B) 2
C) 3
D) 4

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
30
Which of the following bonds is the most polar?
A) F-F
B) H-F
C) C-H
D) C-Si
A) F-F
B) H-F
C) C-H
D) C-Si

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
31
Which of the following bonds has the smallest dipole moment?
A) Li-Cl
B) C-H
C) O-H
D) H-Cl
A) Li-Cl
B) C-H
C) O-H
D) H-Cl

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
32
What is the approximate C-C-C bond angle in propyne, HCºCCH3?
A) 90
B) 109
C) 120
D) 180
A) 90
B) 109
C) 120
D) 180

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
33
Which of the following bonds is the most polar?
A) O-H
B) C-H
C) C-C
D) H-H
A) O-H
B) C-H
C) C-C
D) H-H

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
34
Which of the following bonds has the smallest dipole moment?
A) C-N
B) C-O
C) C-F
D) O-H
A) C-N
B) C-O
C) C-F
D) O-H

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
35
What is the approximate value of the H-C-H bond angles in methane, CH4?
A) 90
B) 109
C) 120
D) 180
A) 90
B) 109
C) 120
D) 180

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
36
What is the approximate H-C-O bond angle in formaldehyde, H2C=O?
A) 90
B) 109
C) 120
D) 180
A) 90
B) 109
C) 120
D) 180

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
37
What is the approximate C-C-C bond angle in propene, CH3CH=CH2?
A) 90
B) 109
C) 120
D) 180
A) 90
B) 109
C) 120
D) 180

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
38
Which of the following is an ionic bond?
A) Br-Br
B) C-Cl
C) C-S
D) Na-O
A) Br-Br
B) C-Cl
C) C-S
D) Na-O

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
39
Which of the following is an ionic bond?
A) F-F
B) C-H
C) Li-O
D) C-N
A) F-F
B) C-H
C) Li-O
D) C-N

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
40
Which of the following is a polar covalent bond?
A) Na-Cl
B) C-Cl
C) C-H
D) Cl-Cl
A) Na-Cl
B) C-Cl
C) C-H
D) Cl-Cl

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
41
What is the approximate value of the length of the CºC bond in ethyne, HCºCH?
A) 121 pm
B) 134 pm
C) 142 pm
D) 154 pm
A) 121 pm
B) 134 pm
C) 142 pm
D) 154 pm

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
42
Which of the following statements is not true regarding resonance structures?
A) Each resonance structure is in rapid equilibrium with all of the other structures
B) The resonance structures may have different energies
C) All resonance structures must have the same arrangement of atoms
D) All resonance structures must have the same number of electrons
A) Each resonance structure is in rapid equilibrium with all of the other structures
B) The resonance structures may have different energies
C) All resonance structures must have the same arrangement of atoms
D) All resonance structures must have the same number of electrons

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
43
What is the approximate value of the H-C-H bond angles in a methyl anion, CH3-?
A) 90
B) 109
C) 120
D) 180
A) 90
B) 109
C) 120
D) 180

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
44
Which atomic orbitals overlap to form the carbon-hydrogen s bonding molecular orbitals of ethane, CH3CH3?
A) C2p + H1s
B) C2sp + H1s
C) C2sp2 + H1s
D) C2sp3 + H1s
A) C2p + H1s
B) C2sp + H1s
C) C2sp2 + H1s
D) C2sp3 + H1s

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
45
What is the approximate value of the H-C-H bond angles in a methyl cation, CH3+?
A) 90
B) 109
C) 120
D) 180
A) 90
B) 109
C) 120
D) 180

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
46
Which of the following resonance structures is the least important contributor to the resonance hybrid of the acetate anion, CH3COO-? 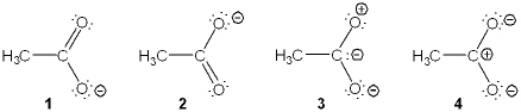
A) 1
B) 2
C) 3
D) 4

A) 1
B) 2
C) 3
D) 4

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
47
How many electrons are there in the valence shell of the carbon atom of a methyl cation, CH3+?
A) 4
B) 5
C) 6
D) 7
A) 4
B) 5
C) 6
D) 7

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
48
Which atomic orbitals overlap to form the C=O bond of acetone, (CH3)2C=O?
A) C 2sp3 + O 2sp2
B) C 2sp2 + O 2p
C) C 2sp2 + O 2sp2
D) C 2sp3 + O 2sp
A) C 2sp3 + O 2sp2
B) C 2sp2 + O 2p
C) C 2sp2 + O 2sp2
D) C 2sp3 + O 2sp

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
49
Which of the following best represents an sp2 hybridized atomic orbital of carbon which overlaps with the 1s atomic orbital of hydrogen to form a C-H s bonding molecular orbital in ethene, H2C=CH2 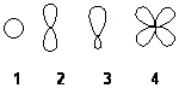
A) 1
B) 2
C) 3
D) 4

A) 1
B) 2
C) 3
D) 4

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
50
Which atomic orbitals overlap to form the C-O bond of dimethyl ether, (CH3)2O?
A) C 2sp3 + O 2sp2
B) C 2sp2 + O 2p
C) C 2sp2 + O 2sp2
D) C 2sp3 + O 2sp3
A) C 2sp3 + O 2sp2
B) C 2sp2 + O 2p
C) C 2sp2 + O 2sp2
D) C 2sp3 + O 2sp3

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
51
Which of the following statements is not true about the carbonate anion, CO32-?
A) All of the oxygen atoms bear the same amount of charge
B) All of the carbon-oxygen bonds are the same length
C) The carbon atom bears the negative charge
D) It is basic
A) All of the oxygen atoms bear the same amount of charge
B) All of the carbon-oxygen bonds are the same length
C) The carbon atom bears the negative charge
D) It is basic

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
52
Rank the following in order of decreasing importance as a contributing resonance structure to the molecular structure of acetone, CH3COCH3 (more important > less important) 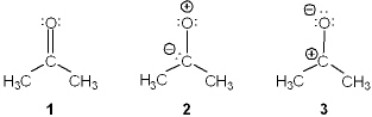
A) 1 > 2 > 3
B) 1 > 3 > 2
C) 2 > 1 > 3
D) 3 > 1 > 2

A) 1 > 2 > 3
B) 1 > 3 > 2
C) 2 > 1 > 3
D) 3 > 1 > 2

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
53
Which of the following statements is not true regarding resonance structures?
A) All resonance structures must have the same number of electrons
B) Each atom in all of the resonance structures must have a complete shell of valence electrons
C) All resonance structures must have the same arrangement of atoms
D) All resonance structures must be valid Lewis structures
A) All resonance structures must have the same number of electrons
B) Each atom in all of the resonance structures must have a complete shell of valence electrons
C) All resonance structures must have the same arrangement of atoms
D) All resonance structures must be valid Lewis structures

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
54
How many electrons are there in the valence shell of the oxygen atom of water?
A) 2
B) 4
C) 6
D) 8
A) 2
B) 4
C) 6
D) 8

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
55
How many electrons are there in the valence shell of the nitrogen atom of ammonia?
A) 4
B) 5
C) 6
D) 8
A) 4
B) 5
C) 6
D) 8

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
56
How many electrons are there in the valence shell of the carbon atom of the methyl anion, CH3-?
A) 2
B) 4
C) 6
D) 8
A) 2
B) 4
C) 6
D) 8

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
57
What is the approximate value of the length of the C=C bond in ethane, CH2=CH2?
A) 121 pm
B) 134 pm
C) 142 pm
D) 154 pm
A) 121 pm
B) 134 pm
C) 142 pm
D) 154 pm

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
58
Which of the following statements is not true about the acetate anion, CH3CO2-?
A) The oxygen atoms bear the same amount of charge
B) The two carbon-oxygen bonds are the same length
C) The carbon atom bears the negative charge
D) It is basic
A) The oxygen atoms bear the same amount of charge
B) The two carbon-oxygen bonds are the same length
C) The carbon atom bears the negative charge
D) It is basic

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
59
Which of the following best represents an sp3 hybridized atomic orbital containing the lone pair of electrons of ammonia, NH3? 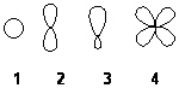
A) 1
B) 2
C) 3
D) 4

A) 1
B) 2
C) 3
D) 4

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
60
Which of the following best represents the shape of a 2p atomic orbital of carbon? 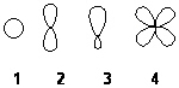
A) 1
B) 2
C) 3
D) 4

A) 1
B) 2
C) 3
D) 4

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
61
Which atomic orbitals overlap to form the carbon-hydrogen s bonding molecular orbitals of ethene, H2C=CH2?
A) C2p + H1s
B) C2sp + H1s
C) C2sp2 + H1s
D) C2sp3 + H1s
A) C2p + H1s
B) C2sp + H1s
C) C2sp2 + H1s
D) C2sp3 + H1s

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
62
Which of the circled bonds is the strongest? 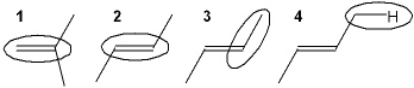
A) 1
B) 2
C) 3
D) 4
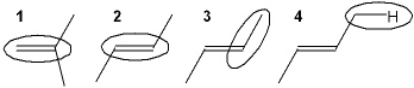
A) 1
B) 2
C) 3
D) 4

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
63
Which of the following shows curved arrows that correctly accounts for the differences between the two structures? 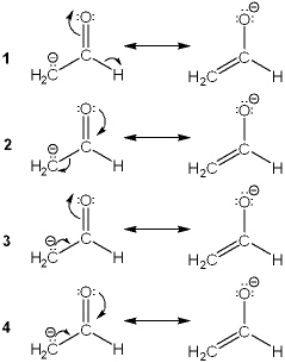
A) 1
B) 2
C) 3
D) 4

A) 1
B) 2
C) 3
D) 4

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
64
Which of the following is a tertiary (3 ) alcohol? 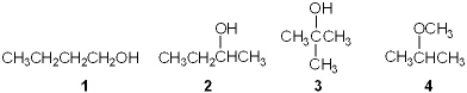
A) 1
B) 2
C) 3
D) 4

A) 1
B) 2
C) 3
D) 4

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
65
Draw bond-line structures of all of the alkanes that have the formula C5H12.

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
66
Which atomic orbitals overlap to form the carbon-carbon s and p bonding molecular orbitals of ethene, H2C=CH2?
A) C2sp3 + C2sp3, and C2p + C2p
B) C2sp2 + C2sp2, and C2sp2 + C2sp2
C) C2sp2 + C2sp2, and C2p + C2p
D) C2sp3 + C2sp3, and C2sp2 + C2sp2
A) C2sp3 + C2sp3, and C2p + C2p
B) C2sp2 + C2sp2, and C2sp2 + C2sp2
C) C2sp2 + C2sp2, and C2p + C2p
D) C2sp3 + C2sp3, and C2sp2 + C2sp2

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
67
Which of the following resonance structures makes the largest contribution to the structure of [H2CCHO] -? ![<strong>Which of the following resonance structures makes the largest contribution to the structure of [H<sub>2</sub>CCHO]<sup> -</sup>? </strong> A) 1 B) 2 C) 3 D) 4](https://storage.examlex.com/TB7077/11eb135c_c799_b370_8021_4b082ca9a792_TB7077_00.jpg)
A) 1
B) 2
C) 3
D) 4
![<strong>Which of the following resonance structures makes the largest contribution to the structure of [H<sub>2</sub>CCHO]<sup> -</sup>? </strong> A) 1 B) 2 C) 3 D) 4](https://storage.examlex.com/TB7077/11eb135c_c799_b370_8021_4b082ca9a792_TB7077_00.jpg)
A) 1
B) 2
C) 3
D) 4

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
68
Which of the following is a primary (1 ) alcohol? 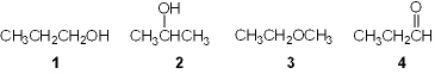
A) 1
B) 2
C) 3
D) 4
A) 1
B) 2
C) 3
D) 4

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
69
Provide a neatly drawn figure to show the atomic orbitals that overlap to form each of the bonds in ethyne (acetylene, HCºCH). Label each bond (e.g., C-H s bond) and indicate which atomic orbitals contribute to this bond (e.g., C 2sp3 + H 1s).

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
70
Provide a neatly drawn figure to show the atomic orbitals that overlap to form each of the bonds in ammonia (NH3) and which contain the lone pair of electons. Label each orbital with its hybridization.

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
71
Provide a neatly drawn figure to show the atomic orbitals that overlap to form each of the bonds in water (H2O) and which contain the lone pair of electrons. Label each orbital with its hybridization.

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
72
Which of the following statements is not true?
A) The sp3C-H bond of an alkane is weaker than the spC-H bond of an alkyne.
B) The carbon-carbon triple bond of an alkyne is shorter than the carbon-carbon bond of alkenes.
C) The carbon-carbon triple bond of an alkene is exactly three times as strong as a carbon-carbon single bond of an alkane.
D) The sp3C-H bond of an alkane is longer than the spC-H bond of an alkyne.
A) The sp3C-H bond of an alkane is weaker than the spC-H bond of an alkyne.
B) The carbon-carbon triple bond of an alkyne is shorter than the carbon-carbon bond of alkenes.
C) The carbon-carbon triple bond of an alkene is exactly three times as strong as a carbon-carbon single bond of an alkane.
D) The sp3C-H bond of an alkane is longer than the spC-H bond of an alkyne.

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
73
Which of the following is/are tetrahedral? 
A) only 1 and 2
B) only 1 and 3
C) only 1 and 4
D) only 2 and 3

A) only 1 and 2
B) only 1 and 3
C) only 1 and 4
D) only 2 and 3

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
74
What is the approximate strength of the C-C bond of ethane?
A) 376 kJ/mol (90 kcal./mol)
B) 422 kJ/mol (101 kcal./mol)
C) 556 kJ/mol (133 kcal./mol)
D) 727 kJ/mol (174 kcal./mol)
A) 376 kJ/mol (90 kcal./mol)
B) 422 kJ/mol (101 kcal./mol)
C) 556 kJ/mol (133 kcal./mol)
D) 727 kJ/mol (174 kcal./mol)

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
75
Which of the following is a primary (1 ) amine? 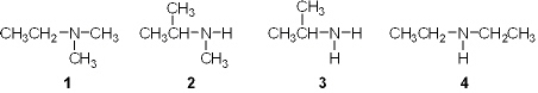
A) 1
B) 2
C) 3
D) 4

A) 1
B) 2
C) 3
D) 4

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
76
Which of the following is an ester? 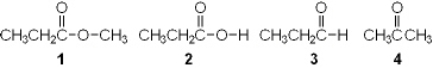
A) 1
B) 2
C) 3
D) 4

A) 1
B) 2
C) 3
D) 4

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
77
Which of the following is a secondary (2 ) amine? 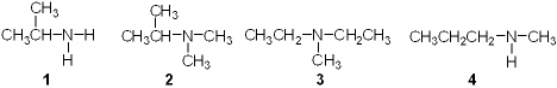
A) 1
B) 2
C) 3
D) 4

A) 1
B) 2
C) 3
D) 4

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
78
Which atomic orbitals overlap to form the carbon-carbon s molecular bonding orbital of ethyne, HCºCH?
A) C2p + C2p
B) C2sp + C2sp
C) C2sp2 + C2sp2
D) C2sp3 + C2sp3
A) C2p + C2p
B) C2sp + C2sp
C) C2sp2 + C2sp2
D) C2sp3 + C2sp3

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
79
Which atomic orbitals overlap to form the carbon-hydrogen s bonding molecular orbitals of ethyne, HCºCH?
A) C2p + H1s
B) C2sp + H1s
C) C2sp2 + H1s
D) C2sp3 + H1s
A) C2p + H1s
B) C2sp + H1s
C) C2sp2 + H1s
D) C2sp3 + H1s

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
80
Provide a neatly drawn figure to show the atomic orbitals that overlap to form each of the bonds in ethene (ethylene, H2C=CH2). Label each bond (e.g., C-H s bond) and indicate which atomic orbitals contribute to this bond (e.g., C 2sp3 + H 1s).

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck


