Deck 27: Nuclear Physics
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/94
Play
Full screen (f)
Deck 27: Nuclear Physics
1
The alpha particles from a naturally radioactive decay have a kinetic energy of 4.25 MeV. How close does an alpha particle come within a gold nucleus if the particle is heading directly toward the nucleus? Give your answer in terms of number of radii of the gold atom, r = 7.0 fm.
A) 2
B) 8
C) 12
D) 15
E) 19
A) 2
B) 8
C) 12
D) 15
E) 19
8
2
Which of the following is a negatively charged particle found in the nucleus of an atom?
A) proton
B) electron
C) neutron
D) nucleon
E) None of these is correct.
A) proton
B) electron
C) neutron
D) nucleon
E) None of these is correct.
None of these is correct.
3
The superscript before the symbol for an element represents the number of
A) protons.
B) neutrons.
C) protons plus neutrons.
D) protons minus neutrons.
E) neutrons minus protons.
A) protons.
B) neutrons.
C) protons plus neutrons.
D) protons minus neutrons.
E) neutrons minus protons.
protons plus neutrons.
4
The volume of a nucleus is directly proportional to the number of _______ it contains.
A) electrons
B) protons
C) neutrons
D) isotopes
E) nucleons
A) electrons
B) protons
C) neutrons
D) isotopes
E) nucleons

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
5
The chemical characteristics of an atom what element it is) are determined by
A) its atomic number.
B) its mass number.
C) its neutron number.
D) its nucleon number.
E) All of these are correct.
A) its atomic number.
B) its mass number.
C) its neutron number.
D) its nucleon number.
E) All of these are correct.

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
6
How many times larger is the radius of the nucleus 208Pb Z = 82) compared to the nucleus 40Ca Z = 20)?
A) 0.58
B) 1.7
C) 5.2
D) 4.1
E) 2.3
A) 0.58
B) 1.7
C) 5.2
D) 4.1
E) 2.3

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
7
Why is the ratio of neutrons to protons greater in the heavier elements?
A) The greater the number of protons, the weaker the proton-neutron strong nuclear force.
B) The greater the number of neutrons, the weaker the proton-neutron strong nuclear force.
C) The greater the number of protons, the greater the electrostatic force of repulsion.
D) The greater the number of neutrons, the greater the electrostatic force of repulsion.
E) There is actually a lesser ratio of neutrons to protons in the heavier elements.
A) The greater the number of protons, the weaker the proton-neutron strong nuclear force.
B) The greater the number of neutrons, the weaker the proton-neutron strong nuclear force.
C) The greater the number of protons, the greater the electrostatic force of repulsion.
D) The greater the number of neutrons, the greater the electrostatic force of repulsion.
E) There is actually a lesser ratio of neutrons to protons in the heavier elements.

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
8
Atoms of an element that have different atomic masses are called
A) ions.
B) isobars.
C) isotopes.
D) metastable.
E) dielectrics.
A) ions.
B) isobars.
C) isotopes.
D) metastable.
E) dielectrics.

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
9
The strong nuclear force acts between which of the following?
A) pairs of protons in the nucleus
B) pairs of neutrons in the nucleus
C) members of proton-neutron pairs in the nucleus
D) the nucleus and the electrons
E) Answers a, b, and c are correct.
A) pairs of protons in the nucleus
B) pairs of neutrons in the nucleus
C) members of proton-neutron pairs in the nucleus
D) the nucleus and the electrons
E) Answers a, b, and c are correct.

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
10
Estimate the mass density of the nucleus of an atom.
A) 103 kg/m3
B) 106 kg/m3
C) 109 kg/m3
D) 1014 kg/m3
E) 1017 kg/m3
A) 103 kg/m3
B) 106 kg/m3
C) 109 kg/m3
D) 1014 kg/m3
E) 1017 kg/m3

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
11
The subscript before the symbol for an element represents the number of
A) protons.
B) neutrons.
C) protons plus neutrons.
D) protons minus neutrons.
E) neutrons minus protons.
A) protons.
B) neutrons.
C) protons plus neutrons.
D) protons minus neutrons.
E) neutrons minus protons.

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
12
In this problem, Z is the atomic number, N is the neutron number, and A is the mass number. For stable isotopes with A greater than 44, as A increases, the ratio Z/N
A) remains the same.
B) decreases.
C) equals A - N.
D) increases.
E) None of these is correct.
A) remains the same.
B) decreases.
C) equals A - N.
D) increases.
E) None of these is correct.

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
13
The pair of nuclides that represents isotopes is
A)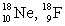 .
.
B)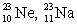 .
.
C)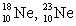 .
.
D)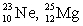 .
.
E)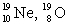 .
.
A)
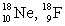 .
.B)
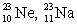 .
.C)
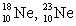 .
.D)
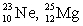 .
.E)
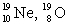 .
.
Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
14
The word isotopes describes elements that have
A) the same atomic number but different atomic mass.
B) the same atomic mass but different atomic number.
C) the same atomic mass and the same atomic number but different chemical properties.
D) similar chemical properties, though they differ in both atomic mass and atomic number.
E) the same number of valence electrons.
A) the same atomic number but different atomic mass.
B) the same atomic mass but different atomic number.
C) the same atomic mass and the same atomic number but different chemical properties.
D) similar chemical properties, though they differ in both atomic mass and atomic number.
E) the same number of valence electrons.

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
15
The nuclear force is
A) short range and strongly dependent on the charge.
B) short range but proportional to the Coulomb force.
C) strongly dependent on the charge.
D) very strong compared with Coulomb forces) and long range.
E) short range, charge independent, and very strong.
A) short range and strongly dependent on the charge.
B) short range but proportional to the Coulomb force.
C) strongly dependent on the charge.
D) very strong compared with Coulomb forces) and long range.
E) short range, charge independent, and very strong.

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
16
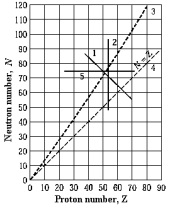 Two nuclides that are isotopes could lie on curve
Two nuclides that are isotopes could lie on curveA) 1.
B) 2.
C) 3.
D) 4.
E) 5.

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
17
The nuclear radius of 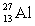 is approximately
is approximately
A) 1.05 fm.
B) 3.60 fm.
C) 0.350 fm.
D) 11.2 fm.
E) 1.85 fm.
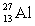 is approximately
is approximatelyA) 1.05 fm.
B) 3.60 fm.
C) 0.350 fm.
D) 11.2 fm.
E) 1.85 fm.

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
18
The radius of the 64Ni nucleus is approximately
A) 1.2 fm.
B) 2.4 fm.
C) 4.8 fm.
D) 16 fm.
E) 64 fm.
A) 1.2 fm.
B) 2.4 fm.
C) 4.8 fm.
D) 16 fm.
E) 64 fm.

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
19
The radius of the 63Cu nucleus is approximately
A) 0.382 fm.
B) 4.78 fm.
C) 12.1 fm.
D) 24.2 fm.
E) 100 fm.
A) 0.382 fm.
B) 4.78 fm.
C) 12.1 fm.
D) 24.2 fm.
E) 100 fm.

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
20
The sodium atom has an atomic number of 11 and an atomic weight of 23. The neutral atom contains
A) 11 neutrons and 12 protons.
B) 11 protons and 12 neutrons.
C) 12 electrons and 11 neutrons.
D) 23 protons and 12 electrons.
E) 23 electrons and 12 neutrons.
A) 11 neutrons and 12 protons.
B) 11 protons and 12 neutrons.
C) 12 electrons and 11 neutrons.
D) 23 protons and 12 electrons.
E) 23 electrons and 12 neutrons.

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
21
The element that is commonly used as a control rod in a nuclear reactor is
A) "1H."
B) "25Mg."
C) "41K."
D) "100Ru."
E) "113Cd."
A) "1H."
B) "25Mg."
C) "41K."
D) "100Ru."
E) "113Cd."

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
22
The nuclear reaction process in which a heavy nucleus splits into two roughly equal parts is known as
A) fission.
B) fusion.
C) spallation.
D) isomerism.
E) internal conversion.
A) fission.
B) fusion.
C) spallation.
D) isomerism.
E) internal conversion.

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
23
The control rods in a nuclear reactor are used to
A) produce neutrons.
B) add fuel to the reactor.
C) fragment elements by fission.
D) absorb γ rays.
E) absorb neutrons.
A) produce neutrons.
B) add fuel to the reactor.
C) fragment elements by fission.
D) absorb γ rays.
E) absorb neutrons.

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
24
About 200 MeV of energy is released in the average fission reaction of a uranium nucleus. Most of this energy is in the form of
A) kinetic energy of fission neutrons.
B) kinetic energy of fission fragments.
C) emitted β- and γ rays.
D) energy of a particles and γ rays.
E) electromagnetic radiation in the visible portion of the spectrum.
A) kinetic energy of fission neutrons.
B) kinetic energy of fission fragments.
C) emitted β- and γ rays.
D) energy of a particles and γ rays.
E) electromagnetic radiation in the visible portion of the spectrum.

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
25
Assuming that 200 MeV of energy is released per fission, the mass of 235U that undergoes fission to operate a 250-MW reactor for 1 y is approximately
A) 100 kg.
B) 200 kg.
C) 400 kg.
D) 500 kg.
E) 1 Mg.
A) 100 kg.
B) 200 kg.
C) 400 kg.
D) 500 kg.
E) 1 Mg.

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
26
Neutrons produced in the fission of uranium are rapidly moving projectiles and must be slowed before they can produce more fission. If the neutrons collide elastically, hydrogen is effective in slowing them down because
A) hydrogen atoms repel neutrons.
B) a hydrogen atom is much more massive than a neutron.
C) a hydrogen atom and a neutron have about the same mass.
D) a neutron is much more massive than a hydrogen atom.
E) hydrogen atoms attract neutrons.
A) hydrogen atoms repel neutrons.
B) a hydrogen atom is much more massive than a neutron.
C) a hydrogen atom and a neutron have about the same mass.
D) a neutron is much more massive than a hydrogen atom.
E) hydrogen atoms attract neutrons.

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
27
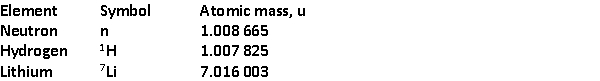 The total binding energy per nucleon of
The total binding energy per nucleon of 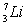 is approximately
is approximatelyA) 0.04215 MeV.
B) 0.00602 MeV.
C) 5.606 MeV.
D) 6.593 MeV.
E) 0.00590 MeV.

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
28
Fission occurs because the average binding energy per nucleon for the fission fragments is higher than that for the original nucleus. The change in binding energy per nucleon is approximately
A) 0.50 MeV.
B) 1.0 MeV.
C) 7.0 MeV.
D) 28 MeV.
E) 0.20 keV.
A) 0.50 MeV.
B) 1.0 MeV.
C) 7.0 MeV.
D) 28 MeV.
E) 0.20 keV.

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
29
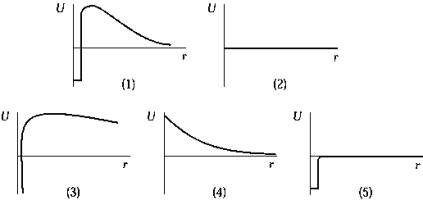 The graph that represents the interaction energy U) of the proton and neutron as a function of distance r is
The graph that represents the interaction energy U) of the proton and neutron as a function of distance r isA) 1).
B) 2).
C) 3).
D) 4).
E) 5).

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
30
How many times larger is the volume of the nucleus 208Pb Z = 82) compared to the nucleus 40Ca Z = 20)?
A) 0.58
B) 1.7
C) 5.2
D) 4.1
E) 2.3
A) 0.58
B) 1.7
C) 5.2
D) 4.1
E) 2.3

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
31
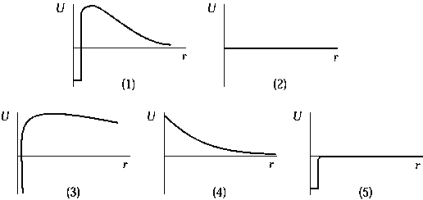 The graph that represents the Coulomb interaction energy U) between two protons as a function of distance r is
The graph that represents the Coulomb interaction energy U) between two protons as a function of distance r isA) 1).
B) 2).
C) 3).
D) 4).
E) 5).

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
32
One way to think about the low binding energy Eb) per nucleon for low A numbers is 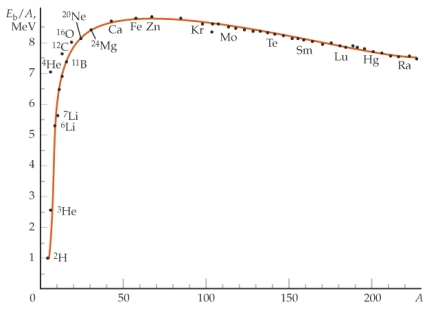
A) that there are few protons, hence there is less electrostatic repulsion.
B) that there are few electrons to provide the electrostatic attraction.
C) at low A the number of nearest neighbors per nucleon is small compared to higher A numbers.
D) at low A, the nucleus is less spherical, hence there is less surface tension.
E) None of the above statements is correct.

A) that there are few protons, hence there is less electrostatic repulsion.
B) that there are few electrons to provide the electrostatic attraction.
C) at low A the number of nearest neighbors per nucleon is small compared to higher A numbers.
D) at low A, the nucleus is less spherical, hence there is less surface tension.
E) None of the above statements is correct.

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
33
The main purpose of a moderator in a nuclear reactor is to
A) absorb the neutrons and thereby decrease the reaction rate.
B) absorb the neutrons and thereby increase the reaction rate.
C) slow the neutrons and thereby decrease the reaction rate.
D) slow the neutrons and thereby increase the reaction rate.
E) cool the reactor chamber.
A) absorb the neutrons and thereby decrease the reaction rate.
B) absorb the neutrons and thereby increase the reaction rate.
C) slow the neutrons and thereby decrease the reaction rate.
D) slow the neutrons and thereby increase the reaction rate.
E) cool the reactor chamber.

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
34
A moderator is used to slow
A) protons.
B) alpha particles.
C) neutrons.
D) beta particles.
E) photons.
A) protons.
B) alpha particles.
C) neutrons.
D) beta particles.
E) photons.

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
35
Which of the following common isotopes has the highest binding energy per nucleon, 4He, 12C, 16O, or 24Mg?
A) "4He"
B) "12C"
C) "16O"
D) "24Mg"
E) "They are all the same."
A) "4He"
B) "12C"
C) "16O"
D) "24Mg"
E) "They are all the same."

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
36
When an alpha particle 4He) with a kinetic energy of 6.54 MeV strikes a 27Al nucleus, a 30P nucleus is created and a neutron is ejected. Assume that the 30P nucleus has no final kinetic energy. Given the following masses for the various particles, what is the kinetic energy of the ejected neutron? 
A) 3.71 MeV
B) 2.83 MeV
C) 6.54 MeV
D) 9.37 MeV
E) 0.45 MeV

A) 3.71 MeV
B) 2.83 MeV
C) 6.54 MeV
D) 9.37 MeV
E) 0.45 MeV

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
37
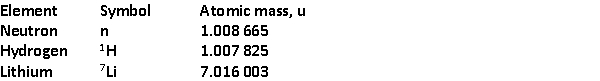 The total binding energy of
The total binding energy of 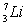 is approximately
is approximatelyA) 8.00 MeV.
B) 56.0 MeV.
C) 39.2 MeV.
D) 37.6 MeV.
E) 22.4 MeV.

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
38
The fact that the binding energy per nucleon is roughly a constant over most of the range of stable nuclei indicates that the nuclear force is
A) short range.
B) long range.
C) weak.
D) strong.
E) repulsive.
A) short range.
B) long range.
C) weak.
D) strong.
E) repulsive.

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
39
For most nuclei, the total binding energy is proportional to the number of nucleons. This leads to the conclusion that nuclear forces show the property of
A) pairing.
B) charge dependence.
C) relativity.
D) magnetism.
E) None of these is correct.
A) pairing.
B) charge dependence.
C) relativity.
D) magnetism.
E) None of these is correct.

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
40
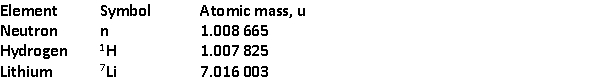 The total binding energy of
The total binding energy of 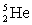 is approximately
is approximatelyA) 16.5 MeV.
B) 40.0 MeV.
C) 8.00 MeV.
D) 26.0 MeV.
E) 27.4 MeV.

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
41
The following fusion reaction occurs in the Sun: 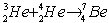 The masses of the nuclei are 3He = 3.016 049 u
The masses of the nuclei are 3He = 3.016 049 u
4He = 4.002 604 u
7Be = 7.016 930 u
The energy released or absorbed by the reaction is
A) 920 MeV, absorbed.
B) 1.60 MeV, absorbed.
C) 920 MeV, released.
D) 1.60 MeV, released.
E) 270 MeV, released.
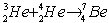 The masses of the nuclei are 3He = 3.016 049 u
The masses of the nuclei are 3He = 3.016 049 u4He = 4.002 604 u
7Be = 7.016 930 u
The energy released or absorbed by the reaction is
A) 920 MeV, absorbed.
B) 1.60 MeV, absorbed.
C) 920 MeV, released.
D) 1.60 MeV, released.
E) 270 MeV, released.

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
42
1 d it has a decay rate of 4.25 mCi. The half-life of 32P is approximately
A) 3.03 d.
B) 7.30 d.
C) 12.1 d.
D) 14.6 d.
E) 24.2 d.
A) 3.03 d.
B) 7.30 d.
C) 12.1 d.
D) 14.6 d.
E) 24.2 d.

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
43
06 d it has a decay rate of 7.5 mCi. The half-life of 32P is approximately
A) 3.03 d.
B) 7.30 d.
C) 12.1 d.
D) 14.6 d.
E) 24.2 d.
A) 3.03 d.
B) 7.30 d.
C) 12.1 d.
D) 14.6 d.
E) 24.2 d.

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
44
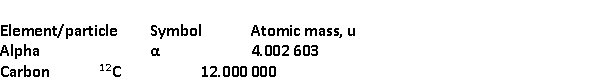 After a star fuses most its hydrogen into helium, it begins to fuse the helium into carbon. The energy released in fusing helium nuclei α particles) into carbon is approximately
After a star fuses most its hydrogen into helium, it begins to fuse the helium into carbon. The energy released in fusing helium nuclei α particles) into carbon is approximatelyA) 0.730 MeV.
B) 1.46 MeV.
C) 7.30 MeV.
D) 14.6 MeV.
E) 73.0 MeV.

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
45
If the radioactivity of thorium C decreases to one-half in 1 h, in another 60 min its activity, compared with that at the beginning of the first hour, will be
A) 1/4.
B) zero.
C) 1/8.
D) 1/16.
E) 1/120.
A) 1/4.
B) zero.
C) 1/8.
D) 1/16.
E) 1/120.

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
46
Radon Rn) is a gas. Its nucleus decays with the emission of an alpha particle to form an isotope of polonium Po): 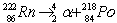 The masses of the particles are:
The masses of the particles are: 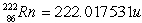
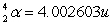
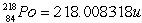 The rest energy of a unified mass unit is 931.5 MeV. The kinetic energy of the ejected particles is
The rest energy of a unified mass unit is 931.5 MeV. The kinetic energy of the ejected particles is
A) 6.26 MeV.
B) 6.16 MeV.
C) 6.04 MeV.
D) 6.61 × 10-3 MeV.
E) 5.73 MeV.
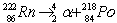 The masses of the particles are:
The masses of the particles are: 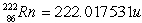
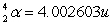
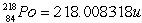 The rest energy of a unified mass unit is 931.5 MeV. The kinetic energy of the ejected particles is
The rest energy of a unified mass unit is 931.5 MeV. The kinetic energy of the ejected particles isA) 6.26 MeV.
B) 6.16 MeV.
C) 6.04 MeV.
D) 6.61 × 10-3 MeV.
E) 5.73 MeV.

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
47
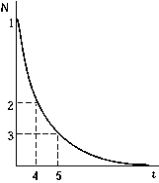 The graph shows the number of radioactive nuclei N remaining as a function of time. The point on the graph that corresponds to the half-life of the decay process is
The graph shows the number of radioactive nuclei N remaining as a function of time. The point on the graph that corresponds to the half-life of the decay process isA) 1.
B) 2.
C) 3.
D) 4.
E) 5.

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
48
What are the numbers of protons Z and neutrons N in the missing fragment of the following fission reaction? 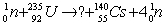
A) Z = 55 and N = 37
B) Z = 37 and N = 55
C) Z = 92 and N = 37
D) Z = 37 and N = 92
E) Z = 37 and N = 58
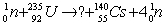
A) Z = 55 and N = 37
B) Z = 37 and N = 55
C) Z = 92 and N = 37
D) Z = 37 and N = 92
E) Z = 37 and N = 58

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
49
The half-life of a radioactive substance is 5 min. Which of the following statements is true of the decay of this substance?
A) After 10 min, one-fourth of the original substance remains.
B) The amount of the substance remaining after a given time is proportional to the number of minutes.
C) The amount of the substance remaining after a given time is inversely proportional to the number of minutes.
D) After 10 min, none of the original substance remains unchanged.
E) None of these is correct.
A) After 10 min, one-fourth of the original substance remains.
B) The amount of the substance remaining after a given time is proportional to the number of minutes.
C) The amount of the substance remaining after a given time is inversely proportional to the number of minutes.
D) After 10 min, none of the original substance remains unchanged.
E) None of these is correct.

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
50
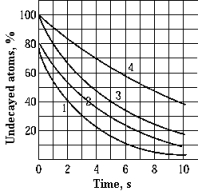 The graph shows the decay curves of four radioactive elements. The half-life is the same for elements
The graph shows the decay curves of four radioactive elements. The half-life is the same for elementsA) 1 and 2.
B) 3 and 4.
C) 1 and 4.
D) 2 and 3.
E) 1 and 3.

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
51
A certain radioactive element has a half-life of 20 d. The time it will take for 7/8 of the atoms originally present to disintegrate is
A) 20 d.
B) 40 d.
C) 60 d.
D) 80 d.
E) 100 d.
A) 20 d.
B) 40 d.
C) 60 d.
D) 80 d.
E) 100 d.

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
52
The decay rate of a radioactive sample is defined as the number of decays per second. For a sample with N particles and a decay constant , the decay rate is
A) proportional to and inversely proportional to N.
B) proportional to but independent of N.
C) directly proportional to both and N.
D) independent of but proportional to N.
E) inversely proportional to both and N.
A) proportional to and inversely proportional to N.
B) proportional to but independent of N.
C) directly proportional to both and N.
D) independent of but proportional to N.
E) inversely proportional to both and N.

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
53
In what type of radioactive decay are the mass numbers of the parent and daughter nuclei the same?
A) alpha
B) beta
C) gamma
D) both alpha and beta
E) both beta and gamma
A) alpha
B) beta
C) gamma
D) both alpha and beta
E) both beta and gamma

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
54
In what type of radioactive decay are the mass numbers of the parent and daughter nuclei different?
A) alpha
B) beta
C) gamma
D) both alpha and beta
E) both beta and gamma
A) alpha
B) beta
C) gamma
D) both alpha and beta
E) both beta and gamma

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
55
Nuclear fusion takes place when
A) a large nucleus splits into two fragments with the release of a few neutrons.
B) an α particle is ejected from the nucleus.
C) a positive β particle is ejected from the nucleus.
D) small nuclei combine to form a larger one.
E) heavy hydrogen is accelerated.
A) a large nucleus splits into two fragments with the release of a few neutrons.
B) an α particle is ejected from the nucleus.
C) a positive β particle is ejected from the nucleus.
D) small nuclei combine to form a larger one.
E) heavy hydrogen is accelerated.

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
56
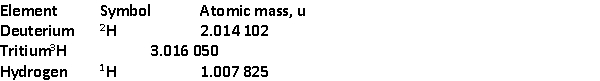 A fusion reaction that is highly probable is
A fusion reaction that is highly probable is 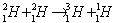 The amount of fusion energy released in this reaction is approximately
The amount of fusion energy released in this reaction is approximatelyA) 1.0 MeV.
B) 2.0 MeV.
C) 3.0 MeV.
D) 4.0 MeV.
E) 5.0 MeV.

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
57
If, on the average, each fission reaction of 235U releases 200 MeV of energy, how many nuclei of 235U fission every second in a 200-MW reactor?
A) 6.3 × 1017.
B) 6.0 × 1023.
C) 1.0 × 1019.
D) 1.6 × 1019.
E) 2.7 × 1018.
A) 6.3 × 1017.
B) 6.0 × 1023.
C) 1.0 × 1019.
D) 1.6 × 1019.
E) 2.7 × 1018.

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
58
The half-life of radium is about 1600 y. If you have 1.00 g of radium today, the amount remaining in 1000 y will be approximately
A) 625 mg.
B) 958 mg.
C) 707 mg.
D) 841 mg.
E) 648 mg.
A) 625 mg.
B) 958 mg.
C) 707 mg.
D) 841 mg.
E) 648 mg.

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
59
The half-life of phosphorus-32 is 14.3 d. If you have 1.00 g of phosphorous-32 today, the amount remaining in 10 d will be approximately
A) 700 mg.
B) 650 mg.
C) 616 mg.
D) 384 mg.
E) 350 mg.
A) 700 mg.
B) 650 mg.
C) 616 mg.
D) 384 mg.
E) 350 mg.

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
60
The "third-life," the time it takes nuclei to decay until one-third of the original amount remains, expressed in terms of the decay constant , is
A) "ln 2)/3 ."
B) "ln 3)/ ."
C) "ln 0.333)/ ."
D) "3ln 2)/ ."
E) " /3."
A) "ln 2)/3 ."
B) "ln 3)/ ."
C) "ln 0.333)/ ."
D) "3ln 2)/ ."
E) " /3."

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
61
A nuclear explosion produces among a lot of other things) 7.9 × 1023 Bq of iodine-131, which has a half-life of 8.05 d, and 5.7 × 1021 Bq of barium-140, which has a half-life of 12.8 d. The total decay rate that remains after 145 d is
A) 3.5 × 1018 Bq.
B) 1.16 × 1020 Bq.
C) 2.2 × 1018 Bq.
D) 5.2 × 1018 Bq.
E) 7.3 × 1018 Bq.
A) 3.5 × 1018 Bq.
B) 1.16 × 1020 Bq.
C) 2.2 × 1018 Bq.
D) 5.2 × 1018 Bq.
E) 7.3 × 1018 Bq.

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
62
3 g/cm3 and its molar mass is 207 g.
A) 3.5 × 10-22 cm2
B) 6.8 × 10-22 cm2
C) 4.6 × 10-23 cm2
D) 2.1 × 10-23 cm2
E) 8.4 × 10-24 cm2
A) 3.5 × 10-22 cm2
B) 6.8 × 10-22 cm2
C) 4.6 × 10-23 cm2
D) 2.1 × 10-23 cm2
E) 8.4 × 10-24 cm2

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
63
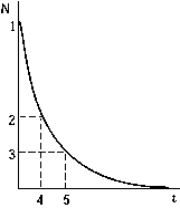 The graph shows the number of nuclei N remaining as a function of time. The point on the graph that corresponds to the number of nuclei remaining after two half-lives have elapsed is
The graph shows the number of nuclei N remaining as a function of time. The point on the graph that corresponds to the number of nuclei remaining after two half-lives have elapsed isA) 1.
B) 2.
C) 3.
D) 4.
E) 5.

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
64
A radioactive source has a half-life of 2 min. At time t = 0 it is placed near a detector and the counting rate is observed to be 3000 counts/s. If the detection efficiency is 25%, how many radioactive nuclei are there at time t = 2 min?
A) 1.04 × 106
B) 1.67 × 106
C) 2.08 × 106
D) 3.41 × 106
E) 3.79 × 106
A) 1.04 × 106
B) 1.67 × 106
C) 2.08 × 106
D) 3.41 × 106
E) 3.79 × 106

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
65
Cesium-137 has a half-life of 31.0 y. The time required for the cesium-137 now present to decrease to 1/30 its present value is
A) 930 y.
B) 30 y.
C) 465 y.
D) 152 y.
E) 1.6 y.
A) 930 y.
B) 30 y.
C) 465 y.
D) 152 y.
E) 1.6 y.

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
66
The intensity of gamma rays passing through a material as a function of thickness, x is given by 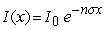 where n is the number of atoms per cm3 and is the absorption cross section. It is observed that the intensity of gamma rays of a particular energy is reduced by half when they passed through 1 cm of lead. What is the thickness of lead needed to reduce the intensity to a quarter?
where n is the number of atoms per cm3 and is the absorption cross section. It is observed that the intensity of gamma rays of a particular energy is reduced by half when they passed through 1 cm of lead. What is the thickness of lead needed to reduce the intensity to a quarter?
A) 1.0 cm
B) 1.5 cm
C) 2.0 cm
D) 3.0 cm
E) 4.0 cm
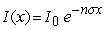 where n is the number of atoms per cm3 and is the absorption cross section. It is observed that the intensity of gamma rays of a particular energy is reduced by half when they passed through 1 cm of lead. What is the thickness of lead needed to reduce the intensity to a quarter?
where n is the number of atoms per cm3 and is the absorption cross section. It is observed that the intensity of gamma rays of a particular energy is reduced by half when they passed through 1 cm of lead. What is the thickness of lead needed to reduce the intensity to a quarter?A) 1.0 cm
B) 1.5 cm
C) 2.0 cm
D) 3.0 cm
E) 4.0 cm

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
67
A radioactive source has a half-life of 2 min. At time t = 0 it is placed near a detector and the counting rate is observed to be 3000 counts/s. The counting rate at t = 10 min will be
A) 1500 counts/s.
B) 750 counts/s.
C) 375 counts/s.
D) 188 counts/s.
E) 94 counts/s.
A) 1500 counts/s.
B) 750 counts/s.
C) 375 counts/s.
D) 188 counts/s.
E) 94 counts/s.

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
68
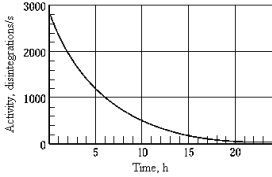 The graph shows the activity as a function of time for a radioisotope. The half-life of this particular radioisotope is approximately
The graph shows the activity as a function of time for a radioisotope. The half-life of this particular radioisotope is approximatelyA) 5 h.
B) 4 h.
C) 8 h.
D) 20 h.
E) 10 h.

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
69
A radioactive nucleus with Z = 92 and A = 235 decays through a series of alpha, beta, and gamma emissions to a stable nucleus with Z = 82 and A = 207. The number of alpha particles and the number of beta particles emitted during the entire process are
A) 8 alpha particles and 6 beta particles.
B) 7 alpha particles and 4 beta particles.
C) 7 alpha particles and 10 beta particles.
D) 14 alpha particles and 7 beta particles.
E) None of these is correct.
A) 8 alpha particles and 6 beta particles.
B) 7 alpha particles and 4 beta particles.
C) 7 alpha particles and 10 beta particles.
D) 14 alpha particles and 7 beta particles.
E) None of these is correct.

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
70
A radiologist uses a radioactive isotope of iodine, 123I, for thyroid scans. She receives a shipment of 15 mCi of this isotope at noon on a Tuesday. The half-life of this isotope is 13.2 h. What is the decay rate at noon on Thursday of the same week?
A) 12 mCi
B) 1.2 mCi
C) 2.4 mCi
D) 4.2 mCi
E) 24 mCi
A) 12 mCi
B) 1.2 mCi
C) 2.4 mCi
D) 4.2 mCi
E) 24 mCi

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
71
A radioactive substance in the laboratory has a half-life of 8 h. At noon today, a Geiger counter reads 480 counts/min above background. At noon tomorrow, the counter should read about
A) 480 counts/min.
B) 240 counts/min.
C) 120 counts/min.
D) 60 counts/min.
E) 30 counts/min.
A) 480 counts/min.
B) 240 counts/min.
C) 120 counts/min.
D) 60 counts/min.
E) 30 counts/min.

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
72
Which of the following radioactive decay products has the largest electric charge?
A) alpha particles
B) beta particles
C) gamma rays
D) neutrons
E) All of these have the same charge.
A) alpha particles
B) beta particles
C) gamma rays
D) neutrons
E) All of these have the same charge.

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
73
The Federal Aviation Administration limits the number of hours flight crews can be at high altitudes, 10, 000 m. One of the reasons is because
A) the crews can suffer from altitude sickness.
B) at high altitudes there are more gamma rays that can damage human tissues.
C) at high altitudes the air is thinner so there is less oxygen.
D) they do not want the crews to accrue too many frequent flyer miles.
E) None of the above statements is correct.
A) the crews can suffer from altitude sickness.
B) at high altitudes there are more gamma rays that can damage human tissues.
C) at high altitudes the air is thinner so there is less oxygen.
D) they do not want the crews to accrue too many frequent flyer miles.
E) None of the above statements is correct.

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
74
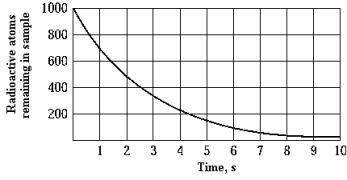 The graph shows the number of undecayed atoms as a function of time. The half-life of this radioactive element is approximately
The graph shows the number of undecayed atoms as a function of time. The half-life of this radioactive element is approximatelyA) 1 s.
B) 2 s.
C) 10 s.
D) 4 s.
E) 5 s.

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
75
A radioactive source has a half-life of 2 min. At time t = 0 it is placed near a detector and the counting rate is observed to be 3000 counts/s. The counting rate at t = 4 min will be
A) 1500 counts/s.
B) 750 counts/s.
C) 375 counts/s.
D) 188 counts/s.
E) 94 counts/s.
A) 1500 counts/s.
B) 750 counts/s.
C) 375 counts/s.
D) 188 counts/s.
E) 94 counts/s.

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
76
A β particle is an energetic
A) photon emitted by a nucleus.
B) electron or positron.
C) proton emitted by a nucleus.
D) neutron emitted by a nucleus.
E) None of these is correct.
A) photon emitted by a nucleus.
B) electron or positron.
C) proton emitted by a nucleus.
D) neutron emitted by a nucleus.
E) None of these is correct.

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
77
Uranium decomposes radioactively to form thorium atomic number 90) by emitting an α particle from its nucleus. The force of repulsion between the thorium nucleus and the α particle when the distance between them is 9.0 fm is approximately
A) 7.2 fN.
B) 57 nN.
C) 4.6 pN.
D) 1.02 kN.
E) 0.51 kN.
A) 7.2 fN.
B) 57 nN.
C) 4.6 pN.
D) 1.02 kN.
E) 0.51 kN.

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
78
A radioactive source has a half-life of 2 min. At time t = 0 it is placed near a detector and the counting rate is observed to be 3000 counts/s. If the detection efficiency is 25%, how many radioactive nuclei are there at time t = 0?
A) 1.04 × 106.
B) 1.67 × 106
C) 2.08 × 106
D) 3.41 × 106
E) 3.79 × 106
A) 1.04 × 106.
B) 1.67 × 106
C) 2.08 × 106
D) 3.41 × 106
E) 3.79 × 106

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
79
When a gamma ray passes through a material,
A) the gamma ray can cause photoelectric effect.
B) the gamma ray can cause the nucleus to disintegrate.
C) the gamma ray can cause pair production.
D) the gamma ray can pass right through the material unchanged.
E) All the above processes can take place.
A) the gamma ray can cause photoelectric effect.
B) the gamma ray can cause the nucleus to disintegrate.
C) the gamma ray can cause pair production.
D) the gamma ray can pass right through the material unchanged.
E) All the above processes can take place.

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
80
At a particular time, a radioactive source A has a decay rate of 1.60 × 1011 Bq and a half-life of 15.0 d, and a second source B has a decay rate of 8.50 × 1011 Bq. Sources A and B have the same decay rate 45.0 d later. The half-life of B is
A) 0.19 d.
B) 8.3 d.
C) 2.8 d.
D) 5.4 d.
E) 28 d.
A) 0.19 d.
B) 8.3 d.
C) 2.8 d.
D) 5.4 d.
E) 28 d.

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck


