Deck 15: Thermodynamics II
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/120
Play
Full screen (f)
Deck 15: Thermodynamics II
1
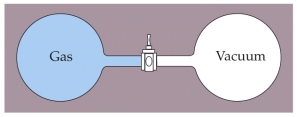 Two containers of equal volume are connected by a stopcock as shown above. One container is filled with a gas at a pressure of 1 atm and temperature of 293 K while the other container is evacuated so that it is under vacuum. The containers are thermally isolated from the surroundings so no heat enters or escapes from the system. The stopcock is then opened allowing the gas from one container to fill the other. What is the final temperature of the gas after it has come to equilibrium?
Two containers of equal volume are connected by a stopcock as shown above. One container is filled with a gas at a pressure of 1 atm and temperature of 293 K while the other container is evacuated so that it is under vacuum. The containers are thermally isolated from the surroundings so no heat enters or escapes from the system. The stopcock is then opened allowing the gas from one container to fill the other. What is the final temperature of the gas after it has come to equilibrium?A) 136.5 K
B) 273 K
C) 293 K
D) 195 K
E) undetermined
293 K
2
During a certain thermodynamic process, 418 J of work are done on a system and 214 cal of heat are transferred to the system. The change in internal energy during the process is
A) 314 cal.
B) 114 cal.
C) 468 cal.
D) 368 cal.
E) 632 cal.
A) 314 cal.
B) 114 cal.
C) 468 cal.
D) 368 cal.
E) 632 cal.
314 cal.
3
The first law of thermodynamics has as its basis the same fundamental principle as
A) the continuity principle.
B) the conservation of energy.
C) Newton's law of universal gravitation.
D) static equilibrium.
E) the conservation of linear momentum.
A) the continuity principle.
B) the conservation of energy.
C) Newton's law of universal gravitation.
D) static equilibrium.
E) the conservation of linear momentum.
the conservation of energy.
4
The percentage of mechanical energy that can theoretically be turned into heat energy according to the first law of thermodynamics is
A) 100%.
B) 90%.
C) 75%.
D) 50%.
E) 0%.
A) 100%.
B) 90%.
C) 75%.
D) 50%.
E) 0%.

Unlock Deck
Unlock for access to all 120 flashcards in this deck.
Unlock Deck
k this deck
5
In physics, we typically write the first law of thermodynamics as ΔU = Q W. The variable Q represents ___ and the variable W represents ___.
A) the internal energy of the system; the work done on a system by its surroundings
B) the internal energy of the system; the work done by a system on its surroundings
C) the heat flow into the system; the work done by a system on its surroundings
D) the temperature of the system; the work done by a system on its surroundings
E) the heat flow into the system; the internal energy of the system
A) the internal energy of the system; the work done on a system by its surroundings
B) the internal energy of the system; the work done by a system on its surroundings
C) the heat flow into the system; the work done by a system on its surroundings
D) the temperature of the system; the work done by a system on its surroundings
E) the heat flow into the system; the internal energy of the system

Unlock Deck
Unlock for access to all 120 flashcards in this deck.
Unlock Deck
k this deck
6
You exercise on a stationary bike and your internal energy decreases by 175 cal. If you did 33 cal of work on the pedals of the bike, how much heat flowed into or out of your system?
A) 208 cal flows out of your system
B) 142 cal flows out of your system
C) 208 cal flows into your system
D) 142 cal flows into your system
E) None of the above.
A) 208 cal flows out of your system
B) 142 cal flows out of your system
C) 208 cal flows into your system
D) 142 cal flows into your system
E) None of the above.

Unlock Deck
Unlock for access to all 120 flashcards in this deck.
Unlock Deck
k this deck
7
There is no heat transfer into or out of a system during an) _____ process.
A) isothermal
B) adiabatic
C) isochoric
D) isobaric
E) There is always heat transfer during thermodynamic processes.
A) isothermal
B) adiabatic
C) isochoric
D) isobaric
E) There is always heat transfer during thermodynamic processes.

Unlock Deck
Unlock for access to all 120 flashcards in this deck.
Unlock Deck
k this deck
8
How much internal energy is contained in 1 mole of monatomic gas at STP?
A) zero
B) 1.11 kJ
C) 2.22 kJ
D) 3.33 kJ
E) 5.55 kJ
A) zero
B) 1.11 kJ
C) 2.22 kJ
D) 3.33 kJ
E) 5.55 kJ

Unlock Deck
Unlock for access to all 120 flashcards in this deck.
Unlock Deck
k this deck
9
The first law of thermodynamics is most closely related to
A) the definition of absolute zero.
B) the definition of an ideal gas.
C) the conservation of energy.
D) thermal expansion.
E) the conservation of momentum.
A) the definition of absolute zero.
B) the definition of an ideal gas.
C) the conservation of energy.
D) thermal expansion.
E) the conservation of momentum.

Unlock Deck
Unlock for access to all 120 flashcards in this deck.
Unlock Deck
k this deck
10
A system absorbs heat Q and has an equal amount of positive work done on it. What is the change in the internal energy of the system?
A) Q
B) 2Q
C) -2Q
D) zero
E) Q/2
A) Q
B) 2Q
C) -2Q
D) zero
E) Q/2

Unlock Deck
Unlock for access to all 120 flashcards in this deck.
Unlock Deck
k this deck
11
In a certain thermodynamic process, 1000 cal of heat are added to a gas confined in a cylinder. At the same time, 1000 J of work are done by the gas as it expands. The increase in internal energy of the gas is
A) zero.
B) 3186 J.
C) -239 J.
D) 5186 J.
E) 1239 J.
A) zero.
B) 3186 J.
C) -239 J.
D) 5186 J.
E) 1239 J.

Unlock Deck
Unlock for access to all 120 flashcards in this deck.
Unlock Deck
k this deck
12
In a certain thermodynamic process, 20 cal of heat are removed from a system and 30 cal of work are done on the system. The internal energy of the system
A) increases by 10 cal.
B) decreases by 10 cal.
C) increases by 50 cal.
D) decreases by 50 cal.
E) decreases by 20 cal.
A) increases by 10 cal.
B) decreases by 10 cal.
C) increases by 50 cal.
D) decreases by 50 cal.
E) decreases by 20 cal.

Unlock Deck
Unlock for access to all 120 flashcards in this deck.
Unlock Deck
k this deck
13
A state variable is one that allows other variables to be determined using a relationship. Which of the following variables are state variables?
A) P, V, and T
B) Internal energy, U
C) W and Q
D) A) and B)
E) A), B), and C)
A) P, V, and T
B) Internal energy, U
C) W and Q
D) A) and B)
E) A), B), and C)

Unlock Deck
Unlock for access to all 120 flashcards in this deck.
Unlock Deck
k this deck
14
In a certain process, 500 cal of heat are supplied to a system consisting of a gas confined in a cylinder. At the same time, 500 J of work are done by the gas by expansion. The increase in thermal energy of the gas is approximately
A) zero.
B) 1.00 kJ.
C) 1.59 kJ.
D) 2.09 kJ.
E) 2.59 kJ.
A) zero.
B) 1.00 kJ.
C) 1.59 kJ.
D) 2.09 kJ.
E) 2.59 kJ.

Unlock Deck
Unlock for access to all 120 flashcards in this deck.
Unlock Deck
k this deck
15
There is no change in the volume of a system during an) _____ process.
A) isothermal
B) adiabatic
C) isochoric
D) isobaric
E) There is always a volume change during thermodynamic processes.
A) isothermal
B) adiabatic
C) isochoric
D) isobaric
E) There is always a volume change during thermodynamic processes.

Unlock Deck
Unlock for access to all 120 flashcards in this deck.
Unlock Deck
k this deck
16
A liquid is irregularly stirred in a well-insulated container and thereby undergoes a rise in temperature. Regarding the liquid as a system, you can say that
A) heat has been transferred.
B) the rise in temperature indicates work done by the system.
C) the internal energy has been unchanged.
D) the work done by the system equals the work done on the system.
E) there is a positive change in internal energy.
A) heat has been transferred.
B) the rise in temperature indicates work done by the system.
C) the internal energy has been unchanged.
D) the work done by the system equals the work done on the system.
E) there is a positive change in internal energy.

Unlock Deck
Unlock for access to all 120 flashcards in this deck.
Unlock Deck
k this deck
17
Suppose you do 75 kJ of work on a system consisting of 10 kg of water by stirring it with a paddle wheel. During this process, 40 kcal of heat is removed. The change in the internal energy of the system is
A) -92 kJ.
B) -115 kJ.
C) -134 kJ.
D) -242 kJ.
E) -156 kJ.
A) -92 kJ.
B) -115 kJ.
C) -134 kJ.
D) -242 kJ.
E) -156 kJ.

Unlock Deck
Unlock for access to all 120 flashcards in this deck.
Unlock Deck
k this deck
18
A 6.0-g lead bullet traveling at 300 m/s penetrates a wooden block and stops. If 50 percent of the initial kinetic energy of the bullet is converted into thermal energy in the bullet, by how much does the bullet's temperature increase? The specific heat of lead is 128 J/kg · K.)
A) 0.17oC
B) 1.8 × 102 oC
C) 17oC
D) 3.5 × 102 oC
E) 35oC
A) 0.17oC
B) 1.8 × 102 oC
C) 17oC
D) 3.5 × 102 oC
E) 35oC

Unlock Deck
Unlock for access to all 120 flashcards in this deck.
Unlock Deck
k this deck
19
An ideal gas is heated so that it expands at constant pressure. The gas does work W. What heat is added to the gas?
A) W
B) -W
C) zero
D) more than W
E) less than W
A) W
B) -W
C) zero
D) more than W
E) less than W

Unlock Deck
Unlock for access to all 120 flashcards in this deck.
Unlock Deck
k this deck
20
An ideal gas undergoes a cyclic process in which total positive) work W is done by the gas. What total heat is added to the gas in one cycle?
A) W
B) -W
C) zero
D) 2W
E) W/2
A) W
B) -W
C) zero
D) 2W
E) W/2

Unlock Deck
Unlock for access to all 120 flashcards in this deck.
Unlock Deck
k this deck
21
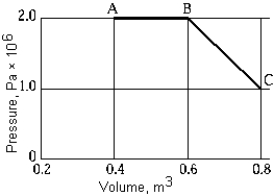 A gas expands along path ABC. The work done by the gas in this expansion is
A gas expands along path ABC. The work done by the gas in this expansion isA) 4.0 × 105 J.
B) 5.0 × 105 J.
C) 6.0 × 105 J.
D) 7.0 × 105 J.
E) 8.0 × 105 J.

Unlock Deck
Unlock for access to all 120 flashcards in this deck.
Unlock Deck
k this deck
22
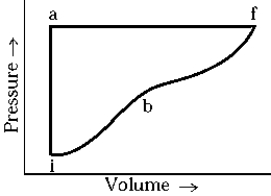 An ideal gas system changes from state i to state f by paths iaf and ibf. If the heat added along iaf is Qiaf = 50 cal, the work along iaf is Wiaf = 20 cal. Along ibf, if Qibf = 40 cal, the work done, Wiaf, is
An ideal gas system changes from state i to state f by paths iaf and ibf. If the heat added along iaf is Qiaf = 50 cal, the work along iaf is Wiaf = 20 cal. Along ibf, if Qibf = 40 cal, the work done, Wiaf, isA) 10 cal.
B) 20 cal.
C) 30 cal.
D) 40 cal.
E) 50 cal.

Unlock Deck
Unlock for access to all 120 flashcards in this deck.
Unlock Deck
k this deck
23
A balloon contains gas at a pressure 1.2 atm 1 atm = 101.3 kPa) and has a volume of 0.10 m3. More gas is pumped into the balloon at constant pressure until the volume is doubled. How much work is done by the pump?
A) 12 J
B) 24 kJ
C) 24 J
D) 12 kJ
E) 6.1 kJ
A) 12 J
B) 24 kJ
C) 24 J
D) 12 kJ
E) 6.1 kJ

Unlock Deck
Unlock for access to all 120 flashcards in this deck.
Unlock Deck
k this deck
24
The work done by an ideal gas in an isothermal expansion from volume V1 to volume V2 is given by the formula: W = nRT lnV2/V1). Standard atmospheric pressure 1 atm) is 101.3 kPa. If 1.0 L of He gas at room temperature 20oC) and 1.0 atm of pressure is compressed isothermally to a volume of 100 mL, how much work is done on the gas?
A) 5.6 kJ
B) 4.7 × 102 J
C) 4.7 × 102 kJ
D) 2.3 × 102 kJ
E) 2.3 × 102 J
A) 5.6 kJ
B) 4.7 × 102 J
C) 4.7 × 102 kJ
D) 2.3 × 102 kJ
E) 2.3 × 102 J

Unlock Deck
Unlock for access to all 120 flashcards in this deck.
Unlock Deck
k this deck
25
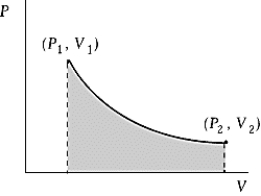 An ideal gas initially at 100oC and pressure P1 = 250 kPa occupies a volume V1 = 4.5 L. It undergoes a quasi-static, isothermal expansion until its pressure is reduced to 150 kPa. How much does the internal energy of the gas change during this process? R = 8.314 J/mol · K = 8.206 L · atm/mol · K.
An ideal gas initially at 100oC and pressure P1 = 250 kPa occupies a volume V1 = 4.5 L. It undergoes a quasi-static, isothermal expansion until its pressure is reduced to 150 kPa. How much does the internal energy of the gas change during this process? R = 8.314 J/mol · K = 8.206 L · atm/mol · K.A) 116 J
B) 320 J
C) 575 J
D) 640 J
E) The internal energy does not change during this process.

Unlock Deck
Unlock for access to all 120 flashcards in this deck.
Unlock Deck
k this deck
26
The equation of state for a certain gas under isothermal conditions is PV = 31.2, where the units are SI. The work done by this gas as its volume increases isothermally from 0.2 m3 to 0.8 m3 is approximately
A) 2.86 J.
B) 28.6 J.
C) 43.3 J.
D) 71.8 J.
E) 115 J.
A) 2.86 J.
B) 28.6 J.
C) 43.3 J.
D) 71.8 J.
E) 115 J.

Unlock Deck
Unlock for access to all 120 flashcards in this deck.
Unlock Deck
k this deck
27
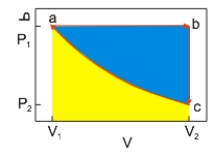 An ideal gas undergoes a cyclic expansion and compression along the path a→b→c→a, as shown above. The work done along a→b of the cycle is the area
An ideal gas undergoes a cyclic expansion and compression along the path a→b→c→a, as shown above. The work done along a→b of the cycle is the areaA) shaded blue.
B) shaded yellow.
C) shaded blue and yellow.
D) negative of the area shaded blue.
E) negative of the area shaded yellow.

Unlock Deck
Unlock for access to all 120 flashcards in this deck.
Unlock Deck
k this deck
28
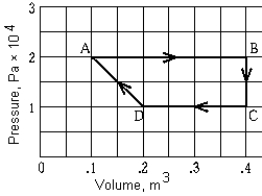 A reversible heat engine has the PV graph shown. The work done during the entire cycle is
A reversible heat engine has the PV graph shown. The work done during the entire cycle isA) zero.
B) 2.5 kJ.
C) 6.0 kJ.
D) 2.0 kJ.
E) 5.0 kJ.

Unlock Deck
Unlock for access to all 120 flashcards in this deck.
Unlock Deck
k this deck
29
A system is said to go through an isothermal process if it
A) remains at a constant temperature.
B) does no work on its surroundings.
C) remains in the same state.
D) neither gains nor loses heat.
E) gains or loses heat at a constant rate.
A) remains at a constant temperature.
B) does no work on its surroundings.
C) remains in the same state.
D) neither gains nor loses heat.
E) gains or loses heat at a constant rate.

Unlock Deck
Unlock for access to all 120 flashcards in this deck.
Unlock Deck
k this deck
30
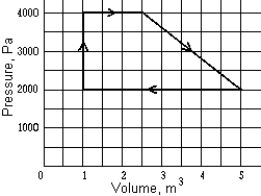 A reversible heat engine has the PV graph shown. The net work performed in one cycle is approximately
A reversible heat engine has the PV graph shown. The net work performed in one cycle is approximatelyA) zero.
B) 2.0 kJ.
C) 4.2 kJ.
D) 5.5 kJ.
E) 10 kJ.

Unlock Deck
Unlock for access to all 120 flashcards in this deck.
Unlock Deck
k this deck
31
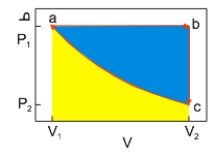 An ideal gas undergoes a cyclic expansion and compression along the path a→b→c→a, as shown above. The work done along c→a of the cycle is the area
An ideal gas undergoes a cyclic expansion and compression along the path a→b→c→a, as shown above. The work done along c→a of the cycle is the areaA) shaded blue.
B) shaded yellow.
C) shaded blue and yellow.
D) negative of the area shaded blue.
E) negative of the area shaded yellow.

Unlock Deck
Unlock for access to all 120 flashcards in this deck.
Unlock Deck
k this deck
32
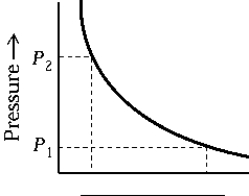 The work done by a gas on a piston can be obtained from the graph, provided the abscissa represents the
The work done by a gas on a piston can be obtained from the graph, provided the abscissa represents theA) internal energy.
B) temperature.
C) density.
D) volume.
E) time.

Unlock Deck
Unlock for access to all 120 flashcards in this deck.
Unlock Deck
k this deck
33
The pressure of a gas in an isobaric expansion remains constant. In such an expansion,
A) no work is done.
B) work is done by the gas.
C) work is done on the gas.
D) "isobaric" and "expansion" are contradictory terms.
E) work is or is not done, depending on whether the temperature of the gas changes.
A) no work is done.
B) work is done by the gas.
C) work is done on the gas.
D) "isobaric" and "expansion" are contradictory terms.
E) work is or is not done, depending on whether the temperature of the gas changes.

Unlock Deck
Unlock for access to all 120 flashcards in this deck.
Unlock Deck
k this deck
34
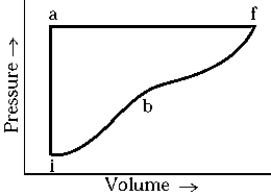 An ideal gas system changes from state i to state f by paths iaf and ibf. If the heat added along iaf is Qiaf = 50 cal, the work along iaf is Wiaf = 20 cal. Along ibf, if Qibf = 40 cal, the work done, Wibf, is
An ideal gas system changes from state i to state f by paths iaf and ibf. If the heat added along iaf is Qiaf = 50 cal, the work along iaf is Wiaf = 20 cal. Along ibf, if Qibf = 40 cal, the work done, Wibf, isA) 10 cal.
B) 20 cal.
C) 30 cal.
D) 40 cal.
E) 50 cal.

Unlock Deck
Unlock for access to all 120 flashcards in this deck.
Unlock Deck
k this deck
35
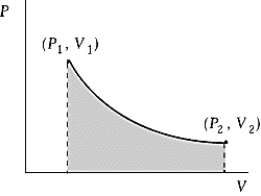 An ideal gas initially at 50oC and pressure P1 = 250 kPa occupies a volume V1 = 4.5 L. It undergoes a quasi-static, isothermal expansion until its pressure is reduced to 150 kPa. How much work was done by the gas during this process? R = 8.314 J/mol · K = 8.206 L · atm/mol · K.
An ideal gas initially at 50oC and pressure P1 = 250 kPa occupies a volume V1 = 4.5 L. It undergoes a quasi-static, isothermal expansion until its pressure is reduced to 150 kPa. How much work was done by the gas during this process? R = 8.314 J/mol · K = 8.206 L · atm/mol · K.A) 116 J
B) 320 J
C) 575 J
D) 640 J
E) 850 J

Unlock Deck
Unlock for access to all 120 flashcards in this deck.
Unlock Deck
k this deck
36
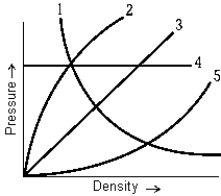 The curve on the graph of pressure versus density that best represents an isothermal process is
The curve on the graph of pressure versus density that best represents an isothermal process isA) 1.
B) 2.
C) 3.
D) 4.
E) 5.

Unlock Deck
Unlock for access to all 120 flashcards in this deck.
Unlock Deck
k this deck
37
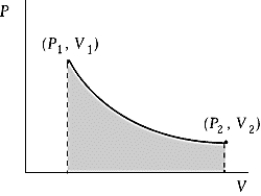 An ideal gas initially at 50oC and pressure P1 = 100 kPa occupies a volume V1 = 3 L. It undergoes a quasi-static, isothermal expansion until its pressure is reduced to 50 kPa. How much does the internal energy of the gas change during this process? R = 8.314 J/mol · K = 8.206 L · atm/mol · K.
An ideal gas initially at 50oC and pressure P1 = 100 kPa occupies a volume V1 = 3 L. It undergoes a quasi-static, isothermal expansion until its pressure is reduced to 50 kPa. How much does the internal energy of the gas change during this process? R = 8.314 J/mol · K = 8.206 L · atm/mol · K.A) 116 J
B) 208 J
C) 256 J
D) 304 J
E) The internal energy does not change during this process.

Unlock Deck
Unlock for access to all 120 flashcards in this deck.
Unlock Deck
k this deck
38
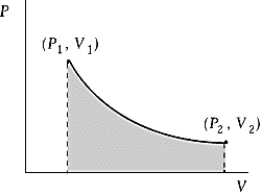 An ideal gas initially at 50oC and pressure P1 = 100 kPa occupies a volume V1 = 3 L. It undergoes a quasi-static, isothermal expansion until its pressure is reduced to 50 kPa. How much work was done by the gas during this process? R = 8.314 J/mol · K = 8.206 L · atm/mol · K.
An ideal gas initially at 50oC and pressure P1 = 100 kPa occupies a volume V1 = 3 L. It undergoes a quasi-static, isothermal expansion until its pressure is reduced to 50 kPa. How much work was done by the gas during this process? R = 8.314 J/mol · K = 8.206 L · atm/mol · K.A) 116 J
B) 208 J
C) 256 J
D) 304 J
E) 416 J

Unlock Deck
Unlock for access to all 120 flashcards in this deck.
Unlock Deck
k this deck
39
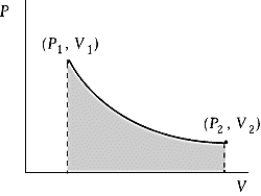 An ideal gas initially at 100oC and pressure P1 = 250 kPa occupies a volume V1 = 4.5 L. It undergoes a quasi-static, isothermal expansion until its pressure is reduced to 150 kPa. How much heat enters the gas during this process? R = 8.314 J/mol · K = 8.206 L · atm/mol · K.
An ideal gas initially at 100oC and pressure P1 = 250 kPa occupies a volume V1 = 4.5 L. It undergoes a quasi-static, isothermal expansion until its pressure is reduced to 150 kPa. How much heat enters the gas during this process? R = 8.314 J/mol · K = 8.206 L · atm/mol · K.A) 116 J
B) 320 J
C) 575 J
D) 640 J
E) 850 J

Unlock Deck
Unlock for access to all 120 flashcards in this deck.
Unlock Deck
k this deck
40
The equation of state for a certain gas under isothermal conditions is PV = 31.2, where the units are SI. The work done by this gas as its volume increases isothermally from 1 L to 10 L is approximately
A) 13.6 J.
B) 31.2 J.
C) 71.8 J.
D) 281 J.
E) 312 J.
A) 13.6 J.
B) 31.2 J.
C) 71.8 J.
D) 281 J.
E) 312 J.

Unlock Deck
Unlock for access to all 120 flashcards in this deck.
Unlock Deck
k this deck
41
A gas can absorb heat without changing temperature if at the same time
A) it is at its critical temperature.
B) it is kept at constant volume.
C) it is slowly compressed.
D) it does sufficient work in expanding.
E) it is confined by an adiabatic envelope.
A) it is at its critical temperature.
B) it is kept at constant volume.
C) it is slowly compressed.
D) it does sufficient work in expanding.
E) it is confined by an adiabatic envelope.

Unlock Deck
Unlock for access to all 120 flashcards in this deck.
Unlock Deck
k this deck
42
The internal energy for a diatomic gas is given by U = 5nRT/2. Calculate the internal energy of a 100 g mixture of oxygen 20%) and nitrogen 80%) gas at 25°C. The molar weight of O2 = 32 g, and the molar weight of N2 = 28 g.)
A) 21.6 kJ
B) 1.80 kJ
C) 12.1 kJ
D) 13.0 kJ
E) 1.10 kJ
A) 21.6 kJ
B) 1.80 kJ
C) 12.1 kJ
D) 13.0 kJ
E) 1.10 kJ

Unlock Deck
Unlock for access to all 120 flashcards in this deck.
Unlock Deck
k this deck
43
The specific heat of a gas is
A) the same for all gases.
B) directly proportional to the absolute temperature.
C) independent of constraints imposed on it while heating.
D) a negligible quantity.
E) greater at constant pressure than at constant volume.
A) the same for all gases.
B) directly proportional to the absolute temperature.
C) independent of constraints imposed on it while heating.
D) a negligible quantity.
E) greater at constant pressure than at constant volume.

Unlock Deck
Unlock for access to all 120 flashcards in this deck.
Unlock Deck
k this deck
44
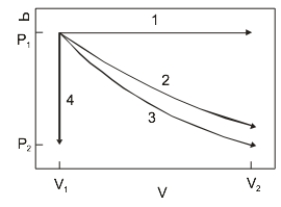 The diagram above show the state of an ideal gas going from V1, P1) to a final state. Which path best represents an isothermal expansion?
The diagram above show the state of an ideal gas going from V1, P1) to a final state. Which path best represents an isothermal expansion?A) 1
B) 2
C) 3
D) 4
E) None of the paths.

Unlock Deck
Unlock for access to all 120 flashcards in this deck.
Unlock Deck
k this deck
45
One mole of an ideal gas γ = 5/3) expands adiabatically and quasi-statically from a pressure P1 = 3 atm and a temperature of 30oC to a pressure P2 = 1 atm. How much work is done by the gas during this process? R = 8.314 J/mol · K = 8.206 L · atm/mol · K.
A) 50.3 kJ
B) 63.5 kJ
C) 95.9 kJ
D) 131 kJ
E) 158 kJ
A) 50.3 kJ
B) 63.5 kJ
C) 95.9 kJ
D) 131 kJ
E) 158 kJ

Unlock Deck
Unlock for access to all 120 flashcards in this deck.
Unlock Deck
k this deck
46
For an ideal gas, the difference in the molar heat capacity at constant P and constant V is
A) equal to R for monotomic gas.
B) equal to 2R for diatomic gas.
C) equal to NR for polyatomic gas, where N is the number of atoms in a polyatomic molecules.
D) equal to R for all gases.
E) A) and D)
A) equal to R for monotomic gas.
B) equal to 2R for diatomic gas.
C) equal to NR for polyatomic gas, where N is the number of atoms in a polyatomic molecules.
D) equal to R for all gases.
E) A) and D)

Unlock Deck
Unlock for access to all 120 flashcards in this deck.
Unlock Deck
k this deck
47
The pressure of a mass of air at 20°C is halved adiabatically. If the ratio of Cp to Cv for air is 1.41, calculate the resulting volume.
A) 2.66 times the original volume
B) 1.63 times the original volume
C) 2.00 times the original volume
D) 0.50 times the original volume
E) 0.61 times the original volume
A) 2.66 times the original volume
B) 1.63 times the original volume
C) 2.00 times the original volume
D) 0.50 times the original volume
E) 0.61 times the original volume

Unlock Deck
Unlock for access to all 120 flashcards in this deck.
Unlock Deck
k this deck
48
A gas has a molar heat capacity at constant volume of 28.39 J/mol · K. Assume the equipartition theorem to be valid. How many degrees of freedom including translational) are there for the molecules of this gas? The ideal-gas law constant is R = 8.31 J/mol · K)
A) 1
B) 3
C) 4
D) 5
E) 7
A) 1
B) 3
C) 4
D) 5
E) 7

Unlock Deck
Unlock for access to all 120 flashcards in this deck.
Unlock Deck
k this deck
49
One mole of an ideal gas γ = 5/3) expands adiabatically and quasi-statically from a pressure P1 = 6 atm and a temperature of 50oC to a pressure P2 = 4 atm. How much work is done by the gas during this process? R = 8.314 J/mol · K = 8.206 L · atm/mol · K.
A) 50.3 kJ
B) 56.2 kJ
C) 95.9 kJ
D) 131 kJ
E) 158 kJ
A) 50.3 kJ
B) 56.2 kJ
C) 95.9 kJ
D) 131 kJ
E) 158 kJ

Unlock Deck
Unlock for access to all 120 flashcards in this deck.
Unlock Deck
k this deck
50
An ideal gas initially at 50oC and pressure P1 = 100 kPa occupies a volume V1 = 3 L. It undergoes a quasi-static, isothermal expansion until its pressure is reduced to 50 kPa. How much heat enters the gas during this process? R = 8.314 J/mol · K = 8.206 L · atm/mol · K.
A) 116 J
B) 208 J
C) 256 J
D) 304 J
E) 416 J
A) 116 J
B) 208 J
C) 256 J
D) 304 J
E) 416 J

Unlock Deck
Unlock for access to all 120 flashcards in this deck.
Unlock Deck
k this deck
51
The specific heat of a gas at constant pressure is
A) directly proportional to the pressure.
B) inversely proportional to the pressure.
C) always greater than the specific heat at constant volume.
D) always less than the specific heat at constant volume.
E) independent of the kind of gas.
A) directly proportional to the pressure.
B) inversely proportional to the pressure.
C) always greater than the specific heat at constant volume.
D) always less than the specific heat at constant volume.
E) independent of the kind of gas.

Unlock Deck
Unlock for access to all 120 flashcards in this deck.
Unlock Deck
k this deck
52
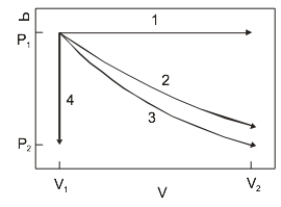 The diagram above shows the state of an ideal gas going from V1,P1) to a final state. Which path best represents adiabatic expansion?
The diagram above shows the state of an ideal gas going from V1,P1) to a final state. Which path best represents adiabatic expansion?A) 1
B) 2
C) 3
D) 4
E) None of the paths.

Unlock Deck
Unlock for access to all 120 flashcards in this deck.
Unlock Deck
k this deck
53
The molar heat capacity at constant volume of a gas is found to be 20.74 J/mol · K. What is the molar heat capacity at constant pressure of this gas? The ideal-gas law constant is R = 8.31 J/mol · K.)
A) 12.4 J/mol · K
B) 29.0 J/mol · K
C) 33.2 J/mol · K
D) 41.5 J/mol · K
E) 8.28 J/mol · K
A) 12.4 J/mol · K
B) 29.0 J/mol · K
C) 33.2 J/mol · K
D) 41.5 J/mol · K
E) 8.28 J/mol · K

Unlock Deck
Unlock for access to all 120 flashcards in this deck.
Unlock Deck
k this deck
54
From the measured molar heat capacities and the equipartition theorem, for a polyatomic gas molecule the number of degrees of freedom due to translational motion are
A) 3.
B) 6.
C) 5.
D) 2.
E) 7.
A) 3.
B) 6.
C) 5.
D) 2.
E) 7.

Unlock Deck
Unlock for access to all 120 flashcards in this deck.
Unlock Deck
k this deck
55
In a system composed of an ideal gas contained in a cylinder fitted with a piston, a reversible adiabatic expansion causes the temperature of the gas to drop because
A) heat is given up by the system when the piston moves.
B) the pressure of the gas remains constant.
C) work is done on the system as the gas expands.
D) work done by the system is done entirely at the expense of its internal energy.
E) heat is absorbed by the piston when it does work.
A) heat is given up by the system when the piston moves.
B) the pressure of the gas remains constant.
C) work is done on the system as the gas expands.
D) work done by the system is done entirely at the expense of its internal energy.
E) heat is absorbed by the piston when it does work.

Unlock Deck
Unlock for access to all 120 flashcards in this deck.
Unlock Deck
k this deck
56
From the measured molar heat capacities and the equipartition theorem, for a diatomic gas molecule the number of degrees of freedom from rotational motion are
A) 3.
B) 0.
C) 5.
D) 2.
E) 6.
A) 3.
B) 0.
C) 5.
D) 2.
E) 6.

Unlock Deck
Unlock for access to all 120 flashcards in this deck.
Unlock Deck
k this deck
57
An ideal monatomic gas has a molar heat capacity Cmp at constant pressure. What is the molar heat capacity at constant volume of an ideal diatomic gas?
A) Cmp
B) Cmp + R
C) Cmp - R
D) Cmp + 3R/2
E) Cmp - 3R/2
A) Cmp
B) Cmp + R
C) Cmp - R
D) Cmp + 3R/2
E) Cmp - 3R/2

Unlock Deck
Unlock for access to all 120 flashcards in this deck.
Unlock Deck
k this deck
58
The pressure of a mass of air at 20°C is halved adiabatically. If the ratio of Cp to Cv for air is 1.41, calculate the resulting temperature.
A) 240°C
B) 85.0°C
C) −33.0°C
D) −126°C
E) 16.0°C
A) 240°C
B) 85.0°C
C) −33.0°C
D) −126°C
E) 16.0°C

Unlock Deck
Unlock for access to all 120 flashcards in this deck.
Unlock Deck
k this deck
59
An ideal gas with an initial volume of 3 L at a pressure of 2 atm is compressed adiabatically until it has a volume of 2 L; then it is cooled at constant volume until its temperature drops to its initial value. The final pressure is
A)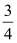 atm.
atm.
B) 2 atm.
C) 3 atm.
D) 4/3 atm.
E) 6 atm.
A)
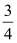 atm.
atm.B) 2 atm.
C) 3 atm.
D) 4/3 atm.
E) 6 atm.

Unlock Deck
Unlock for access to all 120 flashcards in this deck.
Unlock Deck
k this deck
60
Consider the following statement: "The specific heat of an ideal gas at constant pressure Cp is greater than the specific heat of a gas at constant volume Cv." Which of the following describes this statement?
A) The statement is true because there is always more gas at constant pressure.
B) The statement is true because, to raise the temperature of a gas at constant pressure, work must be done by the gas.
C) The statement is true because, to raise the temperature of a gas at constant volume, work must be done by the gas.
D) The statement is not true; Cv > Cp.
E) The statement is not true; Cp = Cv.
A) The statement is true because there is always more gas at constant pressure.
B) The statement is true because, to raise the temperature of a gas at constant pressure, work must be done by the gas.
C) The statement is true because, to raise the temperature of a gas at constant volume, work must be done by the gas.
D) The statement is not true; Cv > Cp.
E) The statement is not true; Cp = Cv.

Unlock Deck
Unlock for access to all 120 flashcards in this deck.
Unlock Deck
k this deck
61
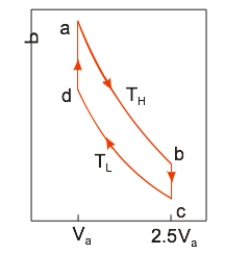 An ideal heat engine uses 0.01 mole of gas and operates between a hot reservoir at TH = 400 K and cold reservoir at TL = 300 K, in a cycle from a→b→c→d→a. From a→b the gas undergoes an isothermal expansion, changing its volume from Va to 2.5Va. From b→c, the pressure is reduced at a constant volume. From c→d, the gas undergoes an isothermal compression, and from d→a, the pressure is increased at a constant volume until the gas is back at the original condition at a. How much work is obtained from the engine in each cycle?
An ideal heat engine uses 0.01 mole of gas and operates between a hot reservoir at TH = 400 K and cold reservoir at TL = 300 K, in a cycle from a→b→c→d→a. From a→b the gas undergoes an isothermal expansion, changing its volume from Va to 2.5Va. From b→c, the pressure is reduced at a constant volume. From c→d, the gas undergoes an isothermal compression, and from d→a, the pressure is increased at a constant volume until the gas is back at the original condition at a. How much work is obtained from the engine in each cycle?A) 22.9 J
B) 30.5 J
C) 7.62 J
D) 8.31 J
E) 0.917 J

Unlock Deck
Unlock for access to all 120 flashcards in this deck.
Unlock Deck
k this deck
62
A heat engine with an output of 300 W has an efficiency of 25% and works at 10 cycles/s. How much heat is absorbed Qh) and how much rejected Qc) in each cycle?
A) Qh = 150 J, Qc = 120 J
B) Qh = 1500 J, Qc = 1200 J
C) Qh = 40 J, Qc = 10 J
D) Qh = 120 J, Qc = 90 J
E) Qh = 1200 J, Qc = 900 J
A) Qh = 150 J, Qc = 120 J
B) Qh = 1500 J, Qc = 1200 J
C) Qh = 40 J, Qc = 10 J
D) Qh = 120 J, Qc = 90 J
E) Qh = 1200 J, Qc = 900 J

Unlock Deck
Unlock for access to all 120 flashcards in this deck.
Unlock Deck
k this deck
63
The diagram below is a schematic of a heat engine. The three quantities, QH, QL, and W are represented, respectively, by 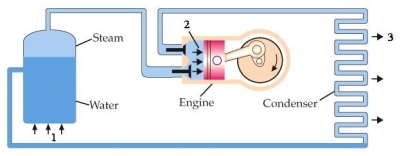
A) 1, 2, 3.
B) 1, 3, 2.
C) 2, 3, 1.
D) 3, 1, 2
E) 3, 2, 1.

A) 1, 2, 3.
B) 1, 3, 2.
C) 2, 3, 1.
D) 3, 1, 2
E) 3, 2, 1.

Unlock Deck
Unlock for access to all 120 flashcards in this deck.
Unlock Deck
k this deck
64
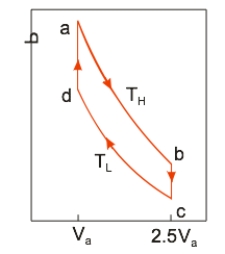 An ideal heat engine uses 0.01 mole of gas and operates between a hot reservoir at TH = 400 K and cold reservoir at TL = 300 K, in a cycle from a→b→c→d→a. From a→b the gas undergoes an isothermal expansion, changing its volume from Va to 2.5Va. From b→c, the pressure is reduced at a constant volume. From c→d, the gas undergoes an isothermal compression, and from d→a, the pressure is increased at a constant volume until the gas is back at the original condition at a. How much heat is absorbed in going from a→b?
An ideal heat engine uses 0.01 mole of gas and operates between a hot reservoir at TH = 400 K and cold reservoir at TL = 300 K, in a cycle from a→b→c→d→a. From a→b the gas undergoes an isothermal expansion, changing its volume from Va to 2.5Va. From b→c, the pressure is reduced at a constant volume. From c→d, the gas undergoes an isothermal compression, and from d→a, the pressure is increased at a constant volume until the gas is back at the original condition at a. How much heat is absorbed in going from a→b?A) 30.5 J
B) 7.62 J
C) 22.9 J
D) 8.31 J
E) 0.917 J

Unlock Deck
Unlock for access to all 120 flashcards in this deck.
Unlock Deck
k this deck
65
A heat engine absorbs 150 J of heat from a hot reservoir and rejects 90 J to a cold reservoir. What is the efficiency of this engine?
A) 20%
B) 40%
C) 60%
D) 67%
E) 90%
A) 20%
B) 40%
C) 60%
D) 67%
E) 90%

Unlock Deck
Unlock for access to all 120 flashcards in this deck.
Unlock Deck
k this deck
66
At a particular point on a PV diagram, the magnitude of the slope of a curve that represents an adiabatic process is
A) zero.
B) infinite.
C) the same as that of an isotherm through the same point.
D) less than that of an isotherm through the same point.
E) greater than that of an isotherm through the same point.
A) zero.
B) infinite.
C) the same as that of an isotherm through the same point.
D) less than that of an isotherm through the same point.
E) greater than that of an isotherm through the same point.

Unlock Deck
Unlock for access to all 120 flashcards in this deck.
Unlock Deck
k this deck
67
A heat engine exhausts heat Q to a cold reservoir. The amount of work done by the engine
A) is Q.
B) must be greater than Q.
C) must be less than Q.
D) could be greater than Q.
E) is zero.
A) is Q.
B) must be greater than Q.
C) must be less than Q.
D) could be greater than Q.
E) is zero.

Unlock Deck
Unlock for access to all 120 flashcards in this deck.
Unlock Deck
k this deck
68
In a system composed of gas contained in a cylinder fitted with a piston, an adiabatic expansion causes the temperature of the gas to drop because
A) heat is given up by the system when the piston moves.
B) the pressure of the gas remains constant.
C) work is done on the system as the gas expands.
D) work done by the system is entirely at the expense of its internal energy.
E) heat is absorbed by the piston when it does work.
A) heat is given up by the system when the piston moves.
B) the pressure of the gas remains constant.
C) work is done on the system as the gas expands.
D) work done by the system is entirely at the expense of its internal energy.
E) heat is absorbed by the piston when it does work.

Unlock Deck
Unlock for access to all 120 flashcards in this deck.
Unlock Deck
k this deck
69
A heat engine absorbs 64 kcal of heat from a hot reservoir and exhausts 42 kcal to a cold reservoir each cycle. Its efficiency is
A) 30%.
B) 34%.
C) 38%.
D) 40%.
E) 42%.
A) 30%.
B) 34%.
C) 38%.
D) 40%.
E) 42%.

Unlock Deck
Unlock for access to all 120 flashcards in this deck.
Unlock Deck
k this deck
70
When a gas is compressed adiabatically,
A) the pressure increases and the internal energy decreases.
B) the pressure increases and work is performed by the gas.
C) the temperature decreases and the internal energy increases.
D) the pressure is unchanged and heat flows out of the system.
E) work is done on the system and the temperature rises.
A) the pressure increases and the internal energy decreases.
B) the pressure increases and work is performed by the gas.
C) the temperature decreases and the internal energy increases.
D) the pressure is unchanged and heat flows out of the system.
E) work is done on the system and the temperature rises.

Unlock Deck
Unlock for access to all 120 flashcards in this deck.
Unlock Deck
k this deck
71
An engine operating in a cycle would violate the second law of thermodynamics if it
A) changed all the heat from a source to mechanical work.
B) changed all of its mechanical work to heat.
C) was irreversible.
D) operated between two isotherms and two adiabats.
E) was less efficient than a Carnot engine.
A) changed all the heat from a source to mechanical work.
B) changed all of its mechanical work to heat.
C) was irreversible.
D) operated between two isotherms and two adiabats.
E) was less efficient than a Carnot engine.

Unlock Deck
Unlock for access to all 120 flashcards in this deck.
Unlock Deck
k this deck
72
A system is said to go through an adiabatic process if, throughout the process,
A) it maintains a constant ratio of pressure to temperature.
B) it remains at a constant temperature.
C) it loses no heat to its surroundings and gains none from them.
D) its total energy increases.
E) it does no work on its surroundings.
A) it maintains a constant ratio of pressure to temperature.
B) it remains at a constant temperature.
C) it loses no heat to its surroundings and gains none from them.
D) its total energy increases.
E) it does no work on its surroundings.

Unlock Deck
Unlock for access to all 120 flashcards in this deck.
Unlock Deck
k this deck
73
A cylinder contains 20 L of air at 1 atm. The ratio of Cp to CV for air is 1.41. If this sample of air is compressed adiabatically to a volume of 5 L, the pressure after compression is approximately
A) 2.7 atm.
B) 7.1 atm.
C) 8.4 atm.
D) 4.0 atm.
E) 9.7 atm.
A) 2.7 atm.
B) 7.1 atm.
C) 8.4 atm.
D) 4.0 atm.
E) 9.7 atm.

Unlock Deck
Unlock for access to all 120 flashcards in this deck.
Unlock Deck
k this deck
74
If you run a refrigerator in a closed room with the door to the refrigerator open, the temperature of the room
A) increases.
B) remains the same.
C) decreases.
D) Any of these can happen depending on how efficient the refrigerator is.
E) Any of these can happen depending on the relative sizes of the room and the refrigerator.
A) increases.
B) remains the same.
C) decreases.
D) Any of these can happen depending on how efficient the refrigerator is.
E) Any of these can happen depending on the relative sizes of the room and the refrigerator.

Unlock Deck
Unlock for access to all 120 flashcards in this deck.
Unlock Deck
k this deck
75
A substance undergoes a series of reversible processes that bring it back to its initial state. In this cycle, heat Qx is absorbed by the substance and heat Qy is rejected. The net amount of work performed by the substance is
A) Qy - Qx.
B) Qx - Qy.
C) Qy - Qx)/Qy.
D) Qx - Qy)/Qx.
E) Qx - Qy)/Qy.
A) Qy - Qx.
B) Qx - Qy.
C) Qy - Qx)/Qy.
D) Qx - Qy)/Qx.
E) Qx - Qy)/Qy.

Unlock Deck
Unlock for access to all 120 flashcards in this deck.
Unlock Deck
k this deck
76
A heat engine absorbs 70 kcal of heat from a hot reservoir and exhausts 50 kcal to a cold reservoir each cycle. Its efficiency is
A) 20%.
B) 24%.
C) 29%.
D) 33%.
E) 37%.
A) 20%.
B) 24%.
C) 29%.
D) 33%.
E) 37%.

Unlock Deck
Unlock for access to all 120 flashcards in this deck.
Unlock Deck
k this deck
77
A heat engine operating between the temperatures T1 and T2 takes in Q1 calories at temperature T1 and gives up Q2 calories at temperature T2. The efficiency of this heat engine is
A) Q1 - Q2)/Q2.
B) Q1 - Q2)/Q1.
C) T2 - T1)/T2.
D) Q2/Q1 - Q2).
E) T1/ T1 - T2).
A) Q1 - Q2)/Q2.
B) Q1 - Q2)/Q1.
C) T2 - T1)/T2.
D) Q2/Q1 - Q2).
E) T1/ T1 - T2).

Unlock Deck
Unlock for access to all 120 flashcards in this deck.
Unlock Deck
k this deck
78
In an adiabatic reversible compression of an ideal gas, there is a decrease in
A) pressure.
B) temperature.
C) internal energy.
D) volume.
E) rms molecular velocity.
A) pressure.
B) temperature.
C) internal energy.
D) volume.
E) rms molecular velocity.

Unlock Deck
Unlock for access to all 120 flashcards in this deck.
Unlock Deck
k this deck
79
A heat engine absorbs heat Q from a hot reservoir. The amount of work done by the engine
A) is Q.
B) must be greater than Q.
C) must be less than Q.
D) could be greater than Q.
E) is zero.
A) is Q.
B) must be greater than Q.
C) must be less than Q.
D) could be greater than Q.
E) is zero.

Unlock Deck
Unlock for access to all 120 flashcards in this deck.
Unlock Deck
k this deck
80
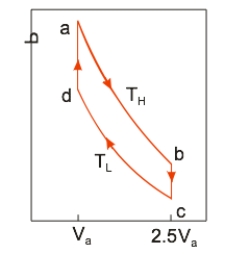 An ideal heat engine uses 0.01 mole of gas and operates between a hot reservoir at TH = 400 K and cold reservoir at TL = 300 K, in a cycle from a→b→c→d→a. From a→b the gas undergoes an isothermal expansion, changing its volume from Va to 2.5Va. From b→c, the pressure is reduced at a constant volume. From c→d, the gas undergoes an isothermal compression, and from d→a, the pressure is increased at a constant volume until the gas is back at the original condition at a. If the engine operates at 50 cycles per second, the power output is
An ideal heat engine uses 0.01 mole of gas and operates between a hot reservoir at TH = 400 K and cold reservoir at TL = 300 K, in a cycle from a→b→c→d→a. From a→b the gas undergoes an isothermal expansion, changing its volume from Va to 2.5Va. From b→c, the pressure is reduced at a constant volume. From c→d, the gas undergoes an isothermal compression, and from d→a, the pressure is increased at a constant volume until the gas is back at the original condition at a. If the engine operates at 50 cycles per second, the power output isA) 381 W.
B) 45.8 W.
C) 1145 W.
D) 415 W.
E) 1525 W.

Unlock Deck
Unlock for access to all 120 flashcards in this deck.
Unlock Deck
k this deck


