Deck 6: Ground Rules of Metabolism
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/54
Play
Full screen (f)
Deck 6: Ground Rules of Metabolism
1
The second law of thermodynamics holds that
A)matter can be neither created nor destroyed.
B)energy can be neither created nor destroyed.
C)energy of one form is converted to a less concentrated form whenever energy is transformed or transferred.
D)entropy decreases with time.
E)none of these is true.
A)matter can be neither created nor destroyed.
B)energy can be neither created nor destroyed.
C)energy of one form is converted to a less concentrated form whenever energy is transformed or transferred.
D)entropy decreases with time.
E)none of these is true.
C
2
The chemical bond energy in which of the following molecules is a consequence of the second law of thermodynamics?
A)fats
B)glucose
C)proteins
D)ATP
E)all of these
A)fats
B)glucose
C)proteins
D)ATP
E)all of these
E
3
The most common form of low-quality energy released in energy conversions is
A)metabolic.
B)heat.
C)entropy.
D)light.
E)electricity.
A)metabolic.
B)heat.
C)entropy.
D)light.
E)electricity.
B
4
Essentially, the first law of thermodynamics says that
A)one form of energy cannot be converted into another.
B)entropy is increasing in the universe.
C)energy can be neither created nor destroyed.
D)energy cannot be converted into matter or matter into energy.
E)all of these are true.
A)one form of energy cannot be converted into another.
B)entropy is increasing in the universe.
C)energy can be neither created nor destroyed.
D)energy cannot be converted into matter or matter into energy.
E)all of these are true.

Unlock Deck
Unlock for access to all 54 flashcards in this deck.
Unlock Deck
k this deck
5
Which of the following statements is false?
A)The universe has a specific amount of energy.
B)One form of energy can be converted to other forms of energy.
C)Whenever energy conversions occur, some energy is converted to less concentrated forms.
D)Once energy is utilized, it disappears.
E)There are differences in the quality of energy.
A)The universe has a specific amount of energy.
B)One form of energy can be converted to other forms of energy.
C)Whenever energy conversions occur, some energy is converted to less concentrated forms.
D)Once energy is utilized, it disappears.
E)There are differences in the quality of energy.

Unlock Deck
Unlock for access to all 54 flashcards in this deck.
Unlock Deck
k this deck
6
Which of the following violate(s) the second law of thermodynamics?
A)gasoline engines
B)life
C)nuclear power plants
D)stars such as the sun
E)none of these
A)gasoline engines
B)life
C)nuclear power plants
D)stars such as the sun
E)none of these

Unlock Deck
Unlock for access to all 54 flashcards in this deck.
Unlock Deck
k this deck
7
According to the first law of thermodynamics,
A)the energy of a system may increase if there is a corresponding decrease in energy elsewhere in the universe.
B)the amount of energy in the universe is constant.
C)chemical reactions do not create or destroy energy.
D)energy can change from one form to another.
E)all of these are true.
A)the energy of a system may increase if there is a corresponding decrease in energy elsewhere in the universe.
B)the amount of energy in the universe is constant.
C)chemical reactions do not create or destroy energy.
D)energy can change from one form to another.
E)all of these are true.

Unlock Deck
Unlock for access to all 54 flashcards in this deck.
Unlock Deck
k this deck
8
All of the following result from an increase in entropy EXCEPT
A)water from a melting ice cube.
B)destruction from a tsunami.
C)a decomposed body from a living body.
D)glucose molecules from photosynthesis.
E)ashes from burning paper.
A)water from a melting ice cube.
B)destruction from a tsunami.
C)a decomposed body from a living body.
D)glucose molecules from photosynthesis.
E)ashes from burning paper.

Unlock Deck
Unlock for access to all 54 flashcards in this deck.
Unlock Deck
k this deck
9
The enzyme responsible for breaking down alcohol is
A)alcohol methylase.
B)alcohol polyphosphorylase.
C)hydroxyl alcoholgenase.
D)transmethylogenase.
E)alcohol dehydrogenase.
A)alcohol methylase.
B)alcohol polyphosphorylase.
C)hydroxyl alcoholgenase.
D)transmethylogenase.
E)alcohol dehydrogenase.

Unlock Deck
Unlock for access to all 54 flashcards in this deck.
Unlock Deck
k this deck
10
CO2 and H2O will not form glucose on their own because
A)CO2 does not contain sufficient energy.
B)H2O does not contain sufficient energy.
C)neither CO2 nor H2O contain sufficient energy.
D)the concentration of CO2 is too low in the atmosphere.
E)the bonds of CO2 and H2O are too stable to be broken without an input of energy.
A)CO2 does not contain sufficient energy.
B)H2O does not contain sufficient energy.
C)neither CO2 nor H2O contain sufficient energy.
D)the concentration of CO2 is too low in the atmosphere.
E)the bonds of CO2 and H2O are too stable to be broken without an input of energy.

Unlock Deck
Unlock for access to all 54 flashcards in this deck.
Unlock Deck
k this deck
11
Which of the following statements about exergonic reactions is false?
A)They release energy.
B)Glucose metabolism is an example.
C)Their products have more energy than the reactants.
D)Some energy is converted to less biologically useful forms.
E)Bonds are broken.
A)They release energy.
B)Glucose metabolism is an example.
C)Their products have more energy than the reactants.
D)Some energy is converted to less biologically useful forms.
E)Bonds are broken.

Unlock Deck
Unlock for access to all 54 flashcards in this deck.
Unlock Deck
k this deck
12
Currently, the most serious drug problem on campuses is
A)methamphetamine addiction.
B)cocaine addiction.
C)marijuana addiction.
D)codeine addiction.
E)binge drinking.
A)methamphetamine addiction.
B)cocaine addiction.
C)marijuana addiction.
D)codeine addiction.
E)binge drinking.

Unlock Deck
Unlock for access to all 54 flashcards in this deck.
Unlock Deck
k this deck
13
Endergonic reactions
A)result in products with less energy than the reactants.
B)require a net input of energy.
C)occur in the breakdown of glucose.
D)are used by cells to provide energy for biological reactions.
E)break down large molecules into smaller molecules.
A)result in products with less energy than the reactants.
B)require a net input of energy.
C)occur in the breakdown of glucose.
D)are used by cells to provide energy for biological reactions.
E)break down large molecules into smaller molecules.

Unlock Deck
Unlock for access to all 54 flashcards in this deck.
Unlock Deck
k this deck
14
The blood circulatory system transports __________ percent of the absorbed ethanol to the liver.
A)10
B)25
C)50
D)75
E)almost 100
A)10
B)25
C)50
D)75
E)almost 100

Unlock Deck
Unlock for access to all 54 flashcards in this deck.
Unlock Deck
k this deck
15
The second law of thermodynamics states that
A)energy can be transformed into matter, and because of this, something is obtained for nothing.
B)energy can be destroyed during nuclear reactions such as those that occur inside the sun.
C)if energy is gained by one region of the universe, another place in the universe also must gain energy in order to maintain the balance of nature.
D)energy tends to flow from concentrated to less concentrated forms.
E)none of these is true.
A)energy can be transformed into matter, and because of this, something is obtained for nothing.
B)energy can be destroyed during nuclear reactions such as those that occur inside the sun.
C)if energy is gained by one region of the universe, another place in the universe also must gain energy in order to maintain the balance of nature.
D)energy tends to flow from concentrated to less concentrated forms.
E)none of these is true.

Unlock Deck
Unlock for access to all 54 flashcards in this deck.
Unlock Deck
k this deck
16
Which of the following quantities of alcoholic beverages have the least damaging effect on the liver?
A)12 ounces of beer
B)5 ounces of wine
C)1.5 ounces of vodka
D)either 12 ounces of beer or 5 ounces of wine
E)all have the same amount of ethanol and therefore have similar effects on the liver
A)12 ounces of beer
B)5 ounces of wine
C)1.5 ounces of vodka
D)either 12 ounces of beer or 5 ounces of wine
E)all have the same amount of ethanol and therefore have similar effects on the liver

Unlock Deck
Unlock for access to all 54 flashcards in this deck.
Unlock Deck
k this deck
17
Energy is commonly defined as "the capacity to do work." This definition is
A)incorrect.
B)adequate in the physical sciences.
C)adequate in the biological sciences.
D)no more than a clue to the actual properties of the concept.
E)not accepted by most scientists.
A)incorrect.
B)adequate in the physical sciences.
C)adequate in the biological sciences.
D)no more than a clue to the actual properties of the concept.
E)not accepted by most scientists.

Unlock Deck
Unlock for access to all 54 flashcards in this deck.
Unlock Deck
k this deck
18
Which of the following is an application of the first law of thermodynamics?
A)The level of entropy increases as time passes.
B)Living organisms represent an exception to the laws of energy.
C)The quantity of energy does not increase or decrease in the universe.
D)Fungi and plants do not make their own energy but derive it from somewhere else.
E)The amount of energy found in the compounds on one side of an equation is equal to that on the other side.
A)The level of entropy increases as time passes.
B)Living organisms represent an exception to the laws of energy.
C)The quantity of energy does not increase or decrease in the universe.
D)Fungi and plants do not make their own energy but derive it from somewhere else.
E)The amount of energy found in the compounds on one side of an equation is equal to that on the other side.

Unlock Deck
Unlock for access to all 54 flashcards in this deck.
Unlock Deck
k this deck
19
Which reaction is NOT an exergonic reaction?
A)protein synthesis
B)explosion
C)fire
D)respiration
E)movement
A)protein synthesis
B)explosion
C)fire
D)respiration
E)movement

Unlock Deck
Unlock for access to all 54 flashcards in this deck.
Unlock Deck
k this deck
20
The activation energy of a reaction refers to the minimum amount of energy
A)released by the reaction.
B)in the reactants.
C)in the products.
D)necessary to cause it to proceed on its own.
E)difference between the energy of the reactants and the energy of the products.
A)released by the reaction.
B)in the reactants.
C)in the products.
D)necessary to cause it to proceed on its own.
E)difference between the energy of the reactants and the energy of the products.

Unlock Deck
Unlock for access to all 54 flashcards in this deck.
Unlock Deck
k this deck
21
In enzyme-catalyzed reactions, substrate is synonymous with
A)end products.
B)by-products.
C)intermediates.
D)reactants.
E)co-enzyme.
A)end products.
B)by-products.
C)intermediates.
D)reactants.
E)co-enzyme.

Unlock Deck
Unlock for access to all 54 flashcards in this deck.
Unlock Deck
k this deck
22
Which of the following terms best describes the role of certain metal ions and coenzymes in metabolic processes?
A)reactants
B)intermediates
C)cofactors
D)products
E)catalysts
A)reactants
B)intermediates
C)cofactors
D)products
E)catalysts

Unlock Deck
Unlock for access to all 54 flashcards in this deck.
Unlock Deck
k this deck
23
The active site of an enzyme
A)is where the coenzyme is located.
B)is a specific bulge or protuberance on an enzyme.
C)is a groove or crevice in the structure of the enzyme complementary to the substrate.
D)will react with only one substrate no matter how many molecules may resemble the shape of the substrate.
E)rigidly resists any alteration of its shape.
A)is where the coenzyme is located.
B)is a specific bulge or protuberance on an enzyme.
C)is a groove or crevice in the structure of the enzyme complementary to the substrate.
D)will react with only one substrate no matter how many molecules may resemble the shape of the substrate.
E)rigidly resists any alteration of its shape.

Unlock Deck
Unlock for access to all 54 flashcards in this deck.
Unlock Deck
k this deck
24
An enzyme is thought to optimize the fit between substrates by restraining and stretching or squeezing them into certain shapes and moving them to the transition state as described by the __________ model of enzyme activity.
A)lock and key
B)induced-fit
C)template
D)activation
E)conformational
A)lock and key
B)induced-fit
C)template
D)activation
E)conformational

Unlock Deck
Unlock for access to all 54 flashcards in this deck.
Unlock Deck
k this deck
25
All of the following are true of the process of phosphorylation EXCEPT that
A)a molecule gains a phosphate group.
B)a molecule loses a phosphate group.
C)molecules become less stable.
D)it is used to prime molecules to react.
E)it produces ADP from ATP.
A)a molecule gains a phosphate group.
B)a molecule loses a phosphate group.
C)molecules become less stable.
D)it is used to prime molecules to react.
E)it produces ADP from ATP.

Unlock Deck
Unlock for access to all 54 flashcards in this deck.
Unlock Deck
k this deck
26
Which of the following statements is false?
A)Enzymes catalyze reversible reactions in either direction.
B)Enzymes are highly specific.
C)Most enzymes are protein molecules.
D)Enzymes allow some reactions to occur that would never occur without them.
E)Enzymes may be temporarily modified during their involvement with the substrate.
A)Enzymes catalyze reversible reactions in either direction.
B)Enzymes are highly specific.
C)Most enzymes are protein molecules.
D)Enzymes allow some reactions to occur that would never occur without them.
E)Enzymes may be temporarily modified during their involvement with the substrate.

Unlock Deck
Unlock for access to all 54 flashcards in this deck.
Unlock Deck
k this deck
27
ATP
A)easily gives up phosphate groups.
B)forms when ADP covalently bonds to phosphate.
C)is the energy currency in the cell's economy.
D)is made by all cells.
E)is/does all of these.
A)easily gives up phosphate groups.
B)forms when ADP covalently bonds to phosphate.
C)is the energy currency in the cell's economy.
D)is made by all cells.
E)is/does all of these.

Unlock Deck
Unlock for access to all 54 flashcards in this deck.
Unlock Deck
k this deck
28
Enzymatic reactions can be controlled by
A)the amount of substrates available.
B)the concentration of products.
C)activation of enzymes.
D)inhibition of enzymes.
E)all of these.
A)the amount of substrates available.
B)the concentration of products.
C)activation of enzymes.
D)inhibition of enzymes.
E)all of these.

Unlock Deck
Unlock for access to all 54 flashcards in this deck.
Unlock Deck
k this deck
29
All of the following are factors in enzyme-catalyzed reactions that work alone or in combination to lower the activation energy EXCEPT
A)helping substrates get together.
B)orienting substrates in positions favoring reaction.
C)using ATP as an energy source.
D)shutting out water molecules.
E)inducing a fit between enzyme and substrate.
A)helping substrates get together.
B)orienting substrates in positions favoring reaction.
C)using ATP as an energy source.
D)shutting out water molecules.
E)inducing a fit between enzyme and substrate.

Unlock Deck
Unlock for access to all 54 flashcards in this deck.
Unlock Deck
k this deck
30
ATP contains
A)adenine.
B)cytosine.
C)uracil.
D)thymine.
E)guanine.
A)adenine.
B)cytosine.
C)uracil.
D)thymine.
E)guanine.

Unlock Deck
Unlock for access to all 54 flashcards in this deck.
Unlock Deck
k this deck
31
Which of the following may show enzymatic activity? I.lipids
II)proteins
III)RNA
A)I only
B)II only
C)III only
D)I and II
E)II and III
II)proteins
III)RNA
A)I only
B)II only
C)III only
D)I and II
E)II and III

Unlock Deck
Unlock for access to all 54 flashcards in this deck.
Unlock Deck
k this deck
32
The binding between an enzyme and its substrate
A)is weak and temporary.
B)consists of strong covalent bonds.
C)consists of weak ionic bonds.
D)is relatively stable.
E)is none of these.
A)is weak and temporary.
B)consists of strong covalent bonds.
C)consists of weak ionic bonds.
D)is relatively stable.
E)is none of these.

Unlock Deck
Unlock for access to all 54 flashcards in this deck.
Unlock Deck
k this deck
33
The activation energy for a reaction
A)is less when enzymes are present.
B)allows greater interaction of substrate with the active site.
C)is needed to begin a reaction.
D)puts the substrate at its transition state.
E)is all of these.
A)is less when enzymes are present.
B)allows greater interaction of substrate with the active site.
C)is needed to begin a reaction.
D)puts the substrate at its transition state.
E)is all of these.

Unlock Deck
Unlock for access to all 54 flashcards in this deck.
Unlock Deck
k this deck
34
Which of the following is NOT true of enzyme behavior?
A)Enzyme shape may change during catalysis.
B)The active site of an enzyme orients its substrate molecules, thereby promoting interaction of their reactive parts.
C)All enzymes have an active site where substrates are temporarily bound.
D)Each enzyme can catalyze a wide variety of different reactions.
E)Enzyme activity is affected by pH and temperature.
A)Enzyme shape may change during catalysis.
B)The active site of an enzyme orients its substrate molecules, thereby promoting interaction of their reactive parts.
C)All enzymes have an active site where substrates are temporarily bound.
D)Each enzyme can catalyze a wide variety of different reactions.
E)Enzyme activity is affected by pH and temperature.

Unlock Deck
Unlock for access to all 54 flashcards in this deck.
Unlock Deck
k this deck
35
A "high-energy bond" in ATP
A)absorbs a large amount of free energy when the phosphate group is attached during hydrolysis.
B)is formed when ATP is hydrolyzed to ADP and one phosphate group.
C)is similar to the bonds in glucose molecules; that is why glucose can be used as a source of metabolic energy.
D)contributes to the "energy in" part of an endergonic reaction.
E)is/does all of these.
A)absorbs a large amount of free energy when the phosphate group is attached during hydrolysis.
B)is formed when ATP is hydrolyzed to ADP and one phosphate group.
C)is similar to the bonds in glucose molecules; that is why glucose can be used as a source of metabolic energy.
D)contributes to the "energy in" part of an endergonic reaction.
E)is/does all of these.

Unlock Deck
Unlock for access to all 54 flashcards in this deck.
Unlock Deck
k this deck
36
ATP contains
A)alanine.
B)arginine.
C)ribose.
D)tyrosine.
E)glucose.
A)alanine.
B)arginine.
C)ribose.
D)tyrosine.
E)glucose.

Unlock Deck
Unlock for access to all 54 flashcards in this deck.
Unlock Deck
k this deck
37
For an enzyme to function it requires
A)oxygen.
B)a suitable substrate.
C)a considerable amount of activation energy.
D)ATP.
E)all of these.
A)oxygen.
B)a suitable substrate.
C)a considerable amount of activation energy.
D)ATP.
E)all of these.

Unlock Deck
Unlock for access to all 54 flashcards in this deck.
Unlock Deck
k this deck
38
Enzymes
A)control the speed of a reaction.
B)change shapes to facilitate certain reactions.
C)may place physical stress on the bonds of the substrate.
D)may require cofactors.
E)do all of these.
A)control the speed of a reaction.
B)change shapes to facilitate certain reactions.
C)may place physical stress on the bonds of the substrate.
D)may require cofactors.
E)do all of these.

Unlock Deck
Unlock for access to all 54 flashcards in this deck.
Unlock Deck
k this deck
39
The regeneration of ATP
A)is an example of an endergonic reaction.
B)requires inorganic phosphate.
C)occurs through the ATP/ADP cycle.
D)is not the result of entropy.
E)is/does all of these.
A)is an example of an endergonic reaction.
B)requires inorganic phosphate.
C)occurs through the ATP/ADP cycle.
D)is not the result of entropy.
E)is/does all of these.

Unlock Deck
Unlock for access to all 54 flashcards in this deck.
Unlock Deck
k this deck
40
Enzymes
A)are very specific.
B)act as catalysts.
C)are organic molecules.
D)have special shapes that control their activities.
E)are all of these.
A)are very specific.
B)act as catalysts.
C)are organic molecules.
D)have special shapes that control their activities.
E)are all of these.

Unlock Deck
Unlock for access to all 54 flashcards in this deck.
Unlock Deck
k this deck
41
In some cases, inhibitors or activators of enzyme-catalyzed reactions act by
A)binding to the substrates.
B)affecting the supply of ATP.
C)reversibly binding to an enzyme's allosteric site.
D)reducing or increasing the concentration of enzymes.
E)binding to the products.
A)binding to the substrates.
B)affecting the supply of ATP.
C)reversibly binding to an enzyme's allosteric site.
D)reducing or increasing the concentration of enzymes.
E)binding to the products.

Unlock Deck
Unlock for access to all 54 flashcards in this deck.
Unlock Deck
k this deck
42
In oxidation-reduction reactions, all of the following are true EXCEPT
A)one molecule gives up electrons.
B)one molecule gains electrons.
C)cells release energy.
D)the molecule that accepts electrons is oxidized.
E)hydrogen ions are usually released.
A)one molecule gives up electrons.
B)one molecule gains electrons.
C)cells release energy.
D)the molecule that accepts electrons is oxidized.
E)hydrogen ions are usually released.

Unlock Deck
Unlock for access to all 54 flashcards in this deck.
Unlock Deck
k this deck
43
Enzyme activity may be affected by
A)salinity.
B)pH.
C)inhibitors.
D)the presence of cofactors.
E)all of these.
A)salinity.
B)pH.
C)inhibitors.
D)the presence of cofactors.
E)all of these.

Unlock Deck
Unlock for access to all 54 flashcards in this deck.
Unlock Deck
k this deck
44
Bioluminescence involves all of the following EXCEPT
A)stable electrons.
B)ATP.
C)oxygen.
D)luciferin.
E)luciferase.
A)stable electrons.
B)ATP.
C)oxygen.
D)luciferin.
E)luciferase.

Unlock Deck
Unlock for access to all 54 flashcards in this deck.
Unlock Deck
k this deck
45
Allosteric inhibition generally results from
A)excess substrates.
B)binding of regulatory molecules at sites other than the active sites.
C)a change in the temperature of the system.
D)a lack of coenzymes.
E)changes in pH.
A)excess substrates.
B)binding of regulatory molecules at sites other than the active sites.
C)a change in the temperature of the system.
D)a lack of coenzymes.
E)changes in pH.

Unlock Deck
Unlock for access to all 54 flashcards in this deck.
Unlock Deck
k this deck
46
Which of the following statements is false?
A)Enzymes are highly specific and act on chemicals called substrates.
B)Enzymes act as catalysts and speed up chemical reactions within cells.
C)Most enzymes work best under high salt conditions.
D)Most enzymes are proteins.
E)Enzymes can become deactivated by high fevers.
A)Enzymes are highly specific and act on chemicals called substrates.
B)Enzymes act as catalysts and speed up chemical reactions within cells.
C)Most enzymes work best under high salt conditions.
D)Most enzymes are proteins.
E)Enzymes can become deactivated by high fevers.

Unlock Deck
Unlock for access to all 54 flashcards in this deck.
Unlock Deck
k this deck
47
Enzyme activity may be affected by
A)temperature.
B)the presence of chemicals that fit into allosteric sites.
C)feedback inhibition.
D)metabolic conditions in the cell.
E)all of these.
A)temperature.
B)the presence of chemicals that fit into allosteric sites.
C)feedback inhibition.
D)metabolic conditions in the cell.
E)all of these.

Unlock Deck
Unlock for access to all 54 flashcards in this deck.
Unlock Deck
k this deck
48
Allosteric enzymes
A)have active sites where substrate molecules bind and other sites that bind with inhibitor or activator molecules.
B)are associated with important energy-carrying nucleotides.
C)are not affected by temperature or pH.
D)have two active sites.
E)all of these.
A)have active sites where substrate molecules bind and other sites that bind with inhibitor or activator molecules.
B)are associated with important energy-carrying nucleotides.
C)are not affected by temperature or pH.
D)have two active sites.
E)all of these.

Unlock Deck
Unlock for access to all 54 flashcards in this deck.
Unlock Deck
k this deck
49
Which of the following statements is true of metabolic pathways?
A)They are enzyme-mediated sequences of reactions.
B)They may be biosynthetic or degradative.
C)Photosynthesis is a biosynthetic pathway.
D)Catabolism is a degradative pathway.
E)All of these are true.
A)They are enzyme-mediated sequences of reactions.
B)They may be biosynthetic or degradative.
C)Photosynthesis is a biosynthetic pathway.
D)Catabolism is a degradative pathway.
E)All of these are true.

Unlock Deck
Unlock for access to all 54 flashcards in this deck.
Unlock Deck
k this deck
50
Questions refer to the figure above.
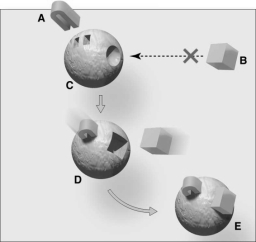
The substance indicated as "A" is a(n)
A)inhibitor.
B)activator.
C)substrate.
D)coenzyme.
E)cofactor.

The substance indicated as "A" is a(n)
A)inhibitor.
B)activator.
C)substrate.
D)coenzyme.
E)cofactor.

Unlock Deck
Unlock for access to all 54 flashcards in this deck.
Unlock Deck
k this deck
51
Questions refer to the figure above.

The substance indicated as "B" is a(n)
A)inhibitor.
B)activator.
C)substrate.
D)coenzyme.
E)cofactor.

The substance indicated as "B" is a(n)
A)inhibitor.
B)activator.
C)substrate.
D)coenzyme.
E)cofactor.

Unlock Deck
Unlock for access to all 54 flashcards in this deck.
Unlock Deck
k this deck
52
Coenzymes such as NAD+ and NADP+
A)are tightly bound to enzymes.
B)are associated with metal ions.
C)diffuse freely through the cytoplasm.
D)are not modified in reactions.
E)all of these.
A)are tightly bound to enzymes.
B)are associated with metal ions.
C)diffuse freely through the cytoplasm.
D)are not modified in reactions.
E)all of these.

Unlock Deck
Unlock for access to all 54 flashcards in this deck.
Unlock Deck
k this deck
53
Questions refer to the figure above.

The figure represents
A)an exergonic reaction.
B)a metabolic pathway.
C)allosteric inhibition.
D)allosteric activation.
E)all of these.

The figure represents
A)an exergonic reaction.
B)a metabolic pathway.
C)allosteric inhibition.
D)allosteric activation.
E)all of these.

Unlock Deck
Unlock for access to all 54 flashcards in this deck.
Unlock Deck
k this deck
54
Questions refer to the figure above.

An enzyme substrate complex is represented by
A)C.
B)D.
C)E.
D)D and E.
E)None of these.

An enzyme substrate complex is represented by
A)C.
B)D.
C)E.
D)D and E.
E)None of these.

Unlock Deck
Unlock for access to all 54 flashcards in this deck.
Unlock Deck
k this deck


